Abstract
The inbred FVB mouse strain is used extensively in cancer research. Transgenic mice with an FVB/N background in which the expression of green fluorescent protein is under the control of various promoters have been used widely for the last decade. However, little is known about the incidence and characteristics of spontaneous tumors in these mice. In addition, only a few tumor lines have been established for use in this particular mouse strain. Our aim was to initiate a database of spontaneous tumors in our retired FVB/N breeders, analyze the histopathologic characteristics of these tumors, and establish novel tumor lines in vivo and in vitro. A total of 234 (40 male, 194 female) breeder mice were observed during their natural lifespans. The incidence of spontaneous tumors was 45.0% in male mice and 52.8% in female mice. All tumors in male mice were lung alveolar–bronchiolar (AB) neoplasms, except for 1 testis interstitial cell tumor. In female mice, histopathologic examination revealed 48 lung AB tumors, 27 mammary gland tumors, 13 ovarian tumors, and 14 other tumors. Several of these spontaneous tumors have been transplanted into FVB/N mice. One mammary adenocarcinoma (MCaP0008) and 1 lung AB carcinoma (LAP0297) were successfully transplanted subcutaneously and passaged serially in vivo. Subsequently, we established cell lines from both tumors, which were maintained in monolayer in vitro. Both of the grafted tumors and cell lines are tumorigenic in VEGFP-GFP/FVB and Tie2P-GFP/FVB mice. Establishment of these novel tumor lines will benefit both in vivo and in vitro studies on the pathophysiology of cancer in this relatively new but widely used mouse strain.
Abbreviation: AB, alveolar–bronchiolar; GFP, green fluorescence protein; VEGF, vascular endothelial growth factor
Currently, the well-defined inbred FVB strain of mice is used extensively for transgenic technology because of the prominent pronuclei of its fertilized zygotes; this characteristic facilitates microinjection of foreign DNA.18 Transgenic mouse lines carrying green fluorescent protein (GFP) under various promoters (such as VEGFP-GFP, EF1αP-GFP, Tie2P-GFP) have been derived from FVB/N mice and bred in our defined-flora animal facility17 for the last decade and show superior reproductive performance; these mouse lines are increasingly being used as novel in vivo models for the study of tumor pathophysiology in our laboratory3-5 and at other institutions.11,16
The FVB/N mouse strain was created in the early 1970s, and a detailed genetic characterization of this strain compared with other inbred mouse strains was published in 1991.18 However, with the exception of 3 recent publications on spontaneous lesions and pathologic changes in pituitary and mammary glands, little is known about the frequency and type of spontaneous tumors in wild-type FVB/N mice.10,12,19 In addition, few established tumor lines can be used in this particular mouse strain.
Our aim was to initiate a database of spontaneous tumors in our defined-flora FVB/N retired breeders, and analyze the histopathologic characteristics of these tumors, and establish novel tumor lines in vivo and in vitro from these tumors to support cancer research in this widely used mouse strain.
Materials and Methods
Animals.
We used retired FVB/N breeders for spontaneous tumor studies and 6- to 8-wk-old male and female FVB/N wild-type mice and VEGFP-GFP/FVB, and Tie-2P-GFP/FVB transgenic mice for tumor transplantation. All mice were bred and maintained in our defined-flora animal facility.17 The FVB/N mice originally were obtained from Taconic Farms (FVB/NTac; Germantown, NY) in July 1999 and have been bred for up to 22 generations in our breeding colony. Every fourth generation (generation 4, 8, 12, and so forth), live sample mice were sent to Charles River Genetic Laboratory (Troy, NY) for microsatellite genetic analysis to monitor their genetic background purity. The VEGFP-GFP/FVB mice express GFP on activation of the promoter for vascular endothelial growth factor (VEGF) in fibroblast-like cells.5 The Tie2P-GFP/FVB mice (The Jackson Laboratory, Bar Harbor, ME) express GFP under the control of the endothelial-specific receptor tyrosine kinase (Tek/Tie2) promoter.11 Under our microbiologic monitoring program, all mice were sampled for aerobic bacteria before being weaned from their parents. Any mice exhibiting aerobic bacterial growth were euthanized and removed from the colony. In addition, sentinel mice from the breeding, stock, and experimental rooms were sent to an independent laboratory (Charles River Laboratory, Wilmington, MA) for complete diagnostic evaluations. The tested items included bacteriology, infectious disease PCR assays, parasitology, Helicobacter PCR tests, pathology, and serology examinations. All sentinel mice were screened for 18 murine viruses, all appropriate pathogenic aerobic bacteria, and parasites.7
All mice in this study were housed in microisolation caging, fed sterile pelletted chow, and given acidified sterile water ad libitum. Animal care and use was in accordance with the Public Health Service Policy on Humane Care of Laboratory Animals15 and approved by the Institution Animal Care and Use Committee and the Subcommittee on Research Animal Care at the Massachusetts General Hospital.
Examination of spontaneous tumors.
A total of 234 (40 male, 194 female) inbred FVB/N retired breeders were used for the spontaneous tumor study and allowed to live to or close to the end of their natural lifespan. These mice were screened tumor development weekly by visual inspection and palpation. When a tumor was found, 2 perpendicular tumor diameters were measured with calipers twice weekly. Tumor volume (V) was calculated as V = a × b2/2, where a and b are the long and short axes, respectively. A complete necropsy was performed on all mice that died during the study, on those euthanized when they were sick or when their tumors reached the maximum of 1700 ± 100 mm3 (approximately 15 × 15 mm), and those euthanized at an age close to 900 d (14 of 234 mice).
Histopathologic examination.
All grossly observed lesions were harvested for histopathologic study. Tissue specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, cut at 5-μm thickness, and stained with hematoxylin and eosin for histologic examination.
Tumor transplantation.
Fresh specimens obtained from spontaneous tumors were excised, cleared of necrotic tissue, cut into small chunks (approximately 1 to 2 mm in diameter), and transplanted subcutaneously into the flanks of 6- to 8-wk-old wild-type FVB/N mice to obtain first-generation (F1) isografts. When F1 tumors reached approximately 15 mm in diameter, 1 was excised and transplanted into the next set of FVB/N mice for F2 tumors. In generations F2 to F5, the tumor tissues also were transplanted subcutaneously into our inhouse Tie2P-GFP/FVB and VEGFP-GFP/FVB transgenic mice for comparative study.
Cell culture.
Fresh tumor tissue obtained from F1-transplanted isografts was quickly cut into small pieces, cleared of necrotic tissue, washed twice in PBS, finely minced, and placed in a T25 culture flask containing 5 ml of DMEM supplemented with 15% heat-inactivated fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). The cells were incubated in 5% CO2 at 37 °C and passaged once or twice weekly to maintain an exponentially growing monolayer.
Tumor growth and metastasis analysis.
The parameters of the percentage of transplanted tumors that grew successfully, latent period (the number of days required for the development of palpable subcutaneous tumors post-transplantation), and growth time (the number of days required for the tumors to grow to 10 mm in diameter [approximately 500 ± 50 mm3 in volume]) were used to describe the growth characteristics of the transplantable tumor models in FVB/N wild-type and transgenic mice. For the purpose of determination of metastatic potential, tumor chunks were transplanted subcutaneously into the right hindlegs of FVB wild-type and transgenic mice. When the tumors reached the size of 10 × 10 mm, the mice were anesthetized (90 mg ketamine and 9 mg xylazine per kilogram of body weight, given by subcutaneous injection), and the tumor-bearing legs were amputated as described previously.6,8 After amputation, the mice were examined at least 3 times weekly for as long as 5 mo after tumor transplantation. At the end of experiment, or when the mouse became moribund, it was euthanized. Macroscopic autopsy and histopathologic examination were performed.
Statistic analysis.
Data are shown as mean ± 1 SD. Statistical differences between groups were compared by Mann–Whitney tests (StatView, Abacus, Berkeley, CA). Differences in metastatic rates were analyzed by χ2 tests (StatView, Abacus). Differences were considered to be significant when P was less than 0.05.
Results
Survival of retired FVB/N breeder mice and age distribution of spontaneous tumors.
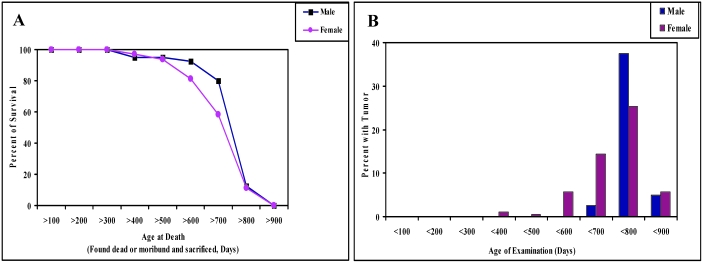
In this study, 194 retired female breeders were observed from 324 to 891 d of age, for a mean observation time of 694 d. Forty male mice were observed from 339 to 856 d of age, with mean observation time of 727 d. Survival analyses showed that about 60% (male mice, 77.5%; female mice, 50.5%) of the retired breeders lived to more than 2 y of age, and more than 10% of the mice (male mice, 12.5%; female mice, 11.3%) lived more than 800 d (Figure 1 A). Natural deaths of these retired breeders included mice found dead and those euthanized due to tumor development or moribundity. In addition, 14 tumor-free mice were euthanized at ages close to 900 d at the end of the study. The age distribution of spontaneous tumors in these retired FVB/N breeders is given in Figure 1 B. Most of the spontaneous tumors were found in mice older than 600 d, and the incidence of spontaneous tumor between 700 to 800 d of age was high in both male (37.5%) and female (25.3%) retired mice. Only about 5% of spontaneous tumors were detected at ages older than 800 d in our retired male (5.0%) and female (5.7%) mice (Figure 1 B).
Figure 1.
(A) Survival curves for 40 male and 194 female FVB/N retired breeder mice. Data are presented as the percentage of live mice throughout the course of observation. (B) The percentages of male and female mice that developed spontaneous tumors during the course of observation.
Incidence of spontaneous tumors in retired FVB/N breeders.
The incidences of spontaneous tumors in male and female retired FVB/N breeders are shown in Table 1. The overall incidence of spontaneous tumors is 45.0% in male mice and 52.8% in female mice. Almost all (17 of 18) of the spontaneous tumors in the male mice were alveolar–bronchiolar (AB) tumors of the lungs, except one tumor found in the testis. In female mice, the most common neoplasm was AB tumor of the lungs (48 of 102 tumors), followed by mammary gland tumor (27 of 102) and ovarian tumor (13 of 102). The other spontaneous tumors in females included 1 squamous cell carcinoma of the skin; 1 carcinosarcoma, 2 fibrosarcomas, 2 lipomas, and 1 histiocytic sarcoma from subcutaneous tissues; 1 squamous cell carcinoma of the stomach; 2 hepatocellular carcinomas; 1 lymphocytic leukemia involving multiple organs; 1 adenocarcinoma from Harderian gland; and 2 pituitary adenomas in the brain.
Table 1.
Incidence of spontaneous tumors in retired FVB/N breeder mice
| Male | Female | |
| No. of mice examined | 40 | 194 |
| No. of mice with tumorsa | 18 (45.0%) | 102 (52.8%) |
| Lung tumors | 17 (42.5%) | 48 (24.7%) |
| AB adenoma | 2 | 12 |
| AB adenoma + carcinoma | 2 | 1 |
| AB carcinoma | 13 | 35 |
| Mammary gland tumors | 0 | 27 (13.9%) |
| Adenocarcinoma | 0 | 19 |
| Adenocanthoma | 0 | 8 |
| Ovary tumors | 0 | 13 (6.7%) |
| Granulosa cell tumor | 0 | 6 |
| Teratoma | 0 | 4 |
| Cyst–adenoma | 0 | 2 |
| Fibrosarcoma | 0 | 1 |
| Other tumors | 1 (2.5%) | 14 (7.2%) |
| Testis, interstitial cell tumor | 1 | 0 |
| Skin, squamous cell carcinoma | 0 | 1 |
| Subcutaneous carcinoma | 0 | 1 |
| Subcutaneous fibrosarcoma | 0 | 2 |
| Subcutaneous lipoma | 0 | 2 |
| Subcutaneous histiocytic sarcoma | 0 | 1 |
| Stomach, squamous cell carcinoma | 0 | 1 |
| Liver, hepatocellular carcinoma | 0 | 2 |
| Lymphoctyic leukemia | 0 | 1 |
| Hardarian gland, adenocarcinoma | 0 | 1 |
| Pituitary adenoma | 0 | 2 |
Six female mice had multiple tumors.
Examination of pathomorphology.
As just mentioned, the most common neoplasm of both male and female retired FVB/N breeders was AB tumor of the lungs. Gross examination of these AB tumors revealed solid, white or gray nodules ranging from 1 to 13 mm in diameter and raised above the pleural surface of the lungs. Malignant AB tumors usually were large, and most tumor nodules ranged from 3 to 10 mm or more in diameter, with displacement of the normal pulmonary parenchyma (Figure 2 A). Nodules less than 3 mm generally corresponded microscopically to AB adenomas. Most AB adenomas were detected by macroscopic examination at necropsy or diagnosed by microscopic examination, without any clinical symptoms in host mice. However, clinical signs of dyspnea and body weight loss occurred in more than half of the mice with AB carcinomas.
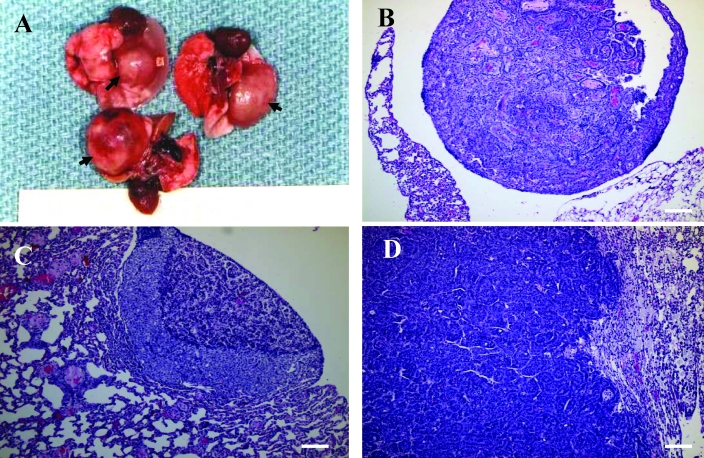
Figure 2.
(A) AB carcinomas in the lungs of FVB/N retired breeder mice. These tumors presented as single, solid, large, raised masses (arrows) in the pleural surface of the lungs, with displacement of the normal pulmonary parenchyma. (B and C) AB adenomas with (B) papillary and (C) solid growth patterns. (D) AB carcinoma with invasive growth. Hematoxylin and eosin stain; bar, 100 μm.
Solid, papillary, and mixed AB adenomas and AB carcinomas were diagnosed microscopically (Figure 2 B to D, and Figure 3 A to D). AB adenomas usually caused slight to moderate compression of the surrounding uninvolved parenchyma (Figure 2 B, C); the tumor cells varied from cuboidal to low columnar or round and had moderate amounts of finely vacuolated cytoplasm (Figure 3 A, B). Occasionally, the focal regions of mucinous cell differentiation were present in the area above the cuboidal tumor cells (Figure 3 A). In contrast, AB carcinomas were usually highly invasive and poorly demarcated (Figure 2 D, Figure 3 D); tumor cells were typically pleomorphic and ranged from round or oval to cuboidal to columnar, typically arranged in a single or multiple layers around a prominent fibrovascular core (Figure 3 C, D). Nuclei of AB carcinoma cells were atypical and pleomorphic. Mitotic figures were uncommon (Figure 3 D). Histomorphologically, a total of 14 AB adenomas and 48 AB carcinomas were present in our retired FVB/N breeders, as were 3 AB carcinomas arising from AB adenomas (Table 1).
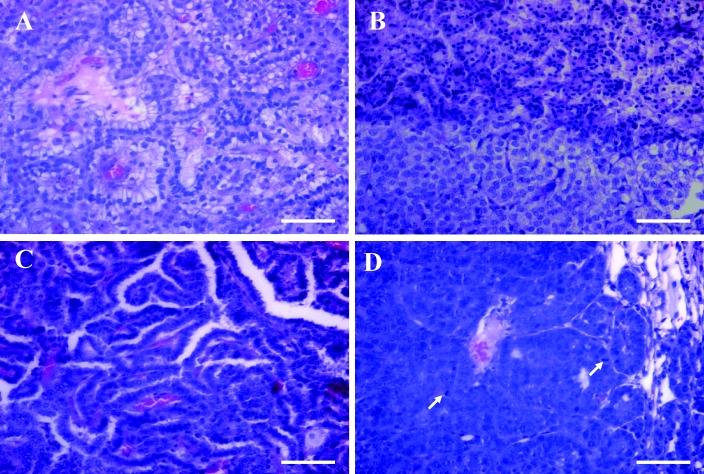
Figure 3.
(A) AB adenoma with mucinous differentiation of tumor cells. (B) Solid AB adenoma (from Figure 2 C) showing atypical changes in regions of benign tumor cells. Hematoxylin and eosin stain; bar, 100 μm. (C) AB carcinoma with a tubulopapillary growth pattern. (D) AB carcinoma showing the anaplastic characteristics of tumor cells with some mitotic figures (arrows). Hematoxylin and eosin stain; bar, 100 μm.
The second most common neoplasm in our female retired FVB/N breeders (incidence, 13.9%) was mammary gland tumor. All mammary gland tumors in our study were grossly visible subcutaneous nodules or masses usually localized in the area along the mammary chain from the cervical region to the inguinal region. A single mass was present in most mice (Figure 4 A). Most of the tumors were well-circumscribed, firm, and lobulated and were white, gray, or yellow on cut surface. Some of the large tumors presented with central necrosis and hemorrhage, with local invasion into the surrounding normal tissue but without distant metastasis. Histologically, the mammary gland tumors comprised 19 adenocarcinomas (Figure 4 B) and 8 adenoacanthomas (adenosquamous carcinomas; Figure 4 C and Table 1). Among the adenocarcinomas, we noted typical histologic patterns, including glandular (Figure 4 B) and papillary (Figure 4 D).
Figure 4.
(A) Mammary gland tumor (T) in a FVB/N retired female breeder. Mammary gland adenocarcinomas with (B) a glandular growth pattern and (C) a papillary growth pattern. (D) A mammary gland adenoacanthoma (adenosquamous carcinoma) with prominent squamous differentiation of the glandular epithelial tumor cells (lower half of image). Hematoxylin and eosin stain; bar, 100 μm.
Ovarian tumors were the third most common tumors in the retired female mice. Most of these ovarian tumors were granulosa cell tumors or teratomas. Ovary cyst–adenoma and fibrosacoma were less frequent in our study population. Most of the ovarian tumors were detected at necropsy as visible ovarian masses and were diagnosed by microscopic examination, except in the few cases of teratomas, which manifested as very large abdominal masses.
We found 14 other pathologic types of spontaneous tumors in our retired female breeders (Table 1), including 2 subcutaneous lipomas and 2 fibrosarcomas; 2 hepatocellular carcinomas; 2 pituitary gland adenomas; 2 squamous cell carcinomas (1 each from skin and stomach); 1 carcinosarcoma and 1 histiocytic sarcoma, both from the subcutaneous tissue; 1 Harderian gland adenocarcinoma, and 1 lymphocytic leukemia involving spleen, liver, and multiple lymph nodes. We found only 1 spontaneous other tumor (interstitial cell tumor of the testis) among retired male breeders.
Establishment of novel tumor lines from spontaneous tumors.
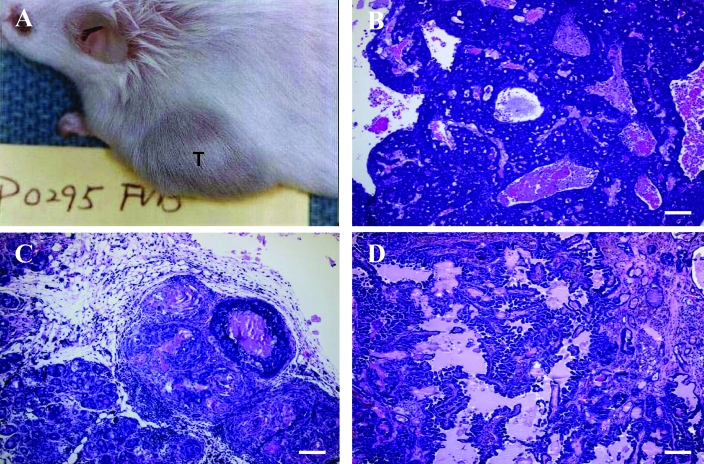
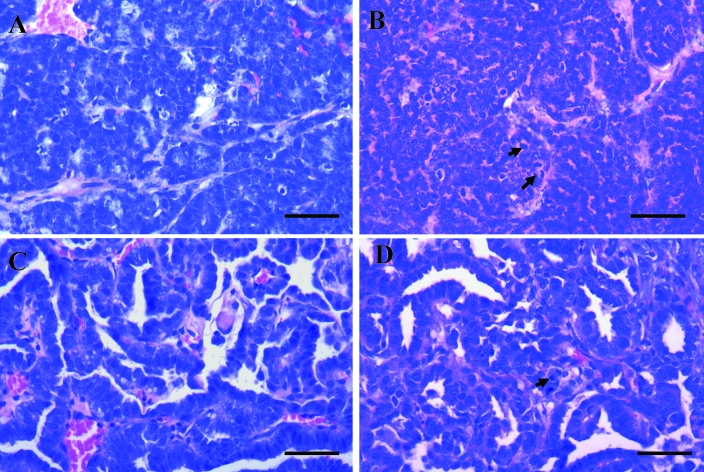
We transplanted 6 of the spontaneous tumors from our retired FVB/N female breeders into 6- to 8-wk-old FVB/N mice. These tumors comprised 4 mammary gland tumors (P0008, P0308, P0495, and P0621), 1 fibrosarcoma (P0477), and 1 lung AB carcinoma (P0297). The P0008 spontaneous tumor arose as an oval mass (1.5 × 1.4 × 1.0 cm) in the right cervical region of a 378-d-old mouse. Microscopic examination showed it to be a typical mammary adenocarcinoma (type B) with a glandular–epithelial growth pattern (Figure 5 A). The P0308, P0495, and P0621 mammary gland tumors were obtained from mice at 710, 677, and 746 d age from the left thoracic, left cervical, and left abdominal region, respectively. All 3 of these tumors appeared as solid masses and were removed when approximately 1.5 cm in diameter. The pathohistologic diagnosis was spontaneous mammary adenocarcinoma (type C) for P0308 (Figure 6 B), mammary adenocarcinoma (type B) for P0495 (morphologically similar to P0008), and mammary adenoacanthoma for P0621 (Figure 6 C). The P0477 spontaneous tumor was diagnosed as a fibrosarcoma (Figure 6 D). This mass was found in the right axilla of an 818-d-old mouse and presented as a subcutaneous solid mass 1.4 × 1.0 × 0.9 cm in size, with invading growth to surrounding musculature. The P0297 tumor was detected in the left lung of a 369-d-old mouse. This lesion appeared as a single, white, solid, 1.1 × 0.8 × 0.6 cm nodule which protruded above the pleural surface of the lung. Pathohistologic examination revealed a spontaneous AB carcinoma with a tubulopapillary growth pattern (Figure 5 C).
Figure 5.
(A) P0008 mammary adenocarcinoma (type B) with a glandular–epithelial growth pattern. (B) MCaP0008 (F4) isograft showing the adenocarcinoma histologic features similar to those in the original P0008 tumor but with more mitotic figures (arrows). (C) P0297 AB carcinoma with a tubulopapillary growth pattern. (D) LAP0297 (F3) isograft that retained the histologic features of the original P0297 tumor type but with more mitotic figures (arrow). Hematoxylin and eosin stain; bar, 100 μm.
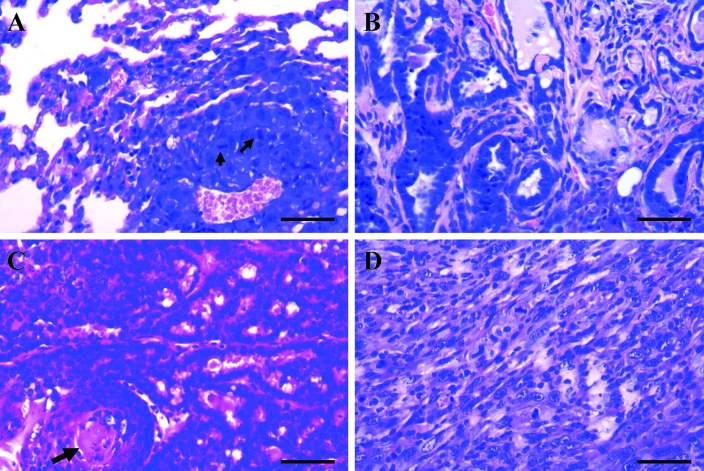
Figure 6.
(A) Metastatic focus of LAP0297 AB carcinoma in lung, showing similar histologic characteristics as but more mitotic figures (arrows) as the primary P0297 tumor. (B) Spontaneous P0308 mammary adenocarcinoma (type C) with prominent stroma component. (C) P0261 mammary gland adenoacanthoma, in which the glandular epithelial tumor tissue shows squamous differentiation and keratinized material (arrow). (D) P0477 fibrosarcoma showing neoplastic fibroblasts with spindle or fusiform shapes, mitotic figures, and surrounding collagen. Hematoxylin and eosin stain; bar, 100 μm.
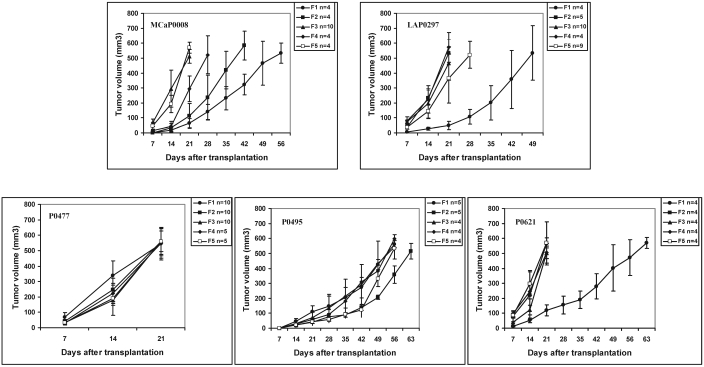
Only the P0308 mammary tumor failed to grow when transplanted in FVB/N mice; the other 5 spontaneous tumors were transplanted successfully into the subcutaneous tissue and passaged serially in vivo (at least 5 generations) in secondary FVB/N hosts. Their growth characteristics (derived from F1 to F5 isografted tumors) are shown in Figure 7 and Table 2. These results show that the P0477 isografts had the fastest growth rate, whereas the P0495 tumors were slow-growing. The other 3 tumor lines (P0008, P0297, and P0621) clearly showed a tendency toward increased growth rate with in vivo passages (Figure 7). All isografted tumors and their metastatic foci (P0008 and P0297) in the lungs and lymph nodes were identified macroscopically and confirmed histologically. The isografts retained the histologic features of their original tumor types (Figure 5 B, D). Subcutaneous isografts and their metastatic colonies in distant organs closely resembled each other morphologically in all cases examined (Figure 6 A). However, isografts and their metastatic tumors had more mitotic figures than did the parental tumors (Figure 5 A to D, Figure 6 A). After their successful F1 passage, 2 tumor lines—the P0008 mammary adenocarcinoma and the P0297 lung AB carcinoma—were transplanted into our inhouse VEGFP-GFP/FVB and Tie2P-GFP/FVB transgenic mice and passaged for 2 to 5 generations (Table 3).
Figure 7.
Growth curves of 3 mammary gland tumors (P0008, P0495, and P0621), 1 fibrosarcoma (P0477), and 1 lung AB carcinoma (P0297) transplanted subcutaneously in FVB/N mice and serially passaged in vivo from F1 to F5. Data are presented as mean tumor volume; bars indicate 1 SD.
Table 2.
Growth characteristics of 5 spontaneous tumors isografted into FVB/N mice
| No. of tumors that grew/no. of tumors transplanted (%) | Latent period (d)a | Growth time (d)b | |
| P0008 | 26/27 (96.3) | 7.9 ± 3.6 | 29.4 ± 12.9 |
| P0297 | 32/33 (97.0) | 4.9 ±1.3 | 26.3 ± 9.7 |
| P0477 | 40/40 (100) | 5.9 ± 1.1 | 20.9 ± 3.3 |
| P0495 | 22/23 (95.7) | 13.4 ± 0.8 | 56.2 ± 4.7 |
| P0621 | 20/20 (100) | 4.9 ± 1.5 | 29.2 ± 16.6 |
Data are given as mean ± 1 SD.
No. of days required after transplantation for development of palpable subcutaneous tumor.
No. of days needed for the tumor to reach 1500 mm3.
Table 3.
Growth characteristics of LAP0297 and MCaP0008 isografts
| No. of tumors that grew/ no. of tumors transplanted (%) | Latent period (d)a | Growth time (d)b | |
| LAP0297 in FVB (F1∼F5) | 32/33 (97.0) | 4.9 ± 1.3 | 26.3 ± 9.7 |
| LAP0297 in Tie2P-GFP/FVB (F2∼F5) | 24/24 (100) | 5.3 ± 1.2 | 26.3 ± 4.4 |
| LAP0297 in VEGFP-GFP/FVB (F2∼F3) | 13/15 (86.7) | 6.0 ±1.2 | 24.3 ± 6.7 |
| MCaP0008 in FVB (F1∼F5) | 26/27 (96.3) | 7.9 ± 3.6 | 29.4 ± 12.9 |
| MCaP0008 in Tie2P-GFB/FVB (F2∼F3) | 10/10 (100) | 6.7 ± 0.7 | 27.0 ± 1.9 |
Data are given as mean ± 1 SD.
No. of days required after transplantation for development of palpable subcutaneous tumor.
No. of days needed for the tumor to reach 1500 mm3.
In addition, we established cell lines from F1 tumors of both P0008 and P0297 and have maintained them as monolayers in vitro. These 2 tumor lines have been named LAP0297 and MCaP0008. The results in Table 3 shows that differences in the latent periods and growth times between LAP0297 and MCaP0008 masses implanted in wild-type FVB/N mice and those implanted in Tie2P-GFP/FVB and VEGFP-GFP/FVB transgenic mice were not significant (Mann–Whitney t test, P > 0.5). In addition, both MCaP0008 and LAP0297 tumor cell lines were tumorigenic in Tie2P-GFP/FVB, VEGFP-GFP/FVB, and EF1αP-GFP/FVB transgenic mice.4 When grown in wild-type FVB/N mice, the LAP0297 isografts showed a high incidence of distant lung metastases (94.4%, Table 4, Figure 6 A, 8 A). However, the incidence of metastasis significantly decreased when LAP0297 tumors were implanted in Tie2P-GFP/FVB (16.7%) or VEGFP-GFP/FVB (7.7%; Table 4 and Figure 8 B; P < 0.01 for both). MCaP0008 isografts showed an 18.2% incidence of metastases to the lung or lymph nodes when grown in FVB/N mice, but no metastasis was detected when this tumor was implanted into Tie2P-GFP/FVB transgenic mice (Table 4).
Table 4.
Metastases of LAP0297 and MCaP0008 isografts
| No. of mice with metastasis/no. of mice implanted (%) | Analysis of incidence of metastasisa | Sites of metastasis | |||||
| Tumor implantation to necropsy (d) | χ2 | P | Lung | Lymph node | |||
| LAP0297 in FVB | 75.2 ± 23.5 | 17/18 (94.4) | not applicable | not applicable | 17 | 2 | |
| LAP0297 in Tie2P-GFP/FVB | 153.7 ± 48.1 | 3/18 (16.7) | 22.1 | <0.01 | 3 | 0 | |
| LAP0297 in VEGF-GFP/FVB | 135.5 ± 41.3 | 1/13 (7.7) | 23.3 | <0.01 | 1 | 0 | |
| MCaP0008 in FVB | 113.0 ± 19.9 | 2/11 (18.2) | not applicable | not applicable | 1 | 2 | |
| MCaP0008 in Tie2P-GFP/FVB | 153.0 ± 0.0 | 0/7 (0) | 2.37 | >0.05 | 0 | 0 | |
Data are given as mean ± 1 SD.
LAP0209 in FVB versus LAP0209 in Tie2P-GFP/FVB or VEGFP-GFP/FVB; MCaP0008 in FVB versus MCaP0008 in Tie2-GFP/FVB
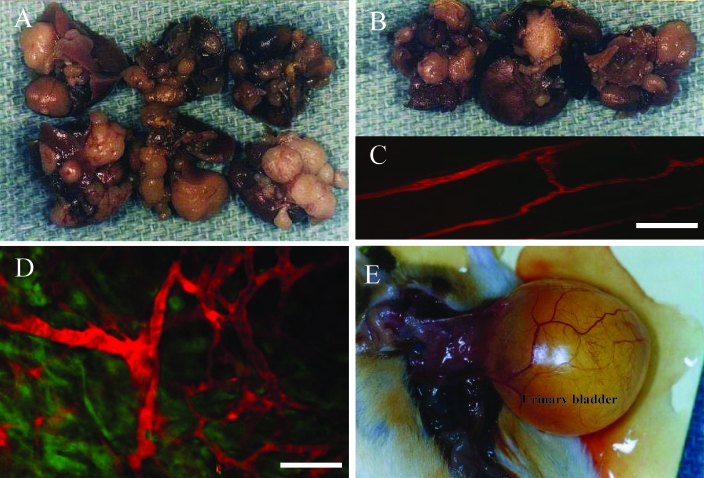
Figure 8.
LAP0297 isograft metastases to the lungs of (A) wild-type FVB/N mice and (B) Tie2P-GFP/FVB transgenic mice. (C) In vivo multiphoton laser-scanning microscopy image of an LAP0297 tumor grown in a VEGFP-GFP/FVB transgenic mouse. The normal vasculature adjacent to the tumor is highlighted by systemically injected TMR-BSA (red). (D) Tumor vasculature (red) and VEGF-expressing tumor-associated stromal cells (green) in a VEGFP-GFP/FVB transgenic mouse. Bar, 100 μm. (E) Aged tumor-free FVB/N female breeder mouse with uroschesis.
Discussion
Compared with other inbred strains, FVB/N mice are a relatively new but widely used strain in cancer research involving transgenic mice technology. However, little is known about spontaneous tumors in this strain of mice beyond a study on spontaneous lesions in aging FVB/N mice10 and pathologic studies on pituitary and mammary glands.12,19 Another recent study evaluated squamous cell carcinomas that had developed from the skin at the ear tag site in 9% of aged FVB/N mice.1 These squamous cell carcinomas likely were induced by the nickel–copper alloy ear tags and not are truly spontaneous tumors of FVB/N mice. In the present study, we report the incidence of spontaneous tumors and their histopathologic patterns in our aged wild-type FVB/N breeders housed under defined flora conditions and the establishment of 2 novel tumor lines (MCaP0008 and LAP0297) from a spontaneous mammary adenocarcinoma and a lung AB carcinoma in FVB/N mice.
The study population comprised 40 male and 194 female FVB/N retired breeders for which complete breeding records, necropsies, and pathologic examinations were available. These mice showed normal sexual maturity, mating, pregnancy, and reproduction; usually were retired after 10 mo of age; and were maintained for observation until their natural deaths (including mice found dead and those euthanized when they were moribund or had large tumors) or until euthanized when approximately 900 d old. Further, 31 male and 98 female mice were observed for more than 2 y. Therefore, the 240-mo survival rate in our study was 77.5% for male mice and 50.5% for female mice. These survival rates are comparable to the findings of a previous study,10 which we believe is the only prior comprehensive study of spontaneous tumor in FVB/N mice; the 24-mo survival rates in that study were 58% for male mice and 61% for female mice. However, the follow-up period was longer in our study, as we monitored 12.5% of male mice and 11.3% of female mice until they were older than 800 d, which is usually considered to be close to their full natural lifespan.14
According to our findings, spontaneous AB tumor of the lung was the most common neoplasm in our defined-flora retired FVB/N breeders. The incidences of spontaneous lung AB tumors in our study (male, 42.5%; female, 24.7%) also were comparable with previously reports of 41% and 37% in male and female mice, respectively.10 In our study, we found only 2 hepatocellular carcinomas (incidence, less than 2%), 1 lymphocytic leukemia, and 1 histiocytic sarcoma (both also less than 2% incidence) in female mice. Neither liver tumor nor lymphoma was found in our male mice. Our results are consistent with previous findings that suggested a higher than typical rate of lung tumors and a lower than usual incidence of liver tumors and lymphomas in FVB/N mice.10
Our results also indicate that the second most common spontaneous tumor in FVB/N female mice is mammary gland tumor (27 of 194 mice, 13.9%). Previous data are surprising in that there were no mammary tumors among the 25 spontaneous tumors in 14-mo-old females and the 47 tumors in 24-mo-old female mice.10 Although clearly different from that study,10 our findings are indirectly supported by another recent study that reported persistent mammary hyperplasia in FVB/N mice and commented that mammary carcinomas may develop sporadically in this strain of mice.12 Of the 6 mammary gland tumors identified in that study from almost 500 wild-type FVB/N mice, 2 were adenosquamous carcinomas, 3 were squamous cell carcinomas, and 1 was an adenocarcinoma.12 In contrast, we diagnosed 19 mammary adenocarcinomas and 8 adenoacanthomas (or adenosqamous carcinomas) in 194 retired female mice by pathohistologic examination. The relatively high incidence of spontaneous mammary gland tumor in our population may reflect the fact that these mice were multiparous breeders. We were unable to determine whether these mammary tumors developed from mammary gland hyperplasia. Other scientists have suggested that persistent mammary gland hyperplasia, including prolactin-positive hyperplasia and adenoma, in female FVB/N mice may be associated with an abnormal pituitary gland and high serum prolactin concentration and that the abnormal pituitary phenotype may correlate with a high prevalence of spontaneous mammary tumors in aged multiparous, but not virgin, FVB/N mice.19 However, we did not find any relationship between the development of mammary gland tumors and pituitary gland tumors in our retired female breeders: we diagnosed 27 mammary carcinomas but only 1 pituitary adenoma in each of 2 female mice.
Our results showed that among 102 spontaneous tumors in female mice, 52.9% (54 mice) tumors developed in mice younger than 2 y, and 47.1% (48 mice) tumors were found after 2 y of age; only 11 cases (10.8% of spontaneous tumors) were detected at ages greater than 800 d. In contrast, among 92 tumor-free retired female mice, 54.4% (50 mice) were older than 2 y, and 12.0% (11 mice) of tumor-free mice survived to ages greater than 800 d; fewer than 45.7% of tumor-free females were found dead or sick and euthanized at less than 2 y. As shown in Figure 1 B, the incidence of spontaneous tumor in our retired FVB/N breeders increased in mice aged from 600 d to close to 800 d and then decreased after age 800 d. This finding agrees with the conclusion of another study on the age distribution of cancer in a different strain of mice.14 In that study, the incidence of cancer rose as a function of age, but then the curve flattened and inverted at an age of about 800 d. This pattern was considered similar to that in humans, for whom the age distribution of cancer incidence inverts at about age 80.14 Approximately 15% to 20% of our aged tumor-free female breeders had uroschesis (Figure 8 E), which was confirmed by necropsy; this condition was 1 of the leading causes of clinical illness among our aged tumor-free female mice. Generally speaking, the retired male breeders survived longer than did the female mice. Among the 18 male mice with spontaneous tumors, 88.9% (16 mice) of the tumors were detected after age 2 y (2 were in mice older than 800 d); and among the 22 tumor-free retired males, 68.2% (15 mice) survived past 2 y (3 mice were over 800 d old). These results indicate that the incidence of spontaneous tumors was not a linear function of age in our male retired breeders.
Finally, as mentioned before, the FVB/N inbred mouse strain has been used widely for creating transgenic mice.18 Over the past 10 years, transgenic mice lines (VEGFP-GFP/FVB, Tie2P-GF/FVB, and EF1αP-GFP/FVB, and so on) have been used extensively in our laboratory and others for cancer research.3-5,11,16 However, few established tumor lines can be used in this particular mice strain. Therefore, 1 of our goals was to establish new tumor lines from spontaneous tumors for use in our cancer studies in FVB/N mice and transgenic mice lines with an FVB/N background. The spontaneous tumors we selected could be transplanted with high efficiency. The 5 transplantable spontaneous tumor lines have been successfully passaged for 1 to 5 generations in vivo with a ‘take rate’ of 95.7% to 100% and a different growth pattern (growth time from 21 to 56 d; Figure 7, Table 2). One mammary gland tumor (P0308) failed to grow after transplantation, possibly because of the presence of prominent stroma and few tumor cells in the transplanted fragment (Figure 6 B). These spontaneous tumors are important resources for cancer studies. Subsequently, tumor lines from a spontaneous mammary adenocarcinoma (MCaP0008) and a lung AB carcinoma (LAP0297) were established successfully in vivo and in vitro. Isografts of LAP0297 showed a high incidence (94.4%) of distant lung metastasis in wild-type FVB/N mice. McaP0008 isografts showed invasive growth in FVB/N mice but a low incidence of metastasis (18.2%). Both tumors and their cell lines were tumorigenic in our inhouse Tie2P-GFP/FVB and VEGFP-GFP/FVB transgenic mice (Table 3). We transplanted LAP0297 and MCaP0008 tumor tissue into dorsal skin-fold chambers, which were implanted in Tie2P-GFP/FVB and VEGFP-GFP/FVB transgenic mice as described previously.9 These tumor models have allowed us to use multiphoton laser-scanning microscopy2,13 at 1 to 3 wk after tumor implantation to observe tumor growth in real time. This availability has allowed quantitative studies of new blood vessels formation, tumor growth, and differential studies of the tumor cells and tumor stroma cells (Figure 8 C, D). However, further studies to better understand the differential metastatic potential within these tumor lines in wild-type and transgenic FVB/N mice are warranted.
In conclusion, spontaneous AB tumor of lung was the most common neoplasm in both male and female retired FVB/N breeders, followed by mammary gland tumors in our female mice. These tumor profiles should be considered during interpretation of neoplastic phenotypes in transgenic lines derived from FVB/N mice and designing long-term lung cancer, breast cancer, and lung metastasis studies involving wild-type or transgenic FVB/N mice. Two novel tumor lines, a mammary adenocarcinoma (MCaP0008) and a lung AB carcinoma (LAP0297), were established in vivo and in vitro. Both tumor lines were tumorigenic in VEGFP-GFP, Tie2P-GFP, and EF1αP-GFP transgenic mice, which had the FVB background. These 2 newly developed tumor lines will benefit studies on the pathophysiology of cancer in this widely used strain of mice.
Acknowledgments
This study was supported by grants R01-CA85140, R01-CA115767 and P01-CA80124 from the National Institutes of Health. We thank the Cox-7 animal facility staff for their excellent animal care; Sylvie Roberge, Julia Kahn, Carolyn Smith, and Chenmei Luo for their excellent technical support; and Leo Gerweck and Robert Sedlacek for helpful discussions.
References
- 1.Baron BW, Langan G, Huo D, Baron JM, Montag A. 2005. Squamous cell carcinomas of the skin at ear-tag sites in aged FVB/N mice. Comp Med 55:231–235 [PubMed] [Google Scholar]
- 2.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. 2001. In vivo measurement of gene expression, angiogenesis, and physiological function in tumor using multiphoton laser-scanning microscopy. Nat Med 7:864–868 [DOI] [PubMed] [Google Scholar]
- 3.Duda DG, Cohen KS, Kozin SV, Perentes JY, Fukumura D, Scadden DT, Jain RK. 2006. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood 107:2774–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duda DG, Fukumura D, Munn LL, Booth MF, Brown EB, Huang P, Seed B, Jain RK. 2004. Differential transplantability of tumor-associated stromal cells. Cancer Res 64:5920–5924 [DOI] [PubMed] [Google Scholar]
- 5.Fukumura D, Xavier R, Sugiura T, Chen Y, Park E, Lu N, Selig M, Nielsen G, Takir T, Jain RK, Seed B. 1998. Tumor induction of VEGF promoter activity in stromal cells. Cell 94:715–725 [DOI] [PubMed] [Google Scholar]
- 6.Huang P, Allam A, Taghian A, Freeman J, Duffy M, Suit HD. 1995. Growth and metastatic behavior of five human glioblastomas compared with nine other histological types of human tumor xenografts in SCID mice. J Neurosurg 83:308–315 [DOI] [PubMed] [Google Scholar]
- 7.Huang P, McKee TD, Jain RK, Fukumura D. 2005. Green fluorescent protein (GFP)-expressing tumor model derived from a spontaneous osteosarcoma in a vascular endothelial growth factor (VEGF)-GFP transgenic mouse. Comp Med 55:236–243 [PubMed] [Google Scholar]
- 8.Huang P, Taghian A, Hsu DW, Perez LA, Allam A, Duffy M, DaCosta A, Suit HD. 1996. Spontaneous metastasis, proliferation characteristics and radiation sensitivity of fractionated irradiation recurrent and unirradiated human xenografts. Radiother Oncol 41:73–81 [DOI] [PubMed] [Google Scholar]
- 9.Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. 1992. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res 52:6553–6560 [PubMed] [Google Scholar]
- 10.Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. 1996. Spontaneous lesions in aging FVB/N mice. Toxicol Pathol 24:710–716 [DOI] [PubMed] [Google Scholar]
- 11.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, Stainier DY, Sato TN. 2000. Universal GFP reporter for the study of vascular development. Genesis 28:75–81 [DOI] [PubMed] [Google Scholar]
- 12.Nieto AI, Shyamala G, Galvez JJ, Thordarson G, Wakefield LM, Cardiff RD. 2003. Persistent mammary hyperplasia in FVB/N mice. Comp Med 53:433–438 [PubMed] [Google Scholar]
- 13.Padera TP, Stool BR, So PTC, Jain RK. 2002. Conventional and high-seed intravital multiphoton laser-scanning microscopy of microvasculature, lymphatics, and leukocyte-endothelial interactions. Mol Imaging 1:9–15 [DOI] [PubMed] [Google Scholar]
- 14.Pompei F, Polkanov M, Wilson R. 2001. Age distribution of cancer in mice: the incidence turnover at old age. Toxicol Ind Health 17:7–16 [DOI] [PubMed] [Google Scholar]
- 15.Public Health Service 1996. Public Health Service policy on humane care and use of laboratory animals. Washington (DC): US Department of Health and Human Services; p 28 [Google Scholar]
- 16.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. 1997. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci USA 94:3058–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedlacek R, Orcutt RP, Suit HD, Rose EF. 1981. A flexible barrier at cage level for existing colonies: production and maintenance of a limited stable anaerobic flora in a closed inbred mouse colony. Sazaki S, Ozawa A, Hashimoto K. Recent advances in germfree research. Tokyo: Tokyo University Press; p 65–69 [Google Scholar]
- 18.Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, Overbeek PA. 1991. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci USA 88:2065–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakefield LM, Thordarson G, Nieto AI, Shyamala G, Galvez JJ, Anver MR, Cardiff RD. 2003. Spontaneous pituitary abnormalities and mammary hyperplasias in FVB/NCr mice: implications for mouse modeling. Comp Med 53:424–432 [PubMed] [Google Scholar]