Abstract
Hematopoietic cell transplantation (HCT) offers potentially curative therapy for patients with myelodysplastic syndromes (MDS). However, who should and can be transplanted, with which approach, and when is a matter of debate. Various classification schemes and prognostic scoring systems have helped in the decision-making process. Offering HCT to patients who were not considered transplant candidates in the past is now possible with the development of new transplant strategies. In addition to disease stage, patient age, comorbid conditions, preHCT chemotherapy, type of donor, source of stem cells, and possibly other factors, need to be considered prior to transplant. Patients without substantial comorbid conditions up to 60 years with the availability of unrelated donor grafts or 65 years with related donor grafts can be transplanted with more conventional (higher dose) regimens (e.g. targeted BU/CY; Flu/BU). Older patients and patients with comorbid conditions should be enrolled in trials aimed at optimizing RIC/nonmyeloablative HCT (e.g. Flu/melphalan; Flu/low dose TBI). Patients who present with ‘advanced’ MDS or are transfusion dependent, and do not have a del(5q), should probably be transplanted early in their course. GVHD (in all patients) and post-HCT relapse (in patients with high risk disease) remain major problems. Modifications of transplant conditioning regimens have reduced transplant-related mortality and allow successful HCT even in older patients. However, prospective randomized trials are needed to determine the role of pre-HCT chemotherapy and the type of transplant conditioning regimen best suited for a given patient.
Keywords: Myelodysplastic syndrome, comorbidities, transplant conditioning, relapse, GVHD
INTRODUCTION
Hematopoietic cell transplantation (HCT) has curative potential for patients with myelodysplastic syndromes (MDS). However, the disease occurs predominantly in older patients, who poorly tolerate high dose therapy. Nevertheless, if the patient is considered a candidate for HCT, the decision to proceed may be easy in high-risk disease with a statistical life expectancy of a few months, but may be rather difficult in a patient who can expect a good quality of life for several years with conservative management.1 Further, recently developed nontransplant modalities may stabilize hematologic parameters, such as transfusion requirements, improve the quality of life, and, in subgroups of patients, even induce remissions, at least for a limited period of time, leading patients and physicians to postpone a possible HCT.
Classification schemes and prognostic scoring systems, such as the French-American-British (FAB) and the World Health Organization (WHO) categorization, and the International Prognostic Scoring System (IPSS),2 have aided in the decision-making process.1 However, not all potential risk factors are considered in these widely used classification schemes. Transfusion dependence has recently been identified as an important risk factor that is being incorporated into a new risk scoring system, the WPSS (WHO Prognostic Scoring System) (Table 1).3 Others factors such as serum LDH, marrow microvascular density, flow cytometric findings, and gene expression profiles are under investigation. How these factors will impact on transplant outcome is not known at present. Frequent reassessments of the best strategy will be required.
Table 1.
WPSS Classification, Median Life Expectancy, and Probability of Leukemic Transformation. Scores are assigned as follows: 0 for WHO categories RA, RARS and 5q-. 1 for RCMD and RCMD-RS, 2 for RAEB-1, and 3 for RAEB-2; 0 for not requiring transfusions, and 1 for regular transfusion needs; 0 for IPSS risk category “good”, 1 for “intermediate”, and 2 for “poor”. Scores are added up to arrive at the overall Risk Score.43
| Risk Score | Survival | AML Evolution |
|---|---|---|
| Very low/0 | 11.3 years | 7% @ 10 years |
| Low/1 | 5.3 | — |
| Intermediate/2 | 3.7 | — |
| High/3–4 | 1.6 | — |
| Very high/5–6 | 0.7 | 50% @ 8 months |
GENERAL CONSIDERATIONS
Age and HCT
Until the mid 1990s few patients above the age of 55 years were offered allogeneic HCT, in particular from unrelated donors. This policy was based on increased severity and frequency of transplant-related toxicity and nonrelapse mortality (NRM) in older patients. However, recent publications describe cohorts of patients with median ages of 55–60 years, and some patients older than 70 years, that have been transplanted successfully.4 This increase in the upper age limit for HCT has become possible with improvements in overall supportive care, substantial changes in the conditioning regimens used in preparation for HCT, and management of the complications related to the procedure.
Timing of Transplantation
An analysis by Cutler et al. of results in patients transplanted from HLA-identical siblings indicated that patients in IPSS risk groups intermediate-2 and higher (most of those patients will have more than 5% myeloblasts in the marrow and often have a high risk karyotype) have the longest overall life expectancy if transplanted without delay assuming that they are interested in HCT, have a donor, and do not have severe comorbid conditions.1 Conversely, patients with low (and less so with intermediate-1) risk disease as classified by IPSS had the longest life expectancy if HCT was delayed, as long as several years, while the disease was closely monitored for progression.1 Of course, this analysis used retrospective data, had to make numerous assumptions, and can only be applied to cohorts, not to individual patients. A given patient with isolated severe neutropenia or transfusion-dependent thrombocytopenia may fall into the low-risk group but should, nevertheless, be considered for earlier HCT because of the risk of infection or hemorrhage. Factors such as comorbid conditions, emphasized in recent publications as risk factors for transplant outcome,5 or treatment with novel nontransplant modalities were not considered in that analysis. A similar analysis for unrelated transplants is not available.
INDUCTION CHEMOTHERAPY BEFORE HCT
Patients with advanced MDS generally have a poor prognosis with conservative management and are, therefore, referred for HCT. Since these patients are at high risk of post-HCT relapse, the question arises as to whether pre-HCT “debulking” chemotherapy would be beneficial in reducing the relapse risk, hopefully without increasing the incidence of NRM. However, the role of pre-HCT chemotherapy has remained controversial. Yacoub-Agha et al. showed that patients with secondary MDS who achieved remissions with pre-HCT chemotherapy had a substantially better relapse-free survival (RFS) than patients who did not achieve remissions.6 Patients who were given induction chemotherapy and failed to respond had a lower probability of a successful post-HCT course than would be expected in untreated patients. A retrospective analysis of results in patients transplanted at the Fred Hutchinson Cancer Research Center (FHCRC) suggested that pre-HCT chemotherapy reduced the risk of post-HCT relapse but did not significantly improve posttransplant RFS. Conceivably, pre-HCT chemotherapy was selected for “treatment sensitive“ patients who might have fared better post-HCT, even without prior therapy.7 A randomized trial comparing HCT with and without pre-HCT induction chemotherapy has been initiated by the European Bone Marrow Transplant (EBMT) group. One should probably view pre-HCT chemotherapy as part of the transplant “package,” and plan the transplant regimen along with pre-HCT therapy. This concept is supported by preliminary data in patients treated with nonmyeloablative conditioning regimens.8 Other factors such as patient age, type of donor, and disease stage must also be considered.
TRANSPLANT REGIMENS
Sources of Hematopoietic Stem Cells
Hematopoietic cells derived from different sources exert different donor-versus-host immune (graft-versus-MDS) effects that must be considered when discussing transplant regimens. A retrospective survey of the EBMT group,9,10 in agreement with our own data,11 showed that the use of granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood mononuclear cells (G-PBMC) for allogeneic HCT from HLA-identical siblings in patients with MDS was associated with lower treatment failure rates than the use of marrow in all MDS subgroups except refractory anemia. The incidence rates of chronic GVHD tend to be higher with G-PBMC than with marrow.12 While GVHD is an undesirable complication, a greater allogeneic and a graft-versus-MDS effect of G-PBMCs may also account for higher engraftment and lower relapse rates (compared to marrow), particularly in patients prepared with reduced intensity/nonmyeloablative conditioning regimens. Preemptive or prophylactic infusion of donor lymphocytes after HCT may further enhance the allogeneic effect.13,14
Umbilical cord blood cells for HCT in adults, long viewed with skepticism, are finding increasing use, particularly in the form of two-unit transplants.15–17 Some advantages of cord blood may be a lower incidence of GVHD and prompt availability.
Major progress has been made with transplants from unrelated donors (due to advances in molecular HLA typing),18 with results now equaling those obtained with HLA genotypically identical siblings.11,19 There is evidence of a lower incidence of post-HCT relapse with unrelated donors after both myeloablative and nonmyeloablative conditioning, and some studies have suggested improved survival.4,20 Some protocols have achieved encouraging results with HLA haploidentical related donors.21,22
Evolution of Conditioning Regimens
The best results with allogeneic HCT are achieved in patients with low marrow myeloblast counts (refractory anemia [RA] with ringed sideroblasts [RARS], refractory cytopenia with multilineage dysplasia [RCMD], or RCMD with ringed sideroblasts [RCMD-RS]) and without poor-risk clonal cytogenetic abnormalities. Those patients also tend to have a good prognosis with less aggressive therapy. Since the success with transplantation declines as the disease progresses and the prognosis without HCT worsens, patients should be followed very closely so that HCT can be carried out at the earliest sign of disease acceleration.
Sierra et al. reported results in 452 patients with MDS transplanted from HLA-identical siblings, including 140 (31%) with less than 5% marrow blasts at HCT.23 Among patients less than 18 years of age, 3-year RFS was 72% compared to 45% for older patients. For the entire cohort, the 3-year NRM was 37%, relapse incidence 23%, and RFS 40%. High marrow blast count, high IPSS score, and T-cell depletion of transplanted cells increased the risk of relapse 2- to 6-fold. Registry data on unrelated donor HCT generally showed lower survival figures than those observed with sibling HCT, though, on average, those patients were younger.24
Several reports suggest improved outcome in patients conditioned with busulfan and cyclophosphamide (BUCY) or fludarabine/BU regimens compared to patients given high-dose TBI.24–27 We used a “targeted” BUCY regimen with oral BU, with dose adjustments to maintain steady state plasma levels of 800–900 ng/mL.11 We observed a 3-year RFS of 68% among 69 patients (up to 66 years of age) with RA/RARS transplanted from HLA-identical sibling donors and 70% with unrelated donors. Most of these patients received marrow as a source of stem cells. NRM (combined for related and unrelated transplants) was 12% at 100 days and 31% at 3 years; relapse occurred in 5% of patients. A recent trial using G-PBMC as a source of stem cells confirms those data, with 78% RFS at 1 year. The incidence of acute GVHD was 85%.12
In patients with more advanced MDS (≥5% marrow myeloblasts; high-risk cytogenetics) relapse rates of 15% to 80% have been reported.11,24,28 With CY+TBI-containing regimens, RFS was 30%–40%. Further increases in intensity, for example, by combining BU (7 mg/kg), CY (120 mg/kg) and TBI (6×200 cGy), reduced the incidence of relapse to 28% (compared to 54% with CY+TBI), but increased NRM to an unacceptable 68%, and resulted in RFS at 3 years of only 23%.
The IBMTR study cited above,23 also included 312 patients with advanced MDS (RAEB, RAEB-T, CMML), most were conditioned with TBI-based regimens. Compared to patients with <5% marrow blasts, the relative risk of relapse was 2.9 fold lower than patients with 5%–20% blasts, and 6.3 fold lower than patients with >20% blasts. RFS was 55% in patients who had been treated with chemotherapy before HCT and who had <5% marrow blasts at HCT, compared to RFS of 26% in patients with 5% or more myeloblasts. While this observation was encouraging in view of the controversy about the importance of pre-HCT induction chemotherapy,7 the analysis (like several others) disregarded patients who never came to HCT.
Russell et al. used intravenous (i.v.) fludarabine (Flu), given over 5 days, and i.v. BU, 3.2 mg/kg, given once a day on 4 consecutive days (concurrently with Flu), combined with rabbit antithymocyte globulin, 4.5 mg/kg, plus methotrexate (MTX) and cyclosporine (CSP) for GVHD prophylaxis.26 The study enrolled 70 patients with various diagnoses, including MDS. The mortality at day 100 was 2% with related donors and 8% with unrelated donors. The incidence of acute GVHD was 8%, and chronic GVH D was 36%. Projected RFS at 2 years was 74% for low-risk and 65% for high-risk disease.26 As patients with various diagnoses were included, results must be interpreted cautiously in regards to the outcome with MDS specifically. A recently concluded FHCRC trial of targeted (oral) BUCY in patients with high-risk MDS also incorporated antithymocyte globulin in a dose escalation design.12 While the rates of GVHD observed at FHCRC were higher (50%) than in the study by Russell et al, they were lower than in concurrent trials not using antithymocyte globulin (85%). NRM at 100 days was 12%, and 1-year RFS was 56%.
The MD Anderson Cancer Center team reported results in 96 patients, including 22 with MDS, who were conditioned with i.v. Flu at 40 mg/m2/day and i.v. BU at 130 mg/m2/day for 4 consecutive days (no antithymocyte globulin was given).29 Regimen-related mortality was 5% at 100 days, and the incidence of acute GVHD (grades II–IV) was 25% and 44% for related and unrelated recipients, respectively.
De Lima and colleagues, also at M.D. Anderson, presented a comparison of two regimens: Flu, 120 mg/m2, plus cytosine arabinoside, 4 g/m2, and idarubicin, 36 mg/m2 (FAI), versus Flu 100–150 mg/m2, plus melphalan, 140 or 180 mg/m2 (FM).30 Among the 94 patients in this report, 26 had MDS. The more intensive FM regimen was associated with a higher degree of donor cell engraftment and a lower incidence of relapse (30% vs. 61%) but also with a higher incidence of NRM (p = 0.036). As a result, the 3-year overall survival rates were not different, 30% for FAI conditioned patients and 35% for FM conditioned patients.
A further reduction in conditioning intensity was used by Ho et al, who combined Flu, BU, and alemtuzumab (anti-CD52) in 62 patients (24 with HLA-identical siblings and 38 with matched unrelated donors).31 Patients with excess myeloblasts had received chemotherapy before transplantation. The day-100 NRM was 0% for HLA-identical siblings, and 11% for unrelated donor transplants. The incidence of relapse ranged from 7% to 50% for intermediate-1 to high-risk IPSS groups. There were 26 patients who received donor lymphocyte infusions at a median of 273 days after HCT to induce remission or convert to complete donor chimerism. RFS ranged from 86% for low-risk IPSS to 33% among IPSS high-risk patients.
Chan and colleagues combined photopheresis with pentostatin and intermediate dose TBI to prepare 18 patients, aged 30–70 (median 54) years (all FAB categories of MDS) for HCT from related or unrelated donors.32 Sixteen patients achieved full donor chimerism, and all patients survived beyond day 100. Relapse occurred in 2 patients. RFS at 1 year was 64%.
A more drastic dose reduction was undertaken by Storb and colleagues, who attempted patient conditioning with 200 cGy of TBI, but added Flu, 3 × 30 mg/m2, because of high rates of graft failure with TBI alone. In one report by this group on 78 patients with MDS, graft failure occurred in 6%, and relapse occurred in 43% of patients. The NRM was 14% at day 100 and 25% at 1 year. Approximately 20% of patients (30% with low-risk, and 10% with high-risk disease) were surviving at 3 years.33 Administration of pre-HCT chemotherapy, low marrow myeloblast counts, and administration of mycophenolate mofetil 3 times a day (instead of twice) after HCT were significantly correlated with more favorable outcome.34
In parallel with the development of RIC/nonmyeloablative regimens, studies analyzing the impact of preexisting comorbid conditions on post-HCT outcome occurred.5,5,35 This work quantified the severity of comorbid conditions and showed, as expected, major differences between patients conditioned with conventional and nonmyeloablative regimens. It also showed a major negative impact of the comorbidity index on post-HCT outcome.36,37 These data clearly indicate that comorbidities must be considered when deciding upon a transplant regimen.
CHOOSING A TRANSLPANT REGIMEN
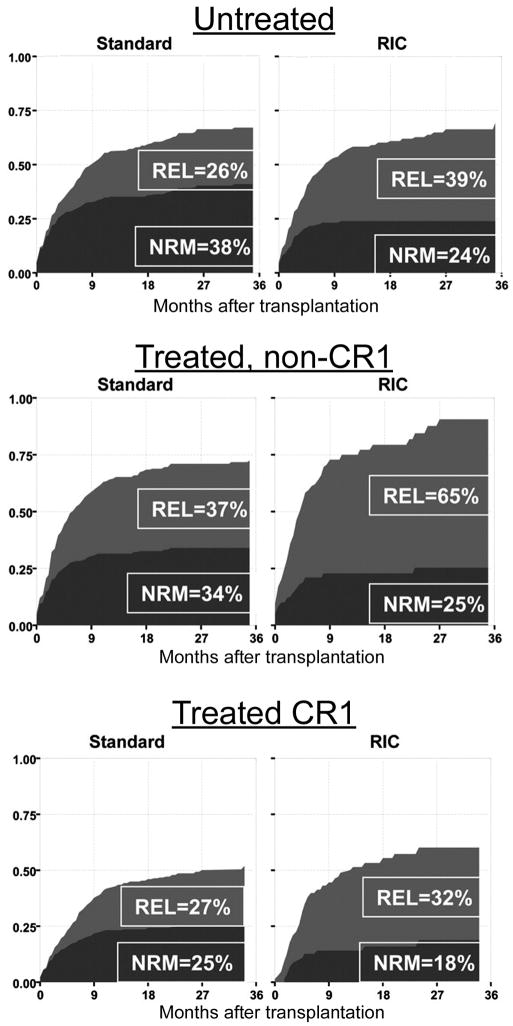
HCT offers potentially curative therapy for patients with MDS at any stage of the disease. The best results are obtained in patients with early stage/low risk MDS. However, a standard transplant regimen has yet to be established. It is likely that different regimens will be optimal for different disease categories, patients in different age groups, and with different comorbid conditions. No prospective data are currently available. Alyea et al. retrospectively compared results with myeloablative and nonmyeloablative transplants carried out at the Dana Farber Cancer Institute for patients with various diseases and found no significant difference in long-term RFS between the 2 groups.38 Scott et al. arrived at similar conclusions in an analysis of results in 172 patients with MDS transplanted at the FHCRC.39 A report by Martino et al. on results in 621 patients conditioned with high-dose and 215 patients prepared with reduced-intensity conditioning (RIC) regimens (including patients in all MDS categories) showed a significantly higher 3-year relapse rate (HR 1.64; p=0.001) but a significantly lower NRM (HR 0.61; p=0.015) in RIC patients.40 As a result, 3-year RFS was comparable, 39% and 33% for high dose and RIC, respectively (Figure 1). It is important to note that RIC patients were older (73% versus 28% >50 years; p< 0.001) and had more adverse pre-HCT conditions, a pattern also present in other studies.5,41 Therefore, the results cannot truly be compared. Only prospective randomized trials will be able to answer the important questions as to whether reduction in NRM is achievable without increasing the probability of relapse after RIC/nonmyeloablative conditioning, and whether such an approach leads to improved RFS.
Figure 1.
Non-relapse mortality (NRM) and relapse (REL) in patients conditioned with standard (conventional intensity) or with reduced intensity conditioning (RIC) regimens. Patients either had not received chemotherapy before HCT (Untreated), had been treated but not achieved remissions (treated, non-CR1) or had achieved complete remissions (CR1). Used with permission from R. Martino et al., Blood; 108:836–846, 2006. This research was originally published in Blood. Rodrigo Martino et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogenic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108 (3):836–46. © the American Society of Hematology.
SUMMARY
HCT offers potentially curative therapy for patients with MDS, and results have improved progressively over the past decade. Nevertheless, the day 100 mortality is in the range of 5%–10% and overall NRM with longer follow-up 25%–30%. Patients with a median life expectancy of 5–10 years without HCT (see Table 1) may not be willing to accept such an upfront risk and may prefer observational follow-up or, possibly, treatment with one of the nontransplant modalities such as DNA methyltransferase inhibitors, lenalidomide, histone deacetylase inhibitors and growth factor stimulants (erythropoietic agents). Patients with “advanced”/high risk MDS, on the other hand, should consider HCT early in their course. These are patients in the intermediate-2 and high risk groups by IPSS criteria (these patients generally have high myeloblast counts, high risk cytogenetics or both), patients with high transfusion needs, and patients in whom the disease has transformed to AML. It is not clear at this time whether pre-HCT induction chemotherapy should be used routinely in the latter patients before they undergo HCT. Patients with severe iron overload should probably be given chelation therapy, as recent data suggest that iron overload is associated with inferior outcome after HCT.42 It remains to be determined to what extent iron overload and transfusion supports rather than the underlying disease biology contributes to the poor outcome. Preliminary data also suggest that patients with marrow fibrosis may do better if transplanted earlier in their course.43
In general, conditioning regimens that do not use high-dose TBI appear to be better tolerated than high-dose TBI regimens. A broad scale of regimens has been developed since one treatment does not work on every type of patient. Disease stage, patient age, comorbid conditions, pre-HCT chemotherapy, type of donor, source of stem cells, and possibly other factors need to be considered. While awaiting the results of controlled prospective trials, patients without substantial comorbid conditions up to 60 years of age (with unrelated donors) or 65 years of age (with related donors) can be transplanted with more conventional (higher dose) regimens (e.g. targeted BU/CY; Flu/BU). Older patients and patients with comorbid conditions should be enrolled in trials aimed at optimizing RIC/nonmyeloablative HCT (for example, Flu/melphalan; Flu/low-dose TBI). GVHD (in all patients) and post-HCT relapse (in patients with high risk disease) have remained problems. Prospective randomized trials are needed to determine the role of pre-HCT chemotherapy and the type of transplant conditioning regimen best suited for a given patient.
Acknowledgments
Supported by grants HL36444 and CA87948, CA119599, CA15704, CA18029, National Institutes of Health, Bethesda, MD, USA. M.M. is supported by a grant from the J.P. McCarthy Fund. We thank Bonnie Larson and Helen Crawford for help with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 3.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 4.Maris MB, Sandmaier BM, Storer BE, Chauncey T, Stuart MJ, Maziarz RT, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakoub-Agha I, de La SP, Ribaud P, Sutton L, Wattel E, Kuentz M, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18:963–971. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Storer B, Loken MR, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11:65–73. doi: 10.1016/j.bbmt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Maris MB, Sandmaier BM, Storer BE, Maloney DG, Shizuru JA, Agura E, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99:4370–4378. doi: 10.1182/blood.v99.12.4370. [DOI] [PubMed] [Google Scholar]
- 10.Maris MB, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 11.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–1207. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 12.Deeg HJ, Appelbaum FR, Storer B, Cassarella M, Scott B, McDonald G, et al. Reduced incidence of acute and chronic graft-versus-host disease (GvHD) without increased relapse in patients with high-risk myeloid disorders given thymoglobulin (THY) as part of the transplant conditioning regimen: a dose-finding study. Blood. 2004;104:56a. (abstr 181) [Google Scholar]
- 13.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]
- 14.Schleuning M, Schmid C, Ledderose G, Tischer J, Humann M, Ullmann J, et al. Durable remission after prophylactic donor lymphocyte transfusion following allogeneic stem cell transplantation with reduced conditioning for high-risk AML and MDS. Blood. 2004;104:89a. (abstr 299) [Google Scholar]
- 15.Koh LP, Chao NJ. Umbilical cord blood transplantation in adults using myeloablative and nonmyeloablative preparative regimens. Biol Blood Marrow Transplant. 2004;10:1–22. doi: 10.1016/j.bbmt.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y, et al. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104:3813–3820. doi: 10.1182/blood-2004-03-1001. [DOI] [PubMed] [Google Scholar]
- 18.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplant outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 19.Petersdorf EW, Malkki M. Human leukocyte antigen matching in unrelated donor hematopoietic cell transplantation. Semin Hematol. 2005;42:76–84. doi: 10.1053/j.seminhematol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Sorror M, Sandmaier B, Maris M, Storer B, Radich J, Agura E, et al. Nonmyeloablative hematopoietic cell transplantation (HCT) for treatment of patients (pts) with fludarabine-refractory chronic lymphocytic leukemia (CLL) results in prolonged median survival. J Clin Oncol. 2006;24:342s. (abstr 6250) [Google Scholar]
- 21.O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa H, Ikegame K, Yoshihara S, Kawakami M, Fujioka T, Masuda T, et al. Unmanipulated HLA 2–3 antigen-mismatched (haploidentical) stem cell transplantation using nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1073–1084. doi: 10.1016/j.bbmt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100:1997–2004. [PubMed] [Google Scholar]
- 24.Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99:1943–1951. doi: 10.1182/blood.v99.6.1943. [DOI] [PubMed] [Google Scholar]
- 25.Bornhauser M, Storer B, Slattery JT, Appelbaum FR, Deeg HJ, Hansen J, et al. Conditioning with fludarabine and targeted busulfan before transplantation of allogeneic hematopoietic stem cells. Blood. 2002;100:213a. doi: 10.1182/blood-2002-11-3567. (abstr 799) [DOI] [PubMed] [Google Scholar]
- 26.Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 27.de Lima M, Couriel D, Shahjahan M, Madden T, Gajewski J, Giralt S, et al. Allogeneic transplantation for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) using a low-toxicity combination of intravenous (IV) busulfan (Bu) and fludarabine (Flu) ± ATG. Blood. 2002;100:853a. (abstr 3366) [Google Scholar]
- 28.Runde V, de WT, Arnold R, Gratwohl A, Hermans J, van BA, et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:255–261. doi: 10.1038/sj.bmt.1701084. [DOI] [PubMed] [Google Scholar]
- 29.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 30.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 31.Ho AY, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004;104:1616–1623. doi: 10.1182/blood-2003-12-4207. [DOI] [PubMed] [Google Scholar]
- 32.Chan GW, Foss FM, Klein AK, Sprague K, Miller KB. Reduced-intensity transplantation for patients with myelodysplastic syndrome achieves durable remission with less graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:753–759. doi: 10.1016/j.bbmt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Stuart MJ, Cao TM, Sandmaier BM, Hegenbart U, Maris M, Agura E, et al. Efficacy of non-myeloablative allogeneic transplant for patients with myelodysplastic syndrome (MDS) and myeloproliferative disorders (MPD) (except chronic myelogenous leukemia) Blood. 2003;102:185a. (abstr 644) [Google Scholar]
- 34.Maris MB, Sandmaier BM, Storer B, Shizuru J, Maloney D, Agura E, et al. Unrelated donor peripheral blood stem cell (PBSC) transplantation using nonmyeloablative conditioning and mycophenolate mofetil (MMF) TID results in high engraftment rates. Blood. 2004;104:503a. (abstr 1818) [Google Scholar]
- 35.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared to myeloablative conditioning before hematopoietic cell transplantation from HLA matched related donors. Blood. 2004 doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 36.Kerbauy DM, Chyou F, Gooley T, Sorror ML, Scott B, Pagel JM, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2005;11:713–720. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Sorror M, Storer B, Sandmaier BM, Maris M, Baron F, Maloney D, et al. Relationship between conditioning intensity and comorbidity in patients (pts) with acute myeloid leukemia (AML) or myelodysplasia (MDS) receiving allogeneic hematopoietic cell transplantation. Blood. 2005;106:208a. (abstr 705) [Google Scholar]
- 38.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 39.Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 40.Martino R, Iacobelli S, Brand R, Jansen T, van BA, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 41.Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplant comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 42.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109:4586–4588. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott BL, Storer BE, Greene JE. Marrow fibrosis as a risk factor for post-transplant outcome in patients with advanced MDS or AML with multilineage dysplasia. Biol Blood Marrow Transplant. 2007 doi: 10.1016/j.bbmt.2006.10.030. In press. [DOI] [PubMed] [Google Scholar]