Abstract
To identify the relevant CpG sites as molecular markers, for the diagnosis and to distinguish the indolent and aggressive prostate tumors, we have determined the methylation status of 8 genes, including FLNC, EFS, ECRG4, RARB2, PITX2, GSTP1, PDLIM4, and KCNMA1 in 32 nonrecurrent, 32 recurrent primary prostate tumors, and 32 benign prostate tissues using EpiTYPER technology. Specific CpG site hypermethylation of RARB2 and GSTP1 CpG sites were useful for diagnosis of prostate cancer. Furthermore, CpG site hypermethylation of genes FLNC, EFS, ECRG4, PITX2, PDLIM4, and KCNMA1 were associated with prediction of biochemical, local, and systemic recurrence of prostate cancer.
Keywords: CpG site methylation, Mass spectrometry assay, Prostate cancer, Recurrence prediction, Molecular markers
INTRODUCTION
Biochemical (prostate-specific antigen) recurrence of prostate cancer after radical prostatectomy remains a major problem (1). Those who experience early PSA recurrence, particularly within the first 2 years of surgery, are prone to develop metastatic lesions and are likely to succumb to their prostate cancer (2, 3). Several clinicopathologic scoring systems have been developed to identify the patients at greatest risk of recurrence after surgery, including the weighted risk of recurrence, determined by the lymph node status, seminal vesicle status, surgical margin status, and postoperative Gleason score and the Kattan nomograms (4–7). These factors are helpful but far from perfect due to significant clinical heterogeneity of the disease. However, many patients defined as being at low risk still develop cancer recurrence (8–12). Clearly, new biological markers are urgently needed to predict more accurately the risk of relapse.
Gene silencing due to DNA methylation of promoter CpG islands (CGIs) is one of the major mechanisms of tumor-suppressor gene inactivation, along with mutations and loss of heterozygosity (13). DNA methylation plays an important role in cancer control and provides potential molecular markers to assess cancer risk, improve cancer detection, monitoring of cancer prognosis, and predict therapy responses (14, 15). Reversal of tumor-associated silencing of tumor suppressor genes is increasingly being targeted for cancer treatment and prevention strategies (16–19). Integration of the methylation status of multiple genes as methylation score was reported to serve as a potential diagnostic and staging biomarker for prostate cancer (20). Detection of altered methylation has a vast diagnostic and prognostic potential but requires a comprehensive characterization of the methylation patterns in tumor and normal tissues to determine which hypermethylation events are disease specific. However, such studies are limited in the literature. The ability to predict the outcome or recurrence of prostate cancer is a worthwhile goal that will enable physicians to tailor treatment recommendations for patients.
Promoter regions of several genes have been found to be hypermethylated in prostate cancer using conventional methylation-specific PCR (MSP) method (20–22). The method only allows assessment of the presence or absence of methylation in a limited region of about 100–150 bases and the MSP product is generated only when the entire amplicon is methylated Consequently, this will not identify the regions with methylation variations in the amplicon. Other methods, such as semiquantitative real-time PCR and methylation microarrays, are limited to a restricted number of CpG sites for analysis. Here, we analyzed the methylation profiles in 64 prostate cancer tissues from patients with and without disease recurrence after radical prostatectomy and in 32 benign prostate tissues obtained after radical prostatectomy by a high-throughput Mass-CLEAVE/MassARRAY method (23). This method of methylation analysis by base-specific cleavage in combination with matrix assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF-MS) detection has enabled us to quantitatively measure the individual CpG unit methylation in more than 500 bases, multiplexing with 20 ng of DNA and at a detection limit approximately 5% methylated DNA (23). In this method, bisulfite-modified DNA is PCR amplified followed by in vitro transcription to generate single-stranded RNA molecule. The RNA strand is then cleaved base specifically and the resulting cleavage products were analyzed by MALDI-TOF MS. This is a rapid and cost-effective quantitative analysis of methylation at individual CpG sites.
We evaluated the promoter CpG unit methylation status of 8 genes. This includes a total of 2,900 base pair regions containing 238 CpG sites within 136 CpG units of the genes filamin C (FLNC), embryonal Fyn-associated substrate (EFS), esophageal cancer–related gene 4 protein (ECRG4), retinoic acid receptor beta 2 (RARB2), paired-like homeodomain transcription factor 2 (PITX2), glutathione s-transferase pi (GSTP1), PDZ and LIM domain 4 (PDLIM4), and potassium large conductance calcium-activated channel, subfamily m, alpha member 1 (KCNMA1). We identified the specific CpG sites methylation for diagnosis and progression of prostate cancer. We assessed the correlations of methylation score for diagnosis of prostate cancer (Mdp) and methylation score for recurrence of prostate cancer (Mrp) with the clinical and histopathological outcome.
MATERIALS AND METHODS
Prostate tissue samples
Surgically resected prostate cancer tissue specimens were obtained from patients who had undergone radical prostatectomy from 1999 to 2003 without prior therapy for prostate cancer at Mayo Clinic with the Institutional Review Board approval (22). Fresh frozen prostate cancer specimens were trimmed to obtain tissue sections containing >70% tumor nuclei. We used organ-confined prostate cancer tissues of patients who were free of disease for >5 years after prostatectomy (n = 32 nonrecurrent) and primary prostate cancer tissues of patients who had recurrence of the disease within 5 years of prostatectomy (n = 32), which included PSA recurrences (n = 10), local recurrences (n = 10), and systemic progressions cases (n = 12) (Table 1). Separately collected nonmalignant benign prostatic hyperplasia tissues (n = 32) obtained after suprapubic prostatectomy were used as controls. Nonrecurrence cases were defined as the prostate cancer patients without any recurrence after radical prostatectomy for >5 years of follow-up, with serum PSA levels <0.2 ng/mL. PSA recurrence was defined as the first postprostatectomy serum PSA value 0.2 ng/mL or above, followed by a value higher than the first (24–27) and excluding any PSA within the first 30 days of surgery. Local recurrence was defined as cancer demonstrated on biopsy of the prostatic bed or the receipt of salvage radiation therapy to the prostatic bed without evidence of systemic recurrence. Systemic progression involved demonstrable metastatic deposits on radionuclide bone scan or on biopsies other than biopsy of the prostatic bed (28).
Table 1.
Prostate tissues used for methylation analysis
| Tissue Group | Total Number | Mean PSA Recurrence Years | Mean Local Recurrence Years | Mean Systemic Recurrence Years | Mean Gleason Score | Mean GPSM Score |
|---|---|---|---|---|---|---|
| Benign prostate | 32 | |||||
| Prostate cancer | ||||||
| Nonrecurrence (>5 years of follow-up) | 32 | 6.53 | 8.37 | |||
| Recurrence (within 5 years of follow-up) | 32 | |||||
| PSA recurrence | 10 | 1.71 years (n = 10) | 7.1 | 9.6 | ||
| Local recurrence | 10 | 1. 87 years (n = 10) | 1.75 years (n= 10) | 7.6 | 9.5 | |
| Systemic recurrence | 12 | 0.677 years (n = 12) | 1.51 years (n = 12) | 8.1 |
Value in parentheses indicates the number of patients in the recurrence group who developed PSA and local and systemic recurrence, respectively.
DNA isolation and bisulfite conversion
DNA was isolated from 10 tissue sections of 20-μm thickness using the ZR Genomic DNA II kit (Zymo Research Corp., Orange, CA, USA). Bisulfite treatment of genomic DNA was performed with a commercial kit from Zymo that combines bisulfite conversion and DNA cleanup (23). The converted DNA was measured using an ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA).
Selection of genomic targets for analysis
From our previous gene expression profiling of prostate cancer tissues (21, 22), we selected the genes FLNC, EFS, ECRG4, and KCNMA1 for methylation analysis primarily because they were most significantly under expressed in organ-confined and metastatic tumors (study name, Vanaja Prostate; www.oncomine.org) and also for the high frequency of CG content in the predicted promoter regions with CpG islands (e.g., http://www.cpgislands.com) (29, 30) (%GC ⇒ 70 and Obs/ExpCpG ⇒ 0.75). In addition, four genes GSTP1 (31), PDLIM4 (21), RARB2 (32), and PITX2 (33) were selected from the literature reported to be hypermethylated in prostate cancer. Methylation primers were designed using the MethPrimer software (http://www.ucsf.edu/urogene.org/methprimer) (34). Primers were selected from the CpG island regions with homogenous CpG site methylation patterns in the amplicon sequence. The target region of the genes used for methylation analysis (Table 2) and the primer sequences used for PCR amplification were shown (Table 3). One of the two primers in the PCR amplification of the target regions is tagged with a T7 promoter sequence: cagtaatacgactcactatagggagaaggct. This includes ggg transcription start and an 8-bp insert (agaaggct) on the 5′ end.
Table 2.
Target region of the genes used for methylation analysis
| Gene | Length (bp) | CpG Sites | CpG Units | Chromosomal Location | Amplicon Location (Transcription Start Site) |
|---|---|---|---|---|---|
| FLNC | 363 | 40 | 20 | chr7: 128257721 – 128258084 | Plus 2 to plus 365 |
| EFS | 371 | 46 | 19 | chr14: 22904250 – 22904621 | Minus 432 to minus 61 |
| ECRG4 | 505 | 48 | 21 | chr2: 106048379 – 106048884 | Minus 166 to plus 339 |
| RARB2 | 391 | 14 | 12 | chr3: 25444596 – 25444987 | Minus 162 to plus 229 |
| PITX2 | 424 | 37 | 24 | chr4: 111762893 – 111763317 | Plus 14640 to plus 15064 |
| GSTP1 | 347 | 23 | 19 | chr11: 67107924 – 67108271 | Plus 62 to plus 409 |
| PDLIM4 | 238 | 11 | 7 | chr5: 131621562 – 131621800 | Plus 276 to plus 514 |
| KCNMA1 | 261 | 19 | 14 | chr10: 79066039 – 79066300 | Minus 1544 to minus 1283 |
CpG sites present in the CpG units of the sequence analyzed are listed with the corresponding amplicon location of the sequence and the amplicon location relative to the transcription start site.
Table 3.
Primers used for PCR amplification of the bisulfite-converted DNA
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| FLNC | TTTGGAGGGAGAGAGAGTTAGAGAG | CACTTAAAATACTCATTACACCAAC |
| EFS | GGTAGGTTAGTTAGGTAGGAGGAGGAG | CAAACCCCAACCCTAAAAACAC |
| ECRG4 | GTGGTTAAGTATTTTTGGTTTTGGAGTTTA | ATCCTCTACCACCCCTTAACCCTAC |
| RARB2 | TTGGTTGTTTGTTTTTGTAGGGTTGT | ATTCCCAAAAAAATCCCAAATTCTC |
| PITX2 | GGTGTGTATTTTTAGTTTGTGTTTGGAG | AATACCCTTCTACCCACATCCCATAT |
| GSTP1 | GGGGTTTAGAGTTTTTAGTATGGGGTTAAT | TAAACTACAACCCCAACCCCTACCT |
| PDLIM4 | GGTTTTTAGGTAGAGGTAGGTAGTTTTAGT | TCATTTTACCCCAAATCTTCAAACAA |
| KCNMA1 | GGGAAGGGAAGATATTTTTAGGGATA | TCTATTCCTTTACCCCCAAACCAC |
PCR and in vitro transcription
The PCR reactions were carried out in a total volume of 5 μL using 1 pmol of each primer, 40 μM dNTP, 0.1 U HotStar Taq DNA polymerase (Qiagen Inc., Valencia, CA), 1.5 mM MgCl2, and 5 × PCR buffer (final concentration 1 ×). The reaction mix was preactivated for 15 min at 95°C. The reactions were amplified in 45 cycles of 95°C for 20s, 62°C for 30s, and 72°C for 30s followed by 72°C for 3 min. Unincorporated dNTPs were dephosphorylated by adding 1.7 μL DNase free water and 0.3 U Shrimp Alkaline Phosphatase (SAP) (Sequenom, Inc., San Diego, CA, USA). The reaction was incubated at 37°C for 20 min and SAP was then heat inactivated for 10 min at 85°C. Typically, 2 μL of the PCR reaction were directly used as template in a 6.5 μL combined transcription-cleavage reaction. Twenty units of T7 R&DNA polymerase (Epicentre, Madison, WI, USA) were used to incorporate either dCTP or dTTP in the transcripts. Ribonucleotides at 1 mM and the dNTP substrate at 2.5 mM were used. RNase A (Sequenom) was included to cleave the in vitro transcript. The mixture was then further diluted with water to a final volume of 27 μL. Conditioning of the phosphate backbone prior to MALDI-TOF MS was achieved by the addition of 6 mg CLEAN resin (Sequenom).
Mass spectrometry measurements
The cleavage reaction samples (15 nL) were dispensed onto silicon chips preloaded with matrix (SpectroCHIP, Sequenom). Mass spectra were collected using a Mass ARRAY mass spectrometer (Sequenom). Spectra were analyzed using proprietary peak picking and spectra interpretation tools (EpiTYPER, Sequenom). For analysis of DNA methylation, we examined the methylation-dependent C/T sequence changes introduced by bisulfite treatment. Those C/T changes are reflected as G/A changes on the reverse strand and hence result in a mass difference of 16 kDa for each CpG site enclosed in the cleavage products generated from the RNA transcript. The mass signals representing nonmethylated DNA and those representing methylated DNA built signal pairs, which are representative for the CpG sites within the analyzed sequence substring. The intensities of the peaks were compared, and the relative amount of methylated DNA was calculated from this ratio. The method yields quantitative results for each of these sequence defined analytic units referred as CpG unit, which contain either one individual CpG site or an aggregate of subsequent CpG sites.
Statistical analysis
The quantitative methylation of each CpG unit measured by the MALDI-TOF-MS assay was filtered to exclude poor-quality and nonvariable data. For the prediction analysis, only the CpG units with values in at least 75% of the 96 samples were selected. This resulted in 101 CpG units from 8 genes. We calculated methylation score for diagnosis of prostate cancer (Mdp score) accounting for the hypermethylation in tumor samples compared with benign tissues and methylation score for recurrence of prostate cancer (Mrp score) separately for PSA recurrence, local recurrence, and systemic recurrence versus nonrecurrence cases, as well as all the recurrence groups compared with nonrecurrence group, as a prediction from a diagonal linear discriminant analysis (DLDA) using combinations of the CpG unit methylation of various genes (35–37). The methylation scores Mdp and Mrp for each sample were defined as the difference of the distance for each class of the methylation value for each CpG weighted by the class average CpG methylation and further divided by the class methylation variance to half the class average CpG unit methylation. The classes were defined as the sum of the corresponding log hazard ratio coefficients, which were derived from multivariate logistic regression analysis of each methylated gene in the benign versus prostate cancer samples and tumor nonrecurrence versus recurrence groups.
Methylation scores of Mdp and Mrp were based on training and test sets of the samples. For the Mdp score calculation, we used CpG feature selection based on the order of p values obtained from paired r-tests comparing tumors versus benign tissues. Both the benign and the tumor samples were randomly divided into two equal groups. Group-1 samples were then used as the prediction training set and group-2 samples were used as the test set to obtain the area under the curve (AUC) performance of the group-2 tumor samples. For the Mrp score calculation, we used CpG feature selection based on the unequal-variance t-tests for the difference in the methylation values of nonrecurrence tumors compared with the recurrence tumor group. DLDA models were built using respective training sets for all increasing combinations of the ranked CpG units beginning with the top two most significant CpG units and increasing until all the CpG units were included in the model. The numbers of CpG units to include in the final Mdp and Mrp score models were determined by the first local maximum AUC value with at least 5 CpG units and at most 20 CpG units. The final Mdp and Mrp scores were the DLDA-predicted values using the respective number of CpG units indicated as the optimal from the test set and using all the samples. The number followed by the CpG unit represents the CpG sites in the annotated gene sequence. The correlation of methylation score and clinicopathologic findings were analyzed using Spearman’s rank correlation test. A p value of <.05 was considered as statistically significant.
RESULTS
Methylation score for diagnosis of prostate cancer
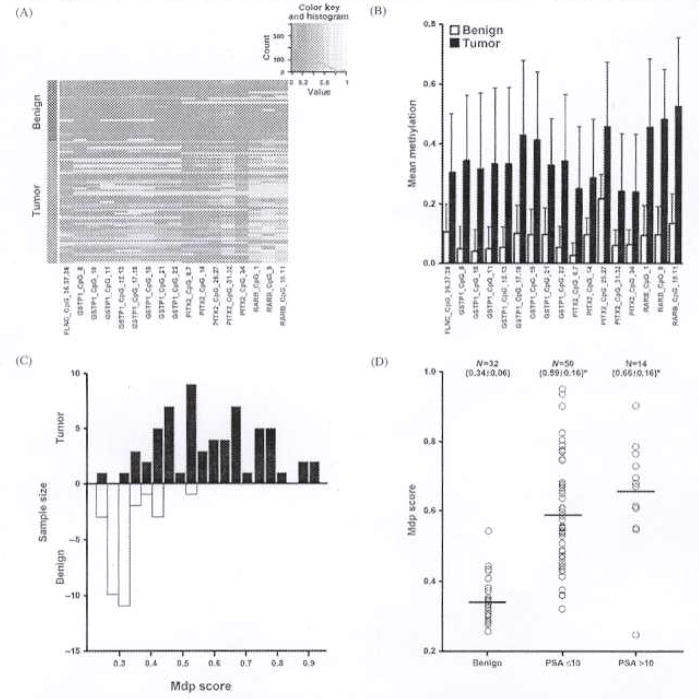
To determine the methylation score for diagnosis of prostate cancer, we evaluated methylation profiles of genes in 64 tumor samples and 32 benign samples. The individual 17 CpG unit methylation of genes that distinguish tumor from the benign tissues were depicted in the cluster diagram [Figure 1(A)]. The number followed by the CpG unit represents the CpG sites in the annotated gene sequence of the amplicon. The mean methylation levels of these CpG units were significantly increased in tumor group compared with the benign [Figure 1(B)]. The DLDA model with the local maximum of 0.9209 at top 13 CpG units using group-2 benign and tumor samples as the test sets was chosen as best fit for the Mdp score. The distribution of methylation similarity score, a cumulative score of the CpG units for diagnosis of prostate cancer, was shown in 32 benign and 64 tumor tissues [Figure 1(C)]. The Mdp score was higher in the tumor tissues when compared with the benign samples, which enabled us to easily distinguish the tumor from the benign tissues. A significant increase in the Mdp score (p < .05) was observed in both preoperative serum PSA of less than 10 ng/mL and greater than 10 ng/mL groups of the cancer patients [Figure 1(D)]. To assess the potential utility of hypermethylation of genes as molecular markers of prostate cancer, we determined the optimal sensitivity and specificity of the CpG units methylation by receiver-operating characteristic (ROC) curves. The sensitivity and specificity of the individual CpG units and the Mdp score for diagnosis of cancer was shown in Table 4. CpG sites 10 and 11 of RARB2 gene were the top most significantly ranked, with a sensitivity and specificity of 90.0% and 92.59%, respectively, for diagnosis of prostate cancer.
Figure 1.
(A) Cluster diagram depicting CpG units methylation of genes in benign (n = 32) and tumor (n = 64) tissues. Each row represents a tissue and each column a CpG unit of the gene. Frequency of methylation levels is color scaled from red to yellow. Red denotes no methylation, while bright yellow denotes complete methylation. Gray represents technically inadequate or missing data. (B) Group mean methylation of the CpG unit of genes in the benign and tumor tissues. (C) Distribution of methylation similarity scores for diagnosis of prostate cancer (Mdp) in benign (black bars) and tumor tissues (white bars). (D) Mdp score in benign and tumor tissues with preoperative serum PSA value of <10 ng/mL and >10 ng/mL groups. n = number of tissues in each group. Mean and standard deviation are shown in parentheses. *p < .05.
Table 4.
Sensitivity and specificity of the Mdp score and the individual CpG units methylation of genes for diagnosis of prostate cancer
| CpG unit | Paired t-Test (p Value) | Sensitivity (95% Confidence Interval) | Specificity (95% Confidence Interval) |
|---|---|---|---|
| MdpScore | 1.90E-19 | 87.3 (76.50–94.30) | 87.10 (70.17–96.37) |
| RARB2_CpG_10.11 | 6.14E-10 | 90 (73.47–97.89) | 92.59 (75.71–99.09) |
| RARB2_CpG_1 | 1.36E-09 | 87.1 (70.17–96.37) | 89.29 (71.77–97.73) |
| RARB2_CpG_9 | 7.89E-09 | 90 (73.47–97.89) | 88.89 (70.84–97.65) |
| GSTP1_CpG_21 | 1.77E-07 | 88.46 (69.85–97.55) | 87.10 (70.17–96.37) |
| GSTP1_CpG_10 | 1.74E-06 | 92.31 (74.87–99.05) | 93.55 (78.58–99.21) |
| GSTP1_CpG_22 | 1.74E-06 | 92.31 (74.87–99.05) | 93.55 (78.58–99.21) |
| GSTP1_CpG_17.18 | 1.75E-06 | 87.5 (67.64–97.34) | 86.21 (68.34–96.11) |
| PITX2_CpG_31.32 | 1.93E-06 | 77.42 (58.90–90.41) | 79.31 (60.28–92.01) |
| GSTP1_CpG_19 | 2.62E-06 | 92 (73.97–99.02) | 89.66 (72.65–97.81) |
| GSTP1_CpG_8 | 5.81E-06 | 81.48 (61.92–93.70) | 76.67 (57.72–90.07) |
| FLNC_CpG_36.37.38 | 6.47E-06 | 80.65 (62.53–92.55) | 80.00 (61.43–92.29) |
| PITX2_CpG_14 | 7.84E-06 | 70.97 (51.96–85.78) | 73.33 (54.11–87.72) |
| PITX2_CpG_6.7 | 1.21E-05 | 70.97 (51.96–85.78) | 75.86 (56.46–89.70) |
| PITX2_CpG_34 | 1.21E-05 | 70.97 (51.96–85.78) | 75.86 (56.46–89.70) |
| GSTP1_CpG_11 | 1.37E-05 | 84.62 (65.13–95.64) | 83.33 (65.28–94.36) |
| GSTP1_CpG_12.13 | 1.61E-05 | 83.33 (62.62–95.26) | 83.33 (65.28–94.36) |
| PITX2_CpG_26.27 | 2.83E-05 | 67.74 (48.63–83.32) | 68.97 (49.17–84.72) |
CpG units were ranked by t-Test, p value comparing tumor (n = 64), and benign (n = 32) tissues.
Methylation score for prediction of prostate cancer recurrence
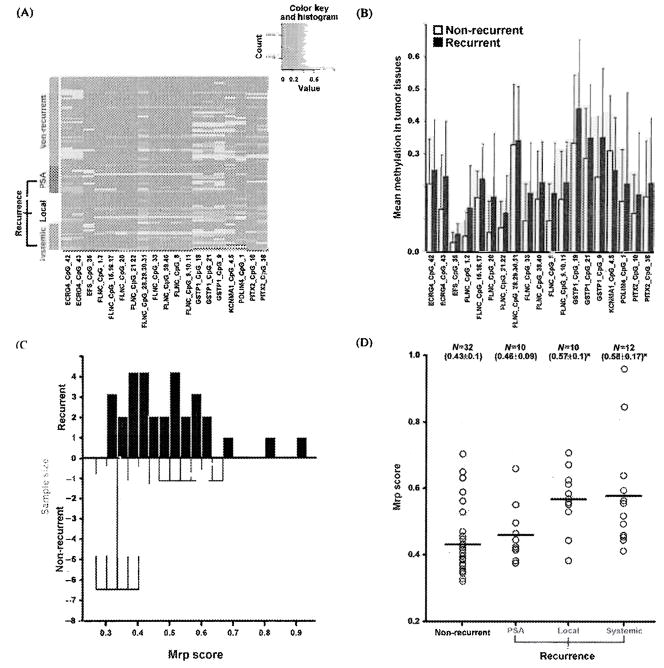
To test the ability of CpG site methylation for prediction of recurrence, we compared 32 organ-confined prostate cancer tissues without recurrence of disease after radical prostatectomy and 32 primary prostate cancer tissues with recurrence of the disease. We found that methylation of 19 CpG units of genes and their mean methylation levels were significantly different in the recurrence group compared with the nonrecurrence group [Figures 2(A) and (B)]. The DLDA model with the local maximum of 0.600 at the top 11 CpG units using the recurrence tumor samples was chosen as best fit for Mrp score. The distribution of methylation similarity score for recurrence prediction was shown in recurrence and nonrecurrence tissues [Figure 2(C)]. A significant increase in the Mrp score was observed in local and systemic recurrence cases [Figure 2(D)]. Mrp score to predict the local and systemic recurrence showed sensitivities of 80.0% and 72.7% and specificities of 81.2% and 75.0%, respectively (Table 5).
Figure 2.
(A) Cluster diagram depicting CpG units methylation of genes in nonrecurrence and recurrence tumor tissues. Each row represents a tissue and each column a CpG unit of the genes. Green represents nonrecurrent (n = 32) tumors. Blue, yellow, and red represents recurrent tumors (n = 32 total) with PSA failure (n = 10), local recurrence (n = 10), and systemic progression (n = 12), respectively. Frequency of methylation levels is color scaled from red to yellow. Red denotes no methylation, while bright yellow denotes complete methylation. Gray represents technically inadequate or missing data. (B) Group mean methylation of the CpG units of genes in nonrecurrence and recurrence tumors (p value < 0.05). (C) Distribution of methylation similarity scores for recurrence of prostate cancer (Mrp) in nonrecurrence (black bars) and recurrence (white bars) tumor tissues. (D) Mrp score in nonrecurrent and recurrent tumors with PSA failure, local, and systemic progression. n = number of tissues in each group. Mean and standard deviation are shown in parentheses. *p < .05.
Table 5.
Sensitivity and specificity of the individual CpG units methylation of genes associated with recurrence of cancer
| CpG Unit | t-Test (p Value) | Sensitivity (95 %Confidence Interval) | Specificity (95% Confidence Interval) |
|---|---|---|---|
| All recurrence prediction | |||
| FLNC_CpG_20 | .01112 | 71.43 (41.92–91.61) | 68.75 (41.34–88.98) |
| FLNC_CpG_1.2 | .01179 | 71.43 (41.92–91.61) | 68.75 (41.34–88.98) |
| FLNC_CpG_33 | .01179 | 78.57 (49.22–95.34) | 75.06 (47.62–92.73) |
| FLNC_CpG_8 | .01179 | 78.57 (49.20–95.34) | 75.12 (47.62–92.73) |
| FLNC_CpG_21.22 | .01542 | 64.29 (35.14–87.24) | 68.75 (41.34–88.98) |
| GSTP1_CpG_9 | .01649 | 75.02 (42.81–94.51) | 72.73 (39.03–93.98) |
| PITX2_CpG_10 | .01866 | 66.67 (38.38–88.18) | 64.29 (51.42–87.24) |
| GSTP1_CpG_21 | .01923 | 73.33 (44.90–92.21) | 72.73 (39.03–93.98) |
| GSTP1_CpG_19 | .01969 | 76.92 (46.19–94.96) | 81.82 (48.22–97.72) |
| FLNC_CpG_15.16.17 | .02248 | 71.43 (41.90–91.61) | 62.52 (35.43–84.84) |
| EFS_CpG_35 | .02338 | 62.50 (24.49–91.48) | 60.02 (32.29–83.66) |
| Local recurrence prediction | |||
| FLNC_CpG_15.16.17 | .00761 | 77.78 (39.99–97.19) | 78.12 (60.03–90.72) |
| FLNC_CpG_20 | .0114 | 77.78 (39.99–97.19) | 81.25 (63.56–92.79) |
| FLNC_CpG_33 | .01249 | 77.78 (39.99–97.19) | 75.04 (56.62–88.54) |
| FLNC_CpG_8 | .01249 | 77.78 (39.99–97.19) | 75.04 (56.63–88.54) |
| FLNC_CpG_1.2 | .01759 | 77.78 (39.99–97.19) | 81.25 (63.56–92.79) |
| FLNC_CpG_21.22 | .03588 | 77.78 (39.99–97.19) | 75.13 (56.62–88.54) |
| FLNC_CpG_39.40 | .04213 | 77.78 (39.99–97.19) | 78.12 (60.03–90.72) |
| FLNC_CpG_9.10.11 | .04213 | 77.78 (39.99–97.19) | 78.12 (60.03–90.72) |
| PITX2_CpG_36 | .04314 | 70.04 (34.75–93.33) | 70.12 (50.64–85.27) |
| Systemic recurrence prediction | |||
| FLNC_CpG_1.2 | .01762 | 72.73 (39.03–93.98) | 68.75 (49.99–83.88) |
| EFS_CpG_35 | .03987 | 72.73 (39.03–93.98) | 60.14 (40.64–77.34) |
| PSA recurrence prediction | |||
| PDLIM4_02_CpG_1 | .00055 | 20.04 (2.52–55.61) | 42.86 (24.46–62.82) |
| EFS_001_CpG_35 | .01324 | 75.02 (34.91–96.81) | 70.22 (50.62–85.27) |
| KCNMA1_03_CpG_4.5 | .02261 | 40.04 (12.16–73.76) | 37.93 (20.69–57.74) |
| ECRG4_001_CpG_42 | .03367 | 60.21 (26.24–87.84) | 64.06 (42.52–82.03) |
| FLNC_002_CpG_28.29.30.31 | .03877 | 33.33 (7.49–70.07) | 31.25 (16.12–50.01) |
| ECRG4_001_CpG_43 | .04627 | 70.02 (34.75–93.33) | 66.67 (46.04–83.48) |
| Mrp score | |||
| All recurrence | .00069 | 71.12 (52.02–85.5) | 71.90 (53.22–86.24) |
| Local recurrence | .00156 | 80.32 (44.04–97.5) | 81.20 (63.62–92.82) |
| Systemic recurrence | .02035 | 72.72 (39.03–94.02) | 75.14 (56.66–88.52) |
| PSA recurrence | .33745 | 60.26 (26.02–87.80) | 59.42 (40.64–76.36) |
All the recurrence groups including PSA (n = 10), local (n = 10), and systemic (n = 12) recurrences were compared with the nonrecurrence group (n = 32). Sensitivity and specificity of the Mrp score is shown for overall recurrence prediction, local, systemic, and PSA recurrences.
Correlation of Mdp and Mrp scores with the clinicopathologic factors of prostate cancer
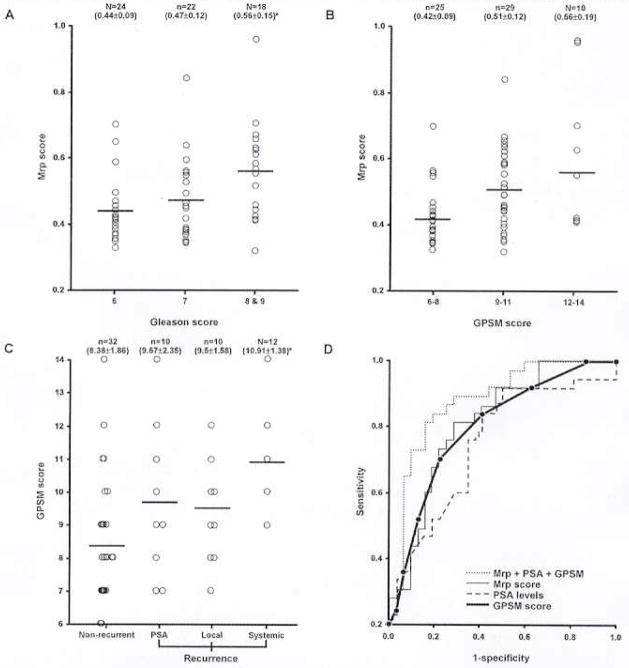
To evaluate methylation scores as an independent or a supplementary approach to the clinicopathologic parameters for diagnosis of prostate cancer and prediction of recurrence, we performed Spearman’s rank correlation test to study the association of Mdp and Mrp scores with the preoperative serum PSA levels, Gleason score, and GPSM score. A GPSM score is a prognostic model using the weighted sum of the pathological Gleason score, preoperative PSA, seminal vesicle involvement, and marginal status to predict biochemical progression after radical prostatectomy. The 5-year progression-free survival was 95% for scores <5, 60% for 10, and 32% for > 12 (8). This predictive model allows identification of patients who are at high risk for cancer progression. Our analysis revealed that both Mdp and Mrp scores had a significant correlation with PSA levels (p = .00086 and p = .000054, respectively). However, significant correlation with pathological Gleason score was observed for only Mrp score (p = .004). The Mrp score is statistically higher in the cases having a Gleason score of 8 or 9 (unequal-variance t-test; p = .005) compared with those with Gleason score of 6 (p = .28) [Figure 3(A)]. Higher Mrp score was observed in patients with higher GPSM scores; however, this trend was not statistically significant [Figure 3(B)], which suggests that Mrp score is an independent predictor of progression. Mrp score was highly significant with the overall recurrence of cancer (p = .00049). Unequal-variance t-tests were used to determine which recurrence groups were statistically different. Both Mdp and Mrp scores were significantly higher in local (p = .017 and p = .0016) and systemic recurrence cases (p = .014 and p = .02) compared with the nonrecurrent tumors; however, they were not significant in cases with only PSA recurrence. The ploidy of tumor tissues did not show any correlation with either Mdp or Mrp scores.
Figure 3.
(A) Correlation of Mrp score with the Gleason score of prostate cancer tissues. Each circle represent a tumor tissue in the arbitrarily grouped Gleason score. (B) GPSM score of the tumor tissues in nonrecurrence and recurrence tumors. (C) Relationship of the Mrp score with GPSM score. Horizontal bars represent the mean in each group. n = number of samples. Mean and standard deviation are shown in parentheses. *p <.05. (D) ROC curves for prediction of recurrence with GPSM score, preoperative PSA, Mrp score, and the combination. GPSM score: Sensitivity = 63.3%, specificity = 78.1% (AUC = 0.755). Preoperative PSA: Sensitivity = 66.7%, specificity = 65.6% (AUC = 0.686). Mrp score: Sensitivity = 71.0%, specificity = 71 9% (AUC = 0.773). Combined: Sensitivity = 80.0%, specificity = 81 2% (AUC = 0.852).
Correlation of recurrence with the clinicopathological factors
We further analyzed the association of prostate cancer recurrence with the Gleason score, GPSM score, and Mrp score. Gleason score based on pathology was significantly higher in local recurrent cases compared with the nonrecurrent cases (unequal-variance t-test; p = .0032), similarly for systemic recurrence and nonrecurrent cases (p = .00057). The mean GPSM score in PSA, local, and systemic recurrence groups was higher than the score in the nonrecurrent group (p = .00174) [Figure 3(C)]. A significant difference in the GPSM score was observed between the nonrecurrent and the systemic recurrence cases (p < .0001). ROC curve analysis revealed that the combination of Mrp score with GPSM score and the preoperative PSA levels has shown an augmentation of sensitivity (80.0%) and specificity (81.2%) with AUC = 0.852 for prediction of recurrence when compared with the individual parameters of risk assessment [Figure 3(D)].
DISCUSSION
Methylation in the promoter region of the genes plays an important role in regulation of gene expression. Rapid and sensitive detection of DNA methylation changes would aid in diagnosis and risk stratification of cancer (15–17). Methylation patterns in the process of tumorigenesis are often very heterogeneous. Methods to analyze the DNA methylation have been hampered by limitations such as no quantification of the methylation variable regions, limited number of analyzed CpG sites, vary labor-intensive methods, and not quantifying the individual CpG site methylation. Our results demonstrate that EpiTYPER technology is able to overcome these limitations. Methylation analysis using the MassARRAY system is an ideal tool for discovery of methylation, for discrimination between methylated and unmethylated regions, and for quantifying the methylation levels to follow the progression of cancer. We quantitatively assessed the methylation of the genes that are underexpressed in prostate cancer (21, 38–41), and identified specific CpG site methylation changes of genes associated with development and progression of prostate cancer.
Methylation of genes, GSTP1, PDLIM4, and RARB2 has been reported in prostate cancer by using MSP method (42–45). We have identified methylation of 17 CpG units of genes, RARB2, GSTP1, PITX2, and FLNC that showed a significant difference in benign prostatic hyperplasia and prostate cancer. We determined the methylation scores for diagnosis of prostate cancer with a sensitivity and specificity of 87.3% and 87.1%, respectively. Enokida et al. were the first to demonstrate multi-gene methylation score (M score) by MSP method as a tool for prostate cancer detection with a sensitivity and specificity of 75.9% and 84.1%, respectively, for the genes GSTP1, APV, and MDR1 (20). The increase in sensitivity and specificity in our study might be due to the identification of specific CpG sites methylation that are of high relevance to the cancer. Our finding highlights the necessity for a comprehensive quantitative methylation analysis before developing a high-throughput assay targeting only a small number of potential methylation sites for implementation in clinical diagnostics. The functional consequences of this variation, of CpG sites methylation, in molecular terms (e.g., accessibility for transcription factors) have to be addressed in future studies. In the prostate cancer patients with PSA levels of 10 ng/mL or less, the Mdp score was significantly increased (p ≤ .05) compared with adjacent benign tissues. These results indicate that analysis of the CpG site methylation of genes in patients with marginal PSA elevation would serve as adjuvant molecular markers for diagnosis of prostate cancer.
Predicting the probability of recurrence will enable us to distinguish between patients who have an indolent cancer not requiring any form of treatment and patients with biochemical relapse who may have a more aggressive course requiring early intervention. Therefore, we compared the methylation patterns in 32 indolent tumors without recurrence of cancer and 32 aggressive tumors with recurrence of disease within 5 years of prostatectomy. We further evaluated the methylation status of the CpG sites to determine their ability to add to the known risk factors in predicting the progression of prostate cancer. Our results showed statistically significant differences in the frequency of methylation at individual CpG units of PITX2, PDLIM4, KCNMA1, GSTP1, FLNC, EFS, and ECRG4 in recurrence and nonrecurrence subtypes of tumors. We found that Mrp score is significantly higher in more advanced tumors in the following order: systemic recurrence → local recurrence → biochemical recurrence → nonrecurrent cases.
GSTP1 promoter hypermethylation is an early event in prostate carcinogenesis (46–49). There are some controversial reports about GSTP1 methylation with the stage and grade of the prostate cancer (43, 50–53). In our study, the CpG sites for all the genes used to derive the Mdp and Mrp scores were different, except for the two CpG sites of GSTP1 (GSTP1 CpG site 19 and 21) that appeared in both Mdp and Mrp scores. The observed difference in the CpG site methylation of GSTP1 associated with recurrence of cancer needs further investigation. We found a significant hypermethylation of RARB2 in benign versus cancer tissues and not in recurrent versus nonrecurrent tumors that indicates that RARB2 methylation could be an early event in the prostate cancer development. The genes FLNC, EFS, KCNMA1, and ECRG4 were significantly down-regulated in our and other microarray expression profiling of prostate cancer tissues (p < .0001) (21, 38, 54). FLNC is an actin-cross-linking protein involved in reorganizing the actin cytoskeleton. Hypermethylation of FLNC in gastric mucosa was reported to correlate with higher risk levels of gastric cancers (55). PITX2, the homeodomain transcription factor methylation, was reported to predict the risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients (56). Our results revealed that 15 CpG sites of the FLNC in the 8 CpG units were highly methylated (p < .042) in local recurrence cases. The methylation of FLNC and PITX2 in prostate cancer might have practical implication for predicting the risk of local recurrence. EFS is a docking protein that plays a central coordinating role for tyrosine-kinase-based signaling related to cell adhesion (57). For prediction of systemic progression, we found that methylation of CpG sites were restricted to FLNC and EFS (p ≤ .03) genes involved in cell attachment. ECRG4 is a tumor suppressor gene downregulated by promoter methylation during the development and progression of esophageal cancer (58). This is the first report to show that methylation of FLNC, PITX2, EFS, and ECRG4 is associated with recurrence of prostate cancer. These results warrant further validation of the methylation status of these genes in a large and unselected cohort of patients to follow the progression of cancer.
The risk of recurrence and metastatic progression for prostate cancer patients who undergo radical prostatectomy can be estimated in relative accuracy based on PSA doubling time and GPSM score. Multigene methylation analysis of GSTP1, APC, and MDR1 was reported to correlate with higher Gleason sum, higher preoperative PSA, and other advanced pathological features (20). In our study, Mrp score showed a strong correlation with worse clinicopathologic features such as higher Gleason sum, higher preoperative PSA values, local, and systemic recurrence. Remarkably, the combination of Mrp score with preoperative PSA and GPSM score improved the theoretical prediction of recurrence. We also observed an apparent association of the Mrp score with the GPSM score, although not statistically significant. The higher GPSM score associated with systemic recurrence cases in our current study confirmed with our previous report that GPSM score is an independent predictor for progression of prostate cancer (12). The results of this study suggest that DNA methylation analysis could augment the ability of currently available predictors of prostate cancer progression. However, this study was not able to identify any CpG site methylation that might be associated with PSA doubling time or mortality with prostate cancer because the vast majority of samples were right censored at the time of data analysis. In this study design, patients were selected on the basis of the end point outcome of cancer; therefore, the analysis of survival curves would not be appropriate to consider. These findings warrant further analysis of the genes in a large study cohort to explore the implications of the CpG site methylation for prediction of prostate cancer risk of recurrence.
In conclusion, our study showed for the first time nonrandom and differential CpG site methylation patterns between nonrecurrence and recurrence tumor tissues. The correlation of PITX2, FLNC, and EFS CpG sites methylation with the malignant progression needs further investigation. These results suggest that analyzing the individual CpG site methylation of genes will identify tumor-type-specific changes associated with risk stratification of patients. The strength of the methylation score depends on the number of the genes analyzed simultaneously. Incorporating the methylation status of additional candidate genes into the Mdp and Mrp scores possibly further improve the sensitivity and specificity for diagnosis of cancer and assessment of progression.
Acknowledgments
Wendy Will Case Cancer Fund, Inc., and National Cancer Institute Grant CA091956 supported this work.
References
- 1.Nielsen ME, Partin AW. The impact of definitions of failure on the interpretation of biochemical recurrence following treatment of clinically localized prostate cancer. Rev Urol. 2007;9(2):57–62. [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen PL, Chen MH, Catalona WJ, Moul JW, Sun L, D’Amico AV. Predicting prostate cancer mortality Among men with intermediate to high-risk disease and multiple unfavorable risk factors. Int J Radiat Oncol Biol Phys. 2008 doi: 10.1016/j.ijrobp.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Hui-Chen M, Renshaw AA, Sussman B, Roehl KA, Catalona WJ. Identifying men diagnosed with clinically localized prostate cancer who are at high risk for death from prostate cancer. J Urol. 2006;176(6 Pt 2):S11–15. doi: 10.1016/j.juro.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Blute ML, Slezak JM, Bergstralh EJ, Leibovich BC. Is the GPSM scoring algorithm for patients with prostate cancer valid in the contemporary era? J Urol. 2007;178(2):459–463. doi: 10.1016/j.juro.2007.03.124. discussion 463. [DOI] [PubMed] [Google Scholar]
- 5.Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the Trifecta nomogram. J Urol. 2008;179(6):2207–2210. doi: 10.1016/j.juro.2008.01.106. discussion 2210–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts WW, Bergstralh EJ, Blute ML, Slezak JM, Carducci M, Han M, Epstein JI, Eisenberger MA, Walsh PC, Partin AW. Contemporary identification of patients at high risk of early prostate cancer recurrence after radical retropubic prostatectomy. Urology. 2001;57(6):1033–1037. doi: 10.1016/s0090-4295(01)00978-5. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Fearn PA, Kattan MW. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98(10):715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351(2):125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 9.Hanks GE, Hanlon AL, Lee WR, Slivjak A, Schultheiss TE. Pretreatment prostate-specific antigen doubling times: clinical utility of this predictor of prostate cancer behavior. Int J Radiat Oncol Biol Phys. 1996;34(3):549–553. doi: 10.1016/0360-3016(95)02154-x. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta S, Myers RP, Slezak JM, Bergstralh EJ, Zincke H, Blute ML. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol. 2005;174(6):2191–2196. doi: 10.1097/01.ju.0000181209.37013.99. [DOI] [PubMed] [Google Scholar]
- 11.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 12.Blute ML, Bergstralh EJ, locca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001;165(1):119–125. doi: 10.1097/00005392-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3(4):253–266. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- 15.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome—components and functional correlates. Genes Dev. 2006;20(23):3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 16.Brueckner B, Kuck D, Lyko F. DNA methyltransferase inhibitors for cancer therapy. Cancer J. 2007;13(1):17–22. doi: 10.1097/PPO.0b013e31803c7245. [DOI] [PubMed] [Google Scholar]
- 17.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 18.Moggs JG, Goodman JI, Trosko JE, Roberts RA. Epigenetics and cancer: implications for drug discovery and safety assessment. Toxicol Appl Pharmacol. 2004;196(3):422–430. doi: 10.1016/j.taap.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5(1):37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 20.Enokida H, Shiina H, Urakami S, Igawa M, Ogishima T, Li LC, Kawahara M, Nakagawa M, Kane CJ, Carroll PR, Dahiya R. Multigene methylation analysis for detection and staging of prostate cancer. Clin Cancer Res. 2005;11(18):6582–6588. doi: 10.1158/1078-0432.CCR-05-0658. [DOI] [PubMed] [Google Scholar]
- 21.Vanaja DK, Ballman KV, Morlan BW, Cheville JC, Neumann RM, Lieber MM, Tindall DJ, Young CY. PDLIM4 repression by hypermethylation as a potential biomarker for prostate cancer. Clin Cancer Res. 2006;12(4):1128–1136. doi: 10.1158/1078-0432.CCR-05-2072. [DOI] [PubMed] [Google Scholar]
- 22.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63(14):3877–3882. [PubMed] [Google Scholar]
- 23.Ehrich M, Field JK, Liloglou T, Xinarianos G, Oeth P, Nelson MR, Cantor CR, van den Boom D. Cytosine methylation profiles as a molecular marker in non-small cell lung cancer. Cancer Res. 2006;66(22):10911–10918. doi: 10.1158/0008-5472.CAN-06-0400. [DOI] [PubMed] [Google Scholar]
- 24.Freedland SJ, Aronson WJ, Presti JC, Jr, Amling CL, Terris MK, Trock B, Kane CJ. Predictors of prostate-specific antigen progression among men with seminal vesicle invasion at the time of radical prostatectomy. Cancer. 2004;100(8):1633–1638. doi: 10.1002/cncr.20122. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Jr, Lilja H, Scardino PT. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24(24):3973–3978. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 26.Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002;20(16):3376–3385. doi: 10.1200/JCO.2002.01.150. [DOI] [PubMed] [Google Scholar]
- 27.Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61(2):365–369. doi: 10.1016/s0090-4295(02)02268-9. [DOI] [PubMed] [Google Scholar]
- 28.Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D’amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179(4):1354–1360. doi: 10.1016/j.juro.2007.11.061. discussion 1360–1351. [DOI] [PubMed] [Google Scholar]
- 29.Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci USA. 2002;99(6):3740–3745. doi: 10.1073/pnas.052410099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 31.Goessl C, Krause H, Muller M, Heicappell R, Schrader M, Sachsinger J, Miller K. Fluorescent methylation-specific polymerase chain reaction for DNA-based detection of prostate cancer in bodily fluids. Cancer Res. 2000;60(21):5941–5945. [PubMed] [Google Scholar]
- 32.Hanson JA, Gillespie JW, Grover A, Tangrea MA, Chuaqui RF, Emmert-Buck MR, Tangrea JA, Libutti SK, Linehan WM, Woodson KG. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98(4):255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 33.Hampton T. New markers may help predict prostate cancer relapse risk. JAMA. 2006;295(19):2234–2238. doi: 10.1001/jama.295.19.2234. [DOI] [PubMed] [Google Scholar]
- 34.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18(11):1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 35.Jornsten R, Yu B. Simultaneous gene clustering and subset selection for sample classification via MDL. Bioinformatics. 2003;19(9):1100–1109. doi: 10.1093/bioinformatics/btg039. [DOI] [PubMed] [Google Scholar]
- 36.Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, Booser D, Theriault RL, Buzdar AU, Dempsey PJ, Rouzier R, Sneige N, Ross JS, Vidaurre T, Gomez HL, Hortobagyi GN, Pusztai L. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24(26):4236–4244. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 37.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 2002;99(10):6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, Mc-Donald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo JH. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22(14):2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 39.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D’Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1(2):203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 40.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA. 2004;101(3):811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412(6849):822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 42.Bastian PJ, Palapattu GS, Lin X, Yegnasubramanian S, Mangold LA, Trock B, Eisenberger MA, Partin AW, Nelson WG. Preoperative serum DNA GSTP1 CpG island hypermethylation and the risk of early prostate-specific antigen recurrence following radical prostatectomy. Clin Cancer Res. 2005;11(11):4037–4043. doi: 10.1158/1078-0432.CCR-04-2446. [DOI] [PubMed] [Google Scholar]
- 43.Yegnasubramanian S, Kowalski J, Gonzalgo ML, Zahurak M, Piantadosi S, Walsh PC, Bova GS, De Marzo AM, Isaacs WB, Nelson WG. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64(6):1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 44.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst. 2005;97(2):103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 45.Lotan R, Lotan Y. Retinoic acid receptor beta2 hypermethylation: implications for prostate cancer detection, prevention, and therapy. Clin Cancer Res. 2004;10(12 Pt 1):3935–3936. doi: 10.1158/1078-0432.CCR-04-0536. [DOI] [PubMed] [Google Scholar]
- 46.Lin X, Tascilar M, Lee WH, Vies WJ, Lee BH, Veeraswamy R, Asgari K, Freije D, van Rees B, Gage WR, Bova GS, Isaacs WB, Brooks JD, DeWeese TL, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation is responsible for the absence of GSTP1 expression in human prostate cancer cells. Am J Pathol. 2001;159(5):1815–1826. doi: 10.1016/S0002-9440(10)63028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henrique R, Jeronimo C. Molecular detection of prostate cancer: a role for GSTP1 hypermethylation. Eur Urol. 2004;46(5):660–669. doi: 10.1016/j.eururo.2004.06.014. discussion 669. [DOI] [PubMed] [Google Scholar]
- 48.Bernardini S, Miano R, lori R, Finazzi-Agro E, Palmieri G, Ballerini S, Angeloni C, Orlandi A, Bellincampi L, Cortese C, Federici G. Hypermethylation of the CpG islands in the promoter region of the GSTP1 gene in prostate cancer: a useful diagnostic and prognostic marker? Clin Chim Acta. 2004;350(1–2):181–188. doi: 10.1016/j.cccn.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 49.Bastian PJ, Nakayama M, De Marzo AM, Nelson WG. GSTP1 CpG island hypermethylation as a molecular marker of prostate cancer. Urologe A. 2004;43(5):573–579. doi: 10.1007/s00120-004-0540-7. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum E, Hoque MO, Cohen Y, Zahurak M, Eisenberger MA, Epstein JI, Partin AW, Sidransky D. Promoter hypermethylation as an independent prognostic factor for relapse in patients with prostate cancer following radical prostatectomy. Clin Cancer Res. 2005;11(23):8321–8325. doi: 10.1158/1078-0432.CCR-05-1183. [DOI] [PubMed] [Google Scholar]
- 51.Jeronimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, Oliveira J, Teixeira MR, Lopes C, Sidransky D. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004;10(24):8472–8478. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 52.Kang GH, Lee S, Lee HJ, Hwang KS. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J Pathol. 2004;202(2):233–240. doi: 10.1002/path.1503. [DOI] [PubMed] [Google Scholar]
- 53.Bastian PJ, Ellinger J, Heukamp LC, Kahl P, Muller SC, von Rucker A. Prognostic value of CpG island hypermethylation at PTGS2, RAR-beta, EDNRB, and other gene loci in patients undergoing radical prostatectomy. Eur Urol. 2007;51(3):665–674. doi: 10.1016/j.eururo.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8(5):393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima T, Maekita T, Oda I, Gotoda T, Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T, Saito D. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2317–2321. doi: 10.1158/1055-9965.EPI-06-0436. [DOI] [PubMed] [Google Scholar]
- 56.Maier S, Nimmrich I, Koenig T, Eppenberger-Castori S, Bohlmann I, Paradise A, Spyratos F, Thomssen C, Mueller V, Nahrig J, Schittulli F, Kates R, Lesche R, Schwope I, Kluth A, Marx A, Martens JW, Foekens JA, Schmftt M, Harbeck N. DNA-methylation of the homeodomain transcription factor PITX2 reliably predicts risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients—technical and clinical validation in a multi-centre setting in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J Cancer. 2007;43(11):1679–1686. doi: 10.1016/j.ejca.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Ohba T, Ishino M, Aoto H, Sasaki T. Dot far-western blot analysis of relative binding affinities of the Src homology 3 domains of Efs and its related proteins. Anal Biochem. 1998;262(2):185–192. doi: 10.1006/abio.1998.2772. [DOI] [PubMed] [Google Scholar]
- 58.Yue CM, Deng DJ, Bi MX, Quo LP, Lu SH. Expression of ECRG4, a novel esophageal cancer-related gene, downregulated by CpG island hypermethylation in human esophageal squamous cell carcinoma. World J Gastroenterol. 2003;9(6):1174–1178. doi: 10.3748/wjg.v9.i6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]