Abstract
The natural isotopic composition of 13C and 12C in tissues is largely determined by the diet. Sources of provitamin A carotenoids (e.g., vegetables) typically have a lower 13C to 12C ratio (13C:12C) than preformed vitamin A sources (i.e., dairy and meat) from corn-fed animals, which are prevalent in the US. The 13C:12C of serum retinol (13C:12C-retinol) was evaluated as a biomarker for vegetable intake in a 3-mo dietary intervention designed to promote weight-loss by increased vegetable consumption or reduced calorie and fat intake. Subjects were 21–50 y of age with a BMI between 30–40 kg/m2 and were enrolled from one geographic area in the US. The high vegetable group (n = 20) was encouraged to increase daily vegetable and fruit consumption to 0.95 liter vegetables and 0.24–0.35 liter fruits. The caloric reduction group (n = 17) was encouraged to lower caloric intake by 500 kcal and consume ≤25% kcal from fat daily. Provided meals supplied 75–100% vegetable and fruit goals and 50–67% kcal and fat g per day. Carotenoid supplementation was discontinued by subjects during the study. Serum retinol and provitamin A carotenoid concentrations; intake of preformed vitamin A, provitamin A, and fat; and body weight, fat mass, and lean mass were analyzed for correlations to 13C:12C-retinol. 13C:12C-Retinol decreased in the vegetable group after intervention (P = 0.050) and the correlation with provitamin A intake was approaching significance (P = 0.079). 13C:12C-Retinol did not change in the caloric reduction group (P = 0.43). 13C:12C-Retinol changes with the vitamin A source in the diet and can be used as a biomarker for increases in dietary provitamin A vegetable intake.
Keywords: β-carotene, 13C, isotope, natural abundance, vegetable intake, vitamin A
Introduction
Stable isotopes as tracers are valuable tools to determine biochemical pathways and mechanisms and nutrient requirements. In the field of vitamin A, isotopically labeled vitamin A has been used in kinetic, metabolic, and status assessment studies (1-9). Provitamin A carotenoids, in both plant and synthetic forms, have also been labeled with stable isotopes to determine bioconversion rates to vitamin A (10-15).
The natural isotopic composition of carbon in animal tissues is largely determined by the diet (16). Plants have different photosynthetic mechanisms that discriminate between the 12CO2 and 13CO2 that is incorporated into organic compounds. Most vegetables and temperate grains (e.g., wheat and rice) are C3 plants. Typically, C4 plants come from hot, dry climates and include crops such as maize, sorghum, and sugarcane, as well as many forage grasses. Different assimilation rates of 13C and 12C by C3 and C4 plants cause the isotopic ratio of plants to differ by 13 – 15‰, enriching C4 plants with 13C (16-18). Animals consuming primarily C3 feeds have a different ratio of 13C to 12C (13C:12C) in milk, serum, meat, and liver compared with animals consuming C4 feeds (16, 18). Similarly, the 13C:12C in human hair differs in vegetarians and omnivores (19-20). Because isotopic differences in tissues are directly related to the diet, one study suggested using 13C:12C as a marker for specific foods such as sweeteners derived from C4 plants (21).
Vitamin A can be obtained from the diet as preformed vitamin A in dairy and organ meats, or as provitamin A carotenoids from vegetables and fruits. Provitamin A is typically in the form of β-carotene, β-cryptoxanthin, and α-carotene, which are commonly found in orange and yellow fruits and vegetables. Most plant sources of provitamin A in the human diet are C3 plants (e.g., carrot, sweet potato, pumpkin, and spinach). One exception is corn (C4 plant), but typical provitamin A concentrations are low (22) and probably do not contribute appreciably to the vitamin A pool of North Americans. The average 13C enrichment, reported as δ 13C, for common C3 fruits and vegetables is -27.16‰ (18). Isotope ratios are reported in standard delta notation relative to Vienna Pee Dee Beleminite (VPDB), where δ 13C = [[Rsample/RVPDB] − 1] × 1000 and R = 13C/12C. All reported δ 13C values are with respect to VPDB and are negative when 13C:12C of the sample is less than VPDB.
In the US, the cattle and dairy industries rely on corn-based diets, while European growers use predominantly C3 plants in their feed (18). Because of the difference in feed, meat and dairy products in the US typically have δ 13C values from -13.5 to -19.2‰, while non-corn-fed European meat and dairy products typically range from -26.02 to -30.38‰ (18, 23). Thus, most dietary sources of preformed vitamin A in the US are enriched with 13C by 10 – 15‰ compared to vegetable sources of provitamin A. If the δ 13C values of animal products correlate with preformed vitamin A in meat and dairy products, altering the ratio of preformed vitamin A to provitamin A in the diet would change the δ 13C value of serum retinol.
The objective of this study was to evaluate the change in δ 13C value of serum retinol after a 3-mo intensive intervention study to promote weight loss. The two dietary weight-loss strategies included increased vegetable intake or reduced calorie and fat intake. Serum retinol and provitamin A carotenoid concentrations; preformed vitamin A, provitamin A carotenoid, and fat intakes; and body weight, fat mass, and lean mass were analyzed for correlations to the δ 13C retinol values using gas chromatography-combustion-isotope ratio mass spectrometry.
Materials and Methods
Subjects
This study was part of a larger weight-loss study designed to compare the effects of two dietary strategies on weight loss, body composition, serum chemistry profile, and serum vitamin A and carotenoids. The complete weight-loss study monitored subjects for 18 mo. This study utilized data collected during the controlled feeding period between 0 and 3 mo. The two dietary strategies were increased vegetable consumption or a 500 kcal/d reduction diet. Of the 60 subjects enrolled in the main trial, those with complete dietary records and adequate serum for carbon isotope analysis [n = 20 and 17 from the vegetable and caloric reduction groups, respectively] were evaluated (Table 1). Subjects were between 21 and 50 y of age, had a BMI between 30 and 40 kg/m2, and visited the study kitchen to collect breakfast and lunch during the 3-mo feeding period. Exclusion criteria before enrollment included consumption of ≥2.5 c/d (0.6 liter/d) vegetables and/or fruits; history of insulin treatment, drug, or alcohol abuse; participation in other research studies that may confound results; pregnancy or lactation; serious medical or psychiatric illness; unwillingness or inability to discontinue use of supplements containing carotenoids; use of drugs that might affect weight loss; and weight change > 3% of body weight during the 3 mo prior to recruitment. Subjects gave written informed consent. The Health Sciences Institutional Review Board at the University of Wisconsin – Medical School approved all aspects of this study.
Table 1.
Characteristics of Subjects in the Vegetable and Reduced Calorie and Fat (Caloric Reduction) Groups at 0 and 3 mo after Intervention. Subjects Were Fed 10 Meals/Wk during the Intervention Designed to Promote Weight-loss1
| Vegetable2 | Caloric Reduction | |||
|---|---|---|---|---|
| 0 Mo | 3 Mo | 0 Mo | 3 Mo | |
| Age (y) | 32.8 ± 8.4 | 32.9 ± 8.9 | 34.5 ± 8.5 | 34.7 ± 8.3 |
| Female/Male (n) | 14/6 | 13/4 | ||
| Weight (kg) | 95.0 ± 15.4a | 93.6 ± 9.8b | 97.2 ± 19.0a | 91.9 ± 19.8b |
| Fat mass (kg) | 39.6 ± 9.8a | 36.8 ± 10.2b | 42.2 ± 11.4a | 36.1 ± 10.7b |
| Fat-free mass (kg) | 56.1 ± 11.8 | 56.3 ± 11.7 | 56.2 ± 13.5 | 56.0 ± 13.9 |
| BMI (kg/m2) | 33.7 ± 3.7 | 33.0 ± 3.6 | 32.4 ± 3.0 | 31.0 ± 3.0 |
Values are mean ± SD for all such values.
Differences between 0 and 3 mo within each treatment group were tested with a paired t-test within a group, significance is indicated with a superscript (α = 0.05). Differences between vegetable and caloric reduction groups at baseline and 3 mo were tested with a 2 sample t-test (α = 0.05) and results are as follows. Age, weight, fat and fat-free mass, and BMI did not differ between vegetable and caloric reduction groups at either 0 or 3 mo.
Body composition
Body composition was measured by air displacement plethysmography (BOD POD®, Life Measurements, Inc., Concord, CA; 24). Weight was measured using the BOD POD® scale, which was tested with calibration weights each day of use. Height was measured at baseline using a wall-mounted stadiometer. All body composition and weight assessments were conducted by BOD POD® certified users. BMI was calculated from the weight and height measurements.
Diets
The subjects in the vegetable group were educated about vegetable and fruit consumption from the Food Guide Pyramid (25), which was based on the Dietary Guidelines for Americans, 5th Ed (26). In the Food Guide Pyramid, consumption is defined by servings [1 serving of vegetable or fruit is equivalent to ½ c (0.12 liter) fresh vegetable or fruit]. Subjects in the vegetable group were given a daily goal of consuming 8 servings (4 c, 0.95 liter) of vegetables and 2 – 3 servings (1 – 1.5 c, 0.24 – 0.35 liter) of fruits. Serving sizes were measured using standard US measuring cups. Subjects were discouraged from consuming potato chips, french fries, or fruit juices to meet their vegetable and fruit goals. Yellow corn was not excluded from the diet. Contributions of provitamin A from yellow corn to the vitamin A pool were expected to be minor due to low provitamin A content of corn and low ratio of corn to C3 vegetables in the diet.
Subjects in the caloric reduction group were given two daily goals: to reduce daily caloric intake by 500 kcal from the estimated caloric intake needed for weight maintenance at baseline, and to consume ≤25% of kcal from fat. Daily caloric need for weight-maintenance at baseline was estimated by multiplying an individual's estimated resting energy expenditure by an individually appropriate activity factor. Resting energy expenditure was estimated based on height, weight, age, and sex using published equations (27). Activity factors were based on those recommended by the Institute of Medicine (28). They were 1.3 for very light activity, 1.5 for light activity, and 1.6 for moderate activity.
All subjects were provided with 2 meals/d, Monday through Friday (10 meals total). Subjects in the vegetable group were provided with 75-88% of their daily vegetable goal and 100% of the fruit goal. The food provided to the caloric reduction group supplied 50-67% kcal and fat g for the day. The vegetable group received twice as many vegetables as the caloric reduction group. The provided protein in the meals and fat-free milk (3.6 liter/wk/person) were the same for both groups.
3-d diet records
Subjects were asked to complete 3-d diet records (2 weekdays and 1 weekend day) at 0 and 3 mo. Completed diet records were analyzed for total vegetable intake (1 c = 0.24 liter) and vitamin A consumption using Nutritionist Pro™ Version 3.1.0 (Axxya Systems; Stafford, TX, © 2007). Foods or their ingredients were classified into two groups, those providing preformed vitamin A and those providing provitamin A derived from carotenoids. Preformed vitamin A included all vitamin A consumed from meat, dairy, and egg products, as well as synthetic vitamin A added for fortification. Provitamin A included all vitamin A attributed to plant-derived and synthetic carotenoids. Foods with multiple sources of vitamin A (e.g., pizza and salad) were divided into preformed vitamin A and provitamin A based on ingredients. Several foods reported in dietary records were not found in the NutritionistPro™ database. Dietary estimates of these foods were based on nutritional information provided on food labels or by restaurants.
Analysis of carotenoids and retinol in serum
Whole blood samples were taken at 0 and 3 mo after an overnight fast (≥8 h). Blood was placed into sterile-interior 6 ml Corvac brand serum separator tubes with clot activator (Tyco Healthcare Group LP, MA). Samples were centrifuged at 2200 × g for 10 min at 4°C after clotting for 10 – 20 min at room temperature. Serum was stored at -80°C until analysis. Serum carotenoids and retinol were analyzed using a modification of a previously published method (29). Briefly, 600 μl ethanol with 0.1% butylated hydroxytoluene was added to 500 μl serum and mixed with a vortex. Retinol and carotenoids were extracted three times with 1 ml hexanes with mixing and centrifugation. The pooled extracts were dried under argon, reconstituted in 100 μl 50:50 (by vol) methanol:dichloroethane, and 50 μl injected into the HPLC system. β-Apo-8′-carotenyl decanoate was used as an internal standard.
The HPLC system consisted of a Resolve C18 (5 μm, 3.9 × 300 mm) column, a Waters 2996 photodiode array detector, 1525 binary pump, and 717 autosampler injector (Milford, MA). The mobile phases were 95:5 (by vol) acetonitrile:water (solvent A) and 85:10:5 (by vol) acetonitrile:methanol:dichloroethane (solvent B), both containing 10 mM ammonium acetate. Samples were analyzed at a flow rate of 2 ml/min using the following gradient: 100% solvent A for 3 min, 7-min linear change to 100% solvent B, 100% solvent B for 10 min, 3-min linear change to 100% solvent A, and 100% solvent A for 1 min. Chromatograms were generated at 450 and 325 nm to quantify carotenoids and retinol, respectively. Standard curves were prepared with purified α-carotene, β-carotene, lutein, lycopene, retinol, and zeaxanthin. Concentrations were determined spectrophotometrically using their respective [i.e., 2800 for α-carotene, 2592 for β-carotene, 2550 for lutein, 3450 for lycopene, 1845 for retinol, and 2348 for zeaxanthin (30-31)].
Isotopic ratio of 13C to 12C in serum retinol
The 13C:12C in serum retinol was determined according to a modification of the method by Tanumihardjo (8). After proteins were precipitated with ethanol (2 ml), retinol was extracted 3 times from serum (1 – 1.5 ml) with hexanes (1 – 2 ml). Extracted layers were combined and dried under argon, reconstituted in 100 μl methanol, frozen for 5 min at -80°C, centrifuged at 1380 × g at room temperature for 30 s, and injected into a 15-cm Resolve® HPLC column (3.9 × 150 mm, 5 μm, Waters Corporation, Milford, MA) equilibrated with 90:10 methanol:water (by vol) at 1 ml/min. Retinol was collected, dried under argon, and further purified on a 30-cm Resolve® HPLC column (3.9 × 300 mm, 5 μm, Waters Corporation) equilibrated with 98:2 methanol:water (by vol) at 1 ml/min. Collected retinol was dried in a Thermo Savant Speed-Vacuum centrifuge (Thermo Scientific; Waltham, MA), reconstituted in 10 μl hexanes, and 1.5 μl injected into a gas chromatography/combustion/isotope ratio mass spectrometer (GCCIRMS). The Trace GC (Thermo Scientific) was equipped with a Programmable Temperature Vaporizing (PTV) injector, a 15-m HP-1MS GC column (Agilent Technologies, Santa Clara, CA), and a 1-m deactivated fused-silica pre-column (0.53 μm i.d.), and was connected to a Combustion III and Advantage Plus isotope ratio mass spectrometer (Thermo Scientific). Samples were injected simulating on-column injection with the PTV injector at 43°C. Temperature of the PTV injector was ramped to 50°C, matching the initial oven temperature, prior to injection. Oven temperature increased at 15°C/s to 300°C. The δ 13C-retinol values were analyzed from serum samples by GCCIRMS in duplicate. Synthetic retinol, prepared by quick saponification of retinyl acetate (Sigma-Aldrich; St Louis, MO), was purified twice similarly to serum retinol and used as an external standard.
Isotopic ratio of 13C to 12C in milk retinol
Vitamin A and D fortified fat-free milk was purchased from the same supplier (University of Wisconsin-Madison Babcock Hall Dairy Store, Madison, WI) that was used during the feeding period and analyzed. Three ml ethanol with 0.1% butylated hydroxytoluene was added to 2 ml milk and mixed by vortex. To remove remaining fat in the sample, the samples were saponified for 30 min at 45°C using 800 μl 500 g/liter potassium hydroxide in water. After saponification, the retinol was extracted from the sample 3 times with 1.5 ml hexanes. After the pooled extracts were dried under argon and reconstituted in 100 μl methanol, retinol was purified and analyzed by GCCIRMS as described for serum retinol.
Isotopic ratio of 13C to 12C in vegetable material
The provitamin A containing C3 plants fed during the intervention were carrots, spinach, and canned pumpkin. These foods were freeze-dried with a Virtis Benchtop 6K freeze-drier (SP Industries; Gardiner, NY) and ground into a powder using a coffee grinder. For comparison, typical yellow field corn (dried), a C4 plant, was provided by the University of Illinois at Urbana-Champaign and analyzed. It was ground to pass a <1 mm screen prior to analysis using a hammer mill. Dried plant material (1 to 2 mg) was weighed into tin capsules and encapsulated into a ball. A Costech ECS 4010 Elemental Combustion System CHNS-O (Costech Analytical Technologies, Inc., Valencia, CA) equipped with a Conflo III (ThermoScientific) and attached to an Advantage Plus isotope ratio mass spectrometer (described previously) was used to determine δ 13C of plant materials. Samples were replicated 10 times.
Statistical analysis
Data were analyzed using Statistical Analysis System software (SAS Institute Inc., Version 8.2, Cary, NC; 2001) to perform paired t-tests, two-sample t-tests, correlations, model building using backward, forward, and step-wise elimination. Significance was evaluated at P ≤ 0.050, unless stated otherwise for the elimination procedures.
Results
Body composition
Body weight and fat mass decreased between 0 and 3 mo for both the vegetable (P = 0.002 and P < 0.001 for body weight and fat mass, respectively) and caloric reduction groups (P < 0.001 for both) (Table 1). Although there was no difference in body weight or fat mass between the groups at either time, there was a significant difference in the change in body weight (P = 0.001) and fat mass (P = 0.020) between the groups. The vegetable group lost -2.2 ± 2.5 kg body weight and -3.0 ± 3.0 kg fat mass compared to -5.8 ± 3.7 and -7.9 ± 5.4 kg in the caloric reduction group, respectively. Fat-free mass and BMI did not differ at 0 or 3 mo or between the dietary groups.
3-d diet records
At baseline, preformed vitamin A and provitamin A intake did not differ between groups (Table 2). Preformed vitamin A intake did not change from 0 to 3 mo in either group, but provitamin A intake increased in both groups (P ≤ 0.002). At 3 mo, preformed vitamin A intake did not differ between groups, but provitamin A was greater in the vegetable group compared with the caloric reduction group (P < 0.001). This corresponded to an increase in total vegetable consumption by the vegetable group on a volume basis (P = 0.018). Almost all of the provitamin A provided to and consumed by subjects was from vegetables that were C3 plants, including carrots, pumpkin, and spinach.
Table 2.
Dietary Intake and Serum Characteristics of Subjects in the Vegetable and Reduced Calorie and Fat (Caloric Reduction) Groups at 0 and 3 mo after Intervention1
| Vegetable2 | Caloric Reduction | ||||
|---|---|---|---|---|---|
| Mo | 0 | 3 | 0 | 3 | |
| Dietary Intake Characteristics3 | |||||
| Vegetable increase4 | c/d | 1.65 ± 1.31a | 0.78 ± 0.88b | ||
| Preformed VA5 | mg VA/d | 0.58 ± 0.19 | 0.71 ± 0.46 | 0.61 ± 0.27 | 0.64 ± 0.22 |
| Provitamin A | mg βC/d | 2.9 ± 4.1b | 12.2 ± 5.3a | 2.7 ± 2.6b | 6.3 ± 3.6a |
| Fat | g/d | 91 ± 19.6a | 61.7 ± 21.7b | 86.6 ± 41.3a | 46.9 ± 22.2b |
| Serum Characteristics | |||||
| α-Carotene | μM | 0.06 ± 0.03b | 0.33 ± 0.20a | 0.07 ± 0.06b | 0.27 ± 0.13a |
| β-Carotene | μM | 0.42 ± 0.24b | 1.17 ± 0.82a | 0.59 ± 0.77b | 1.02 ± 0.65a |
| Retinol | μM | 2.21 ± 0.46 | 2.21 ± 0.44 | 1.96 ± 0.48 | 2.08 ± 0.62 |
Values are mean ± SD, n = 20 (vegetable) and n = 17 (caloric reduction).
Differences between 0 and 3 mo within each treatment groups were tested with a paired t-test, significance is indicated with a superscript (α = 0.05). Differences between vegetable and caloric reduction groups were tested with a 2 sample t-test (α = 0.05) at baseline and 3 mo and results are as follows. Preformed vitamin A, provitamin A, and fat intake did not differ at baseline. At 3 mo, provitamin A and fat intake were significantly higher (P < 0.001 and P < 0.05, respectively) in the vegetable group compared to the caloric reduction group. Serum characteristics (α-carotene, β-carotene, and retinol) did not differ between vegetable and caloric reduction groups at either 0 or 3 mo.
Intake of preformed vitamin A, provitamin A, and fat were evaluated from 3-d diet records. Foods were categorized into either preformed vitamin A or provitamin A and converted to mg retinol and β-carotene as described in the USDA nutrient database. Preformed vitamin A includes meat, dairy, and synthetic vitamin A in foods. Provitamin A includes all plant-derived and synthetic carotenoids.
Significance between groups for daily vegetable increase is indicated with a superscript.
Abbreviations used: VA, vitamin A.
Fat intake was monitored as a possible co-variant because dietary fat is required for absorption of vitamin A and carotenoids (32-34). Fat consumption did not differ between the groups at baseline, and decreased from 0 to 3 mo in both groups (P < 0.001 and P = 0.002 for vegetable and caloric reduction groups, respectively; Table 2). At 3 mo, the fat intake was higher in the vegetable group than the caloric reduction group (P = 0.049).
Milk intake estimated from the 3-d records was monitored to ensure changes in δ 13C were not due to differences in milk intake. Milk intake increased for both groups by approximately 300 ± 300 ml/d from baseline to 3 mo due to the feeding study (P < 0.001). Changes in milk intake did not differ between the groups (P = 0.92).
Serum carotenoids and retinol
From 0 to 3 mo, serum α- and β-carotene increased in both the vegetable (P < 0.001 for both α- and β-carotene; Table 2) and caloric reduction groups (P < 0.001 and P = 0.046 for α- and β-carotene, respectively), but serum retinol did not differ. Serum α-carotene, β-carotene, and retinol did not differ between the groups at baseline or 3 mo.
δ 13C of serum retinol
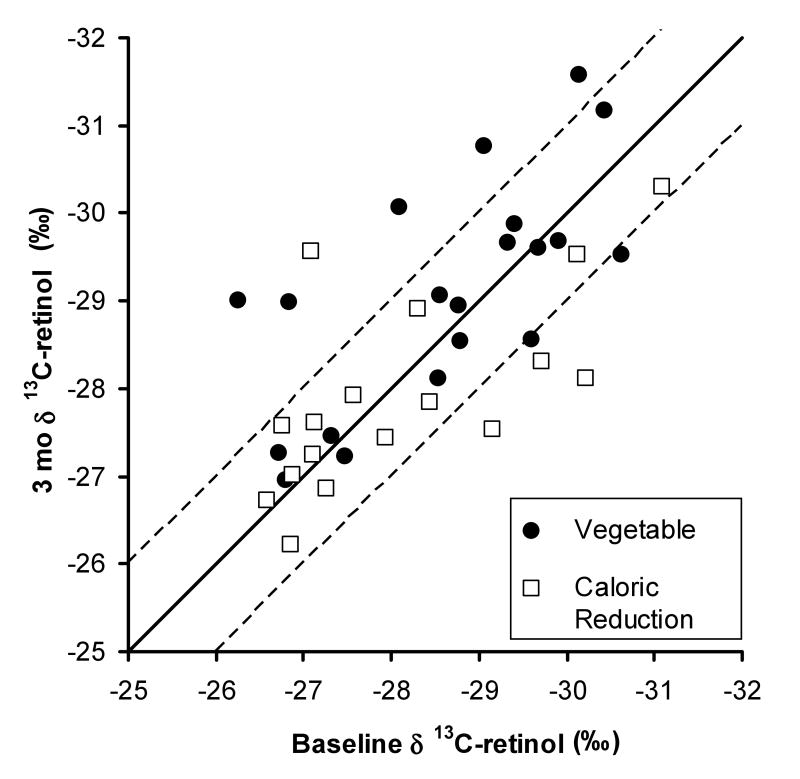
Baseline δ 13C-retinol values ranged from -26.26 to -31.08‰ and 3 mo values ranged from -26.21 to -31.57‰ (Figure 1). Delta 13C-retinol values in the vegetable group decreased from 0 to 3 mo (P = 0.050), but no difference was measured in the caloric reduction group (P = 0.43). A marginal difference was noted in the change in δ 13C-retinol values between the vegetable and caloric reduction groups (P = 0.054).
Figure 1.
Baseline versus 3 mo mean δ 13C-retinol values for subjects in the vegetable and reduced calorie and fat g (caloric reduction) groups (n = 20 and 17, respectively). The solid line represents equivalent 0 and 3 mo values, the dashed lines represents ± 1‰ variation in 3 mo δ 13C values compared to baseline. Average standard deviation for δ 13C-retinol values was ± 0.197. Delta 13C-retinol values in the vegetable group decreased from 0 to 3 mo (P = 0.050), but did not change in the caloric reduction group (P = 0.43). The 3 mo change in δ 13C-retinol values between the vegetable and caloric reduction groups was marginally significant (P = 0.054).
Evaluated parameters (i.e., serum α-carotene, β-carotene, and retinol concentrations; preformed vitamin A, provitamin A, and fat intakes; body weight, fat mass, and lean mass) were used to assess their relationship to δ 13C-retinol measurements. Except for the change in provitamin A intake in the vegetable group (P = 0.079) and the change in serum retinol in the caloric reduction group (P = 0.013), correlations to δ 13C-retinol were not significant at the α = 0.10 level for either group. For the vegetable group, stepwise forward regression analysis (α = 0.15 to include) showed that the decrease in δ 13C-retinol was due primarily to the increase in provitamin A intake (P = 0.079) and all other parameters were not significant. Backward elimination regression (α = 0.10 to remove) revealed that the changes in serum β-carotene, provitamin A intake, fat mass, body weight, and the percent body fat contributed to the changes in δ 13C-retinol for the vegetable group (P = 0.035). For the caloric reduction group, stepwise forward regression analysis (α = 0.15 to include) showed the change in δ 13C-retinol, was correlated to changes in serum retinol and percent body fat (P = 0.004). Backwards elimination regression (α = 0.15 to include) showed that changes in serum retinol, preformed vitamin A intake, body weight, fat mass, and percent body fat were all significant in the model (P = 0.003).
δ 13C of reference foods and vitamin A
The δ 13C of carrots, pumpkin, and spinach was -25.199 ± 0.313, -26.788 ± 0.228, and -27.375 ± 0.175‰, respectively. Corn had a δ 13C of -11.283 ± 0.079‰ and the δ 13C-retinol value of milk provided to study participants was -24.2‰. Synthetic vitamin A prepared from retinyl acetate was -28.779 ± 0.465‰.
Discussion
High intake of fruits and vegetables is associated with reduced risk of several chronic diseases, including cardiovascular disease, type 2 diabetes, and certain cancers (28). These foods are the dietary source for carotenoids, which have also been associated with reduced disease risk (35). Plasma or serum carotenoids are often used as biomarkers for fruit and vegetable intake (36, 37). Although useful as an indicator, conversion of provitamin A carotenoids to retinol will result in a loss of biomarker from serum carotenoid measurements. Because bioconversion is dependent on vitamin A status, the use of serum carotenoids as a biomarker for fruit and vegetable intake in populations with low or highly variable vitamin A status may be erroneous. By using the enrichment of 13C in retinol compared to provitamin A sources, conversion of provitamin A carotenoids to retinol can be confirmed and dietary adherence can be determined. The additional vegetable intake by the vegetable group was almost exclusively from C3 plants (i.e., carrots, pumpkin, and spinach) leading to a significant decrease in the δ 13C-retinol value. Furthermore, the δ 13C-retinol technique was more precise than serum β-carotene analysis. At baseline, the CV for δ 13C-retinol measurements was 4.7 and 4.4% for the vegetable and caloric reduction groups, respectively, while serum β-carotene was 57 and 131%, respectively. The greater variability in serum carotenoid measurements will require more subjects than studies using δ 13C-retinol. Analysis by GCCIRMS is more expensive and less available than HPLC analysis; however, this cost is somewhat offset by the reduction in subject number. Analysis of δ 13C- retinol is a new technique, but it can be adapted to any facility with GCCIRMS and HPLC capabilities.
Although both dietary intervention groups increased vegetable and provitamin A intake over the 3-mo intervention period, the vegetable group consumed significantly more vegetables and provitamin A than the caloric reduction group. The increase in vegetable consumption in both groups was confirmed by elevated serum α- and β-carotene at 3 mo and vegetable intake as indicated by 3-d diet records. Although the increase in provitamin A was confirmed by serum carotenoids, they were not able to distinguish the 2-fold greater intake of provitamin A by the vegetable group. In contrast, the δ 13C-retinol value decreased in the vegetable group (P = 0.050), but not in the caloric reduction group (P = 0.43). Using backward statistical modeling, the change in δ 13C-retinol was attributable to the change in provitamin A intake, serum carotenoids, fat mass, and body weight (P = 0.035). Step-wise modeling found that change in provitamin A intake was the only factor to explain the change in the δ 13C-retinol value at the α = 0.1 level.
Before δ 13C-retinol values are affected by dietary provitamin A carotenoids, provitamin A must be converted to retinol. Bioconversion was likely minimized by the high intake of preformed vitamin A and the adequate vitamin A status of the subjects. Subjects with a lower vitamin A status will have greater bioconversion of provitamin to vitamin A. Thus, the influence of dietary provitamin A carotenoids on δ 13C-retinol will be much greater in subjects with low vitamin A stores and the sensitivity of the technique will be greatly enhanced. Due to the effect of vitamin A status on the effectiveness of this technique, more studies are necessary to determine the absolute sensitivity of the method to modest changes in vegetable intake. In populations with low vitamin A status, sensitivity to changes in dietary vitamin A could be exploited with washout periods to establish a steady baseline prior to intervention.
Previous studies have shown that provitamin A in maize contributes to vitamin A pools similarly to synthetic β-carotene when vitamin A is absent from the diet (38). High consumption of relatively low carotenoid foods such as yellow or orange maize, can contribute to vitamin A stores when vitamin A stores are depleted (38). Substitution of yellow or orange maize for white maize in the diet of a population should increase the serum δ 13C-retinol value with time because maize is a C4 plant. In a vitamin A depleted population, the change in δ 13C-retinol value in response to an intervention would likely be greater than the response observed in this study and would confirm bioconversion to retinol, provided that the population's major vitamin A source was not solely from animals consuming C4 plants.
Serum retinol was not significantly different between groups or over time. This is expected because serum retinol is homeostatically controlled in individuals with adequate vitamin A status (39, 40). Although the caloric reduction group increased their carotenoid intake during the dietary intervention, it was not sufficient to have an effect on the isotopic composition of retinol in 3 mo. Changes in δ 13C-retinol and serum retinol concentrations did not differ between 0 and 3 mo in the caloric reduction group and consequently were correlated.
The decrease in δ 13C-retinol in the vegetable group indicates that the dietary intervention altered the isotopic composition of serum retinol. The increase in dietary provitamin A carotenoids by subjects in the vegetable group reduced the δ 13C-retinol indicating that carotenoids from C3 plants are being absorbed, cleaved into retinol, and circulated in the body. Dietary increase in provitamin A carotenoids was the most significant dietary parameter that correlated with the decrease in δ 13C-retinol in the vegetable group. Results from analysis of high provitamin A carotenoid vegetables (i.e., carrots, -25.102 ± 0.076‰; pumpkin, -26.788 ± 0.228‰; and spinach, -27.375 ± 0.175‰) provided for consumption during the 3 mo intervention were consistent with reported values (-27.16‰) for common fruits and vegetables (18).
Preformed vitamin A is primarily obtained from meat and dairy products, which, in the US, typically have much higher δ 13C values ranging from -13.5 to -19.2‰ (18, 23). The δ 13C-retinol value of fat-free milk, provided to study participants, was -24.2‰ and is much lower than the overall δ 13C of meat and dairy products. Animals must obtain vitamin A by cleaving provitamin A carotenoids or from vitamin A supplements because they cannot synthesize it. Thus, the δ 13C-retinol value of the milk originates from provitamin A in the diet (e.g., maize) that has a relatively high δ 13C value (-11.283 ± 0.079‰) and preformed vitamin A from feed, direct supplementation to the animal, and milk fortification that have a relatively low δ 13C value (-29.779 ± 0.465‰). Although preformed vitamin A is typically considered more bioavailable than provitamin A, the δ 13C-retinol value of milk indicates that provitamin A carotenoids in corn-based diets contribute to vitamin A pools.
The difference in fat intake between groups probably did not influence the decrease in δ 13C-retinol or the increase in serum carotenoids noted in the vegetable group. It is well established that added fat is necessary for carotenoid absorption, especially from raw vegetables (41). However, fat intake greater than 5 g fat/meal is generally accepted as adequate for absorption of carotenoids from supplements or vegetables (41-44). Both dietary groups in this study were well beyond this minimal fat requirement at each meal. Thus, differences in fat intake should not affect absorption of provitamin A carotenoids and would not be reflected in δ 13C-retinol values or serum carotenoids.
In addition to demonstrating the incorporation of carotenoid-derived retinol into the vitamin A pool, the change in δ 13C-retinol due to increased provitamin A intake also illustrates its utility as a biomarker for consumption of provitamin A-rich fruits and vegetables. Changes in serum α- and β-carotene concentrations were unable to differentiate between groups in response to the intervention, but δ 13C-retinol as measured by GCCIRMS differed due to its greater sensitivity toward changes in dietary vitamin A sources. The current guidelines for vegetable and fruit intake are 3.5 – 4.5 c for a 1600 – 2000 kcal diet in MyPyramid (25). The vegetable group was within these guidelines, i.e., 4.3 c/d, while the caloric reduction group was not, i.e., 3.1 c/d. The δ 13C-retinol reflected this difference and could be used in future studies to evaluate adherence to MyPyramid recommendations.
Acknowledgments
The authors thank Tom Tabone from the College of Agriculture Statistical Consulting Services for valuable assistance with statistical analysis and Torbert Rocheford from the University of Illinois at Urbana-Champaign for providing typical maize.
This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-35200-05377 and NIHNIDDK 61973.
References
- 1.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR, 3rd, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 1989;49:713–716. doi: 10.1093/ajcn/49.4.713. [DOI] [PubMed] [Google Scholar]
- 2.Green MH, Green JB. Vitamin-A intake and status influence retinol balance, utilization and dynamics in rats. J Nutr. 1994;124:2477–2485. doi: 10.1093/jn/124.12.477. [DOI] [PubMed] [Google Scholar]
- 3.Adams WR, Green MH. Prediction of liver vitamin-A in rats by an oral isotope-dilution technique. J Nutr. 1994;124:1265–1270. doi: 10.1093/jn/124.8.1265. [DOI] [PubMed] [Google Scholar]
- 4.von Reinersdorff D, Bush E, Liberato DJ. Plasma kinetics of vitamin A in humans after a single oral dose of [8,9,19-13C]retinyl palmitate. J Lipid Res. 1996;37:1875–1885. [PubMed] [Google Scholar]
- 5.von Reinsersdorff D, Green MH, Green JB. Development of a compartmental model describing the dynamics of vitamin metabolism in men. Adv Exp Med Biol. 1998;445:207–223. doi: 10.1007/978-1-4899-1959-5_13. [DOI] [PubMed] [Google Scholar]
- 6.Haskell MJ, Islam MA, Handelman GJ, Peerson JM, Jones AD, Wahed MA, Mahalanabis D, Brown KH. Plasma kinetics of an oral dose of [2H4]retinyl acetate in human subjects with estimated low or high total body stores of vitamin A. Am J Clin Nutr. 1998;68:90–95. doi: 10.1093/ajcn/68.1.90. [DOI] [PubMed] [Google Scholar]
- 7.Ribaya-Mercado JD, Mazariegos M, Tang G, Romero-Abal ME, Mena I, Solomons NW, Russell RM. Assessment of total body stores of vitamin A in Guatemalan elderly by the deuterated-retinol-dilution method. Am J Clin Nutr. 1999;69:279–284. doi: 10.1093/ajcn/69.2.278. [DOI] [PubMed] [Google Scholar]
- 8.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr. 2000;130:2844–2849. doi: 10.1093/jn/130.11.2844. [DOI] [PubMed] [Google Scholar]
- 9.Furr HC, Green M, Haskell M, Mokhtar N, Nestel P, Newton S, Ribaya-Mercado JD, Tang G, Tanumihardjo S, Wasantwisut E. Stable isotope dilution techniques for assessing of vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutrition. 2005;8:596–607. doi: 10.1079/phn2004715. [DOI] [PubMed] [Google Scholar]
- 10.Yao LH, Liang YX, Trahanovsky WS, Serfass RE, White WS. Use of a C-13 tracer to quantify the plasma appearance of a physiological dose of lutein in humans. Lipids. 2000;35:339–348. doi: 10.1007/s11745-000-0531-0. [DOI] [PubMed] [Google Scholar]
- 11.Kelm MA, Flanagan VP, Pawlosky RJ, Novotny JA, Clevidence BA, Britz SJ. Quantitative determination of C-13-labeled and endogenous beta-carotene, lutein, and vitamin A in human plasma. Lipids. 2001;36:1277–1282. doi: 10.1007/s11745-001-0842-1. [DOI] [PubMed] [Google Scholar]
- 12.van Lieshout M, West CE, van Breemen RB. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: a review. Am J Clin Nutr. 2003;77:12–28. doi: 10.1093/ajcn/77.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Kurilich AC, Britz SJ, Clevidence BA, Novotny JA. Isotopic labeling and LC-APCI-MS quantification for investigating absorption of carotenoids and phylloquinone from kale (Brassica oleracea) J Agric Food Chem. 2003;51:4877–4883. doi: 10.1021/jf021245t. [DOI] [PubMed] [Google Scholar]
- 14.Novotny JA, Kurilich AC, Britz SJ, Clevidence BA. Plasma appearance of labeled beta-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. J Lipid Res. 2005;46:1896–1903. doi: 10.1194/jlr.M400504-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Tang GW, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am J Clin Nutr. 2005;82:821–828. doi: 10.1093/ajcn/82.4.821. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell AD, Steele NC, Hare PE. Alteration of tissue levels of 13C in pigs by natural abundance 13C labeling of diets. Growth Dev Aging. 1993;57:205–215. [PubMed] [Google Scholar]
- 17.Smith BN, Epstein S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971;47:380–384. doi: 10.1104/pp.47.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison DJ, Dodson B, Slater C, Preston T. C-13 natural abundance in the British diet: implications for C-13 breath tests. Rapid Commun Mass Spectrom. 2000;14:1321–1324. doi: 10.1002/1097-0231(20000815)14:15<1321::AID-RCM946>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Petzke KJ, Boeing H, Metges CC. Choice of dietary protein of vegetarians and omnivores is reflected in their hair protein C-13 and N-15 abundance. Rapid Commun Mass Spectrom. 2005;19:1392–1400. doi: 10.1002/rcm.1925. [DOI] [PubMed] [Google Scholar]
- 20.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived dietary protein intake in humans. J Nutr. 2005;135:1515–1520. doi: 10.1093/jn/135.6.1515. [DOI] [PubMed] [Google Scholar]
- 21.Jahren AH, Saudek C, Yeung EH, Kao WHL, Kraft RA, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–1384. doi: 10.1093/ajcn/84.6.1380. [DOI] [PubMed] [Google Scholar]
- 22.Kurilich AC, Juvik JA. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J Agric Food Chem. 1999;47:1948–1955. doi: 10.1021/jf981029d. [DOI] [PubMed] [Google Scholar]
- 23.Schoeller DA, Klein PD, Watkins JB, Heim T, MacLean WC., Jr 13C abundances of nutrients and the effect of variations in 13C isotopic abundances of test meals formulated for 13CO2 breath tests. Am J Clin Nutr. 1980;33:2375–2385. doi: 10.1093/ajcn/33.11.2375. [DOI] [PubMed] [Google Scholar]
- 24.Ginde SR, Geliebter A, Rubiano F, Silva AM, Wang J, Heshka S, Heymsfield SB. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13:1232–1237. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 25.US Department of Agriculture. MyPyramid—USDA's New Food Guidance System, peer-to-peer PowerPoint presentation. [4 June 2008]; http://www.mypyramid.gov/professionals/index.html.
- 26.US Department of Health and Human Services, US Department of Agriculture. Nutrition and your health: dietary guidelines for Americans. Washington, DC: US Government Printing Office; 2000. USDA Home and Garden Bulletin no. 232. [Google Scholar]
- 27.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 28.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) Washington, DC: National Academy Press; 2005. [Google Scholar]
- 29.Molldrem KL, Li J, Simon PW, Tanumihardjo SA. Lutein and β-carotene are bioavailable in humans from lutein yellow carrots. Am J Clin Nutr. 2004;80:131–136. doi: 10.1093/ajcn/80.1.131. [DOI] [PubMed] [Google Scholar]
- 30.DeRitter E, Purcell AE. Carotenoid analytical methods. In: Bauerfeind JC, editor. Carotenoids as colorants and vitamin A precursors. Orlando, FL: Academic Press; 1984. p. 883. [Google Scholar]
- 31.Cama HR, Collin FD, Morton RA. Studies in vitamin A. 17. Spectroscopic properties of all-trans-vitamin A and vitamin A acetate. Analysis of liver oils. Biochem J. 1951;50:48–59. doi: 10.1042/bj0500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayarajan P, Reddy V, Mohanram M. Effect of dietary-fat on absorption of beta-carotene from green leafy vegetables in children. Indian J Med Res. 1980;71:53–56. [PubMed] [Google Scholar]
- 33.Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. Serum retinol concentrations in children are affected by food sources of β-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr. 1998;68:623–629. doi: 10.1093/ajcn/68.3.623. [DOI] [PubMed] [Google Scholar]
- 34.van het Hof KH, West CE, Weststrate JA, Hautvast JGAJ. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000;130:503–506. doi: 10.1093/jn/130.3.503. [DOI] [PubMed] [Google Scholar]
- 35.Tanumihardjo SA, Yang Z. Carotenoids: Epidemiology of health effects. In: Caballero B, Allen L, Prentice A, editors. Encyclopedia of Human Nutrition. 2nd. Oxford: Elsevier Ltd; 2005. pp. 339–345. [Google Scholar]
- 36.Brantsaeter AL, Haugen M, Rasmussen SE, Alexander J, Samuelsen SO, Meltzer HM. Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Public Health Nutr. 2007;10:838–847. doi: 10.1017/S1368980007339037. [DOI] [PubMed] [Google Scholar]
- 37.Paterson E, Gordon MH, Niwat C, George TW, Parr L, Waroonphan S, Lovegrove JA. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J Nutr. 2006;136:2849–2855. doi: 10.1093/jn/136.11.2849. [DOI] [PubMed] [Google Scholar]
- 38.Howe JA, Tanumihardjo SA. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J Nutr. 2006;136:2562–2567. doi: 10.1093/jn/136.10.2562. [DOI] [PubMed] [Google Scholar]
- 39.Furr HC, Green MH, Haskell M, Mokhtar N, Nestel P, Ribaya-Mercado J, Tanumihardjo SA, Wasantwisut E. Vitamin A Tracer Task Force. Appropriate uses of vitamin A tracer (stable isotope) methodology. Washington, DC: ILSI Human Nutrition Institute; 2004. Members. Lead drafters: Tanumihardjo SA, Nestel P. [Google Scholar]
- 40.Debier C, Larondelle Y. Vitamins A and E: metabolism, roles and transfer to offspring. Br J Nutr. 2005;93:153–174. doi: 10.1079/bjn20041308. [DOI] [PubMed] [Google Scholar]
- 41.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- 42.Ribaya-Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr. 2007;85:1041–1049. doi: 10.1093/ajcn/85.4.1041. [DOI] [PubMed] [Google Scholar]
- 43.Roodenburn AJC, Leenen R, van het Hof KH, Weststrate JA, Tijberg LBM. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am J Clin Nutr. 2000;71:1187–1193. doi: 10.1093/ajcn/71.5.1187. [DOI] [PubMed] [Google Scholar]
- 44.Nestel P, Nalubola R. As little as one teaspoon of dietary fat in a meal enhances the absorption of β-carotene. Washington, DC: ILSI; 2003. [Google Scholar]