Abstract
Rationale:
The role of dopamine in cocaine abuse has been long recognized. Cocaine use can profoundly alter dopaminergic functioning through depletion of this monoamine and changes in receptor functioning. Based on these facts, levodopa (L-dopa) pharmacotherapy may be helpful in reducing or abolishing cocaine use.
Objective:
The current studies sought to evaluate the safety, tolerability, and efficacy of L-dopa as a treatment for cocaine dependence.
Methods:
In Study 1, 67 cocaine-dependent subjects were randomized in a 5-week, double-blind, placebo-controlled safety trial. Subjects received either placebo, or 400 mg L-dopa plus 100 mg of the peripheral decarboxylase inhibitor, carbidopa, in a sustained-release preparation (Sinemet CR). In Study 2, 122 cocaine-dependent subjects were enrolled in a 9-week, randomized, double-blind, placebo-controlled trial to compare placebo to 400/100 mg and 800/200 mg L-dopa/carbidopa treatments. Placebo or L-dopa were administered twice daily in both studies.
Results:
L-dopa was well tolerated with similar retention and medication adherence rates compared to placebo. Only two side effects occurred more often in L-dopa-treated patients: nausea and dizziness. L-dopa lowered diastolic blood pressure in a dose-dependent fashion. In these trials, L-dopa had no effect on cocaine use, cocaine craving, or mood.
Conclusion:
These two studies demonstrate the safety and tolerability of L-dopa pharmacotherapy in cocaine-dependent patients. No evidence for greater efficacy of L-dopa compared to placebo was observed. The possibility of enhancing treatment effects by combining L-dopa with other behavioral or pharmacological interventions is discussed.
Keywords: L-dopa, levodopa, carbidopa, cocaine, dopamine, side effects
1. Introduction
The search for efficacious pharmacotherapies for cocaine dependence has spanned a broad array of candidate agents and neurotransmitter systems (de Lima et al., 2002; Gorelick et al., 2004; Sofuoglu and Kosten, 2005). Cocaine is a potent inhibitor of dopamine reuptake, and not surprisingly considerable emphasis has been placed on dopaminergic medications. A variety of dopaminergic agonists have been evaluated. Recently, promising findings have been found for dextroamphetamine (Grabowski et al., 2001; Grabowski et al., 2004a; Shearer et al., 2003), a drug that, along with other effects, promotes release of dopamine. Bupropion, an inhibitor of dopamine and noradrenalin reuptake, has had a mixed record as a treatment for cocaine dependence (Margolin et al., 1995; Poling et al., 2006). Other agonist approaches include direct dopamine receptor agonists, such as amantadine, bromocriptine, and pergolide (Eiler et al., 1995; Giannini et al., 1989; Handelsman et al., 1997; Tennant and Sagherian, 1987). These medications have not shown evidence of efficacy (Soares et al., 2003). Finally, other medications with potential for cocaine use treatment, including disulfiram and modafinil, may work in part by facilitating dopaminergic functioning, either directly by inhibiting dopamine beta hydroxylase (disulfiram) or indirectly by enhancing glutamate function (modafinil) (Carroll et al., 2004a; Dackis et al., 2005; Petrakis et al., 2000).
An alternative strategy for the treatment of cocaine dependence has been to antagonize the dopaminergic system. Medications that block dopamine functioning have been extensively considered (e.g., Gorelick et al., 2004; Meltzer et al., 2002; Platt et al., 2002; Rothman, 1990) and evaluated in various research paradigms (e.g., Bergman et al., 1990; Di Ciano et al., 2003; Koob et al., 1987; Vorel et al., 2002; Xi et al., 2005). However, there has been little support from clinical research findings for potential therapeutic benefit of these agents, including risperidone, olazapine, and ecopipam (Grabowski et al., 2000; Grabowski et al., 2004a; Haney et al., 2001; Nann-Vernotica et al., 2001; Reid et al., 2005)
However, another logical treatment strategy is replenishment or stabilization of dopamine stores depleted by repeated cocaine administration. Direct dopamine administration is not feasible but the precursor levodopa (L-dopa, administered in combination with carbidopa, a peripheral decarboxylase inhibitor), can increase brain levels of dopamine while minimizing potential side effects. Yet, little research has examined the toxic and therapeutic effects of dopamine-replenishment pharmacotherapy in cocaine users (Rosen et al., 1986; Shoptaw et al., 2005; Wolfsohn and Angrist, 1990; Wolfsohn et al., 1993). Although theoretically promising, complete safety, tolerability, and efficacy studies of L-dopa in clinical populations of cocaine-dependent individuals are needed.
In this report, we describe the results of two trials. In Study 1, a brief small-N randomized double-blind placebo-controlled trial examined safety and tolerability of a low dose of L-dopa/carbidopa (400/100 mg). Based on initial evidence of safety and tolerability in Study 1, we conducted a larger study to assess efficacy as well as safety and tolerability, given a longer course of treatment. In Study 2, a second randomized double-blind, placebo-controlled trial employed a larger sample, provided medications for a longer duration, and compared two doses of L-dopa/carbidopa (400/100 mg and 800/200/mg). Safety was determined through self-reported side effects and vital signs. Tolerability was operationalized in terms of treatment retention and medication adherence. Efficacy outcomes included cocaine use, cocaine craving, and mood.
2. Methods
Identical methods were employed for the two studies, with the exception of study design and medication doses. Thus, only study design and medications are described separately by study.
2.1 Subjects
The University of Texas-Houston Committee for the Protection of Human Subjects approved the two clinical trials. All participants were recruited through advertisements in local media sources. Initial telephone contact permitted preliminary eligibility screening, which specified inclusion criteria as: (a) English-speakers; (b) between the ages of 18 and 55; and (c) current users of cocaine, and exclusion criteria as: (a) pregnancy or nursing; (b) current dependence on substances other than cannabis or nicotine; (c) current psychotic, affective, or anxiety disorders; or (d) serious medical conditions, including movement disorders.
Eligible callers were invited to an informed consent session and to participate in a 3-10 day pre-treatment, intake evaluation, which included a medical history and complete physical examination. Assessment included laboratory evaluation of liver and thyroid function, cardiac functioning (i.e., 12-lead electrocardiogram), heart rate and blood pressures, and weight. In addition, tests were conducted for pregnancy (serum), drug toxicology (e.g., urine, for over 90 illicit and prescription drugs), tuberculosis, and HIV. Diagnostic interviewing was conducted to assess psychiatric history (i.e., Structured Clinical Interview for DSM-IV Axis I Disorders [SCID], First et al., 1995), as well as substance abuse and psychosocial functioning (Addiction Severity Index [ASI], McLellan et al., 1992). A benzoylecognine (BE; cocaine metabolite) positive urine sample was required during intake (i.e., ≤ 300 ng/mL with creatinine adjustment for urine concentration).
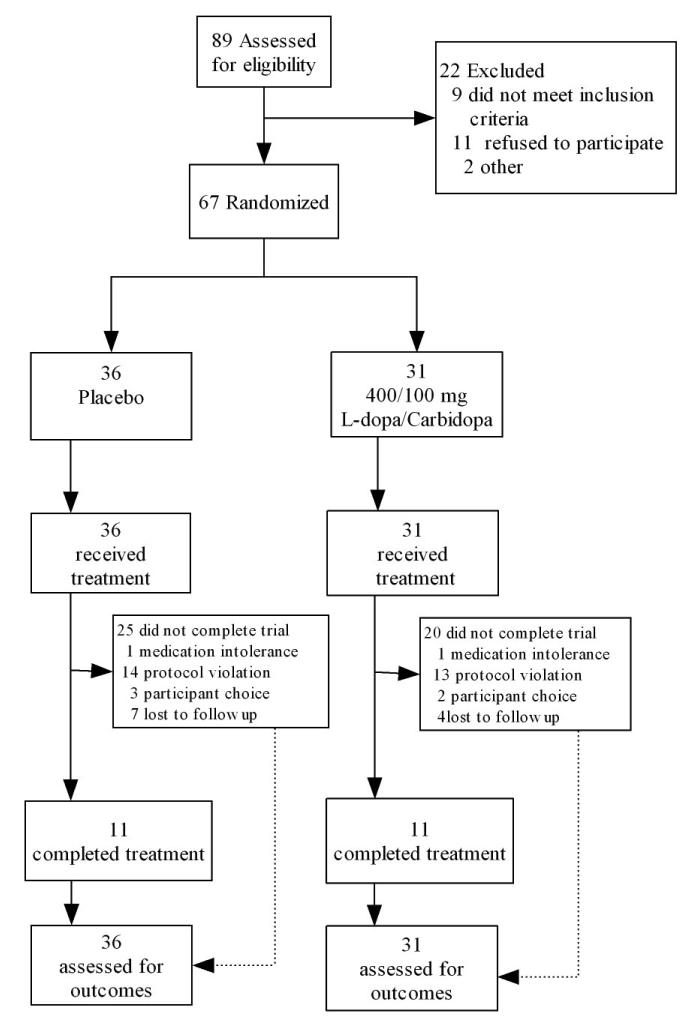
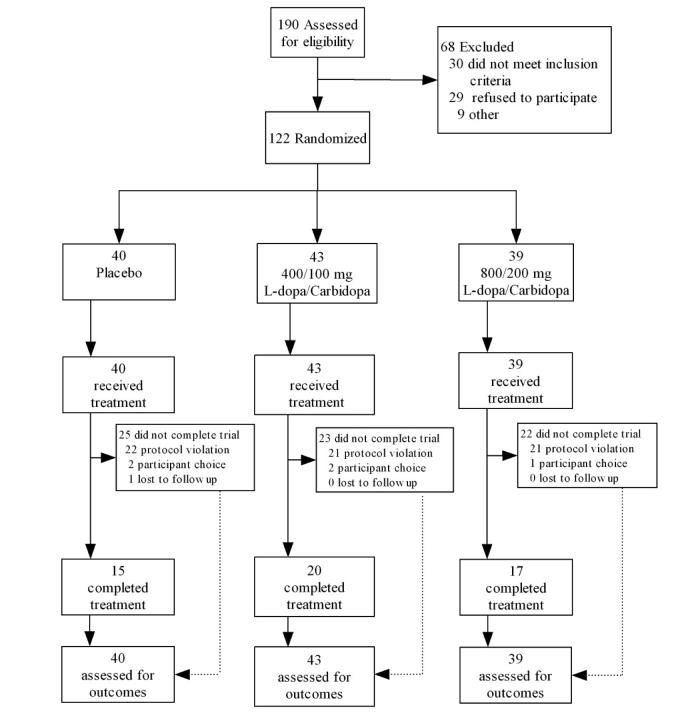
Study enrollment and attrition data are presented in Figures 1 and 2.
Figure 1.
Participant enrollment and retention figure for Study 1. Protocol violations indicate high absenteeism or failure to provide self-report or biological data.
Figure 2.
Participant enrollment and retention figure for Study 2. Protocol violations indicate high absenteeism or failure to provide self-report or biological data.
2.2 Procedures
The studies were conducted at the Treatment Research Clinic (TRC) of the Substance Abuse Research Center, Department of Psychiatry and Behavioral Sciences at the University of Texas Health Science Center at Houston. The clinic itself is described elsewhere (Grabowski et al., 1997).
2.2.1. Treatment and compliance
Subjects received either medication or placebo in identical capsules across both studies. In order to ensure compliance, each day's dose included a total of 100 mg of riboflavin for subsequent evaluation (Del Boca et al., 1996). At each clinic visit, participants provided a urine sample that was submitted to spectrophotoflurometry to obtain a semi-quantitative level of riboflavin using a Model 4-8202 Aminco-Bowman spectrophotofluorometer (American Instrument Co., Silver Springs, Maryland). Riboflavin levels range from 0 to 99 fluorescence units, with levels at or below 35 units considered to reflect non-compliance (previous work in our clinic showed that 99% of subjects at initial intake had levels below 35 fluorescence units). Supportive behavioral counseling was provided for one hour each week by master's-level therapists.
2.2.2. Measures
Each week, participants self-rated 33 side effects on an investigator-authored side effects inventory (listed in Table 2). Known and high frequency side effects of L-dopa therapy in Parkinson's disease patients were incorporated into a broader list of side effects evaluated in our other clinic studies (i.e., nausea, vomiting, anxiety/nervousness, depression, dizziness, problems concentrating, and twitching). Adverse events were evaluated by the study nurse and physician. Craving measures differed between Studies 1 and 2. In Study 1, craving in the preceding week was measured on a 100 mm visual analogue scale (Halikas et al., 1991). In Study 2, craving was measured with the cocaine subscale of the Brief Substance Craving Scale (Mezinskis et al., 1998), comprised of 3 craving items focused on the preceding 24 hours. In both studies, mood was assessed with the Beck Depression Inventory (Beck et al., 1961). Subjects provided urine samples for BE and riboflavin analysis. Biweekly specimens were assessed qualitatively and semi-quantitatively for BE. Samples were analyzed onsite in our analytical neurochemistry lab using the Syva EMIT system and the Abbott Toxi-Lab thin layer chromatographic system. Creatinine-adjusted samples (Wilkins, 1997) were classified as cocaine positive with BE concentrations equaling or exceeding 300 ng/mL. Study entry followed completion of the pre-treatment evaluation. Nursing staff reviewed self-reported side effects and measured vitals and weight. Based on the result of riboflavin levels, patients showing poor compliance were briefly counseled in ways to achieve full dose exposure.
Table 2.
Mean Rates of 33 Self-Reported Medication Side Effects in the Treatment Phase
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Side Effect | 0 | 100/400 | 0 | 100/400 | 200/800 |
| 1. Change in appetite | 0.40 | 0.55 | 0.28 | 0.24 | 0.29 |
| 2. Sleeping More | 0.26 | 0.48 | 0.26 | 0.20 | 0.21 |
| 3. Sleeping Less | 0.21 | 0.08 | 0.17 | 0.17 | 0.30 |
| 4. More Anxiousa | 0.28 | 0.39 | 0.14 | 0.09 | 0.10 |
| 5. Less Anxiousa | 0.13 | 0.21 | 0.12 | 0.12 | 0.14 |
| 6. More Unhappya | 0.06 | 0.23 | 0.09 | 0.11 | 0.13 |
| 7. Happiera | 0.40 | 0.66 | 0.47* | 0.21 | 0.32 |
| 8. Coughing | 0.06 | 0.08 | 0.06 | 0.03 | 0.04 |
| 9. Trouble Concentratinga | 0.10 | 0.14 | 0.09 | 0.10 | 0.11 |
| 10. Weight Changing | 0.29 | 0.41 | 0.31 | 0.29 | 0.27 |
| 11. More Angry | 0.13 | 0.12 | 0.15 | 0.12 | 0.20 |
| 12. Hands Shaking | 0.04 | 0.04 | 0.04 | 0.05 | 0.02 |
| 13. Diarrhea | 0.11 | 0.06 | 0.05 | 0.06 | 0.06 |
| 14. Constipation | 0.07 | 0.14 | 0.09 | 0.02 | 0.13 |
| 15. Nauseaa | 0.13 | 0.07 | 0.03 | 0.13† | 0.21† |
| 16. Vomitinga | 0.08 | 0.02 | 0.03 | 0.03 | 0.07 |
| 17. More Energy | 0.40 | 0.46 | 0.29 | 0.18 | 0.20 |
| 18. Less Energy | 0.12 | 0.12 | 0.07 | 0.13 | 0.16 |
| 19. Felt High | 0.04 | 0.04 | 0.04 | 0.07 | 0.14 |
| 20. Chills | 0.03 | 0.02 | 0.04 | 0.05 | 0.07 |
| 21. Fever | 0.04 | 0.01 | 0.04 | 0.03 | 0.05 |
| 22. Heart Beating Slower | 0.11 | 0.10 | 0.06 | 0.01 | 0.08 |
| 23. Heart Beats Faster | 0.03 | 0.04 | 0.11 | 0.08 | 0.12 |
| 24. Mouth/Tongue Twitcha | –b | –b | 0.03 | 0.04 | 0.02 |
| 25. Runny Nose | 0.23 | 0.3 | 0.14 | 0.09 | 0.13 |
| 26. Trouble with Eyes | 0.13 | 0.15 | 0.12 | 0.10 | 0.06 |
| 27. Muscles Move/Twitcha | 0.10 | 0.11 | 0.20 | 0.16 | 0.07 |
| 28. Drowsya | 0.19 | 0.17 | 0.11 | 0.11 | 0.22 |
| 29. Muscles/Bones Ache | 0.14 | 0.25 | 0.10 | 0.13 | 0.13 |
| 30. Felt Dizzya | 0.05 | 0.07 | 0.02 | 0.04 | 0.15* |
| 31. Sleeping Better | 0.48 | 0.52 | 0.33 | 0.23 | 0.26 |
| 32. Medication Too High | 0.05 | 0.02 | 0.06 | 0.02 | 0.02 |
| 33. Medication Too Low | 0.19 | 0.13 | 0.06 | 0.06 | 0.08 |
Note. Side effect rates are collapsed across treatment period due to low weekly endorsement rate (Study 1, 4 weeks; Study 2, 8 weeks).
p <. 05.
p <.01.
Items 4, 5, 6, 7, 9, 15, 16, 24, 27, 28, and 30 were a priori hypotheses tested without adjustment for Type I error while all other 22 items were tested with a Bonferroni-adjustment.
No maximum likelihood solution due to low endorsement rate for item.
2.3 Design
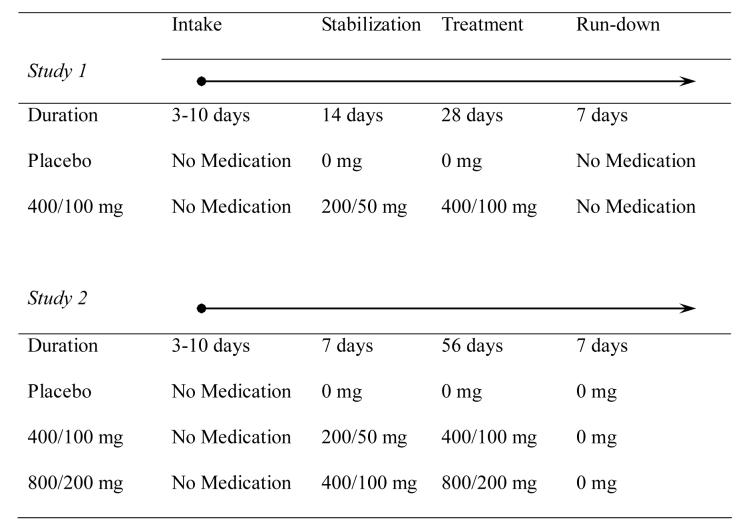
Following completion of the consent process and intake procedures, willing eligible participants were randomized to treatment. Subsequently, stabilization, treatment, and rundown phases occurred (see Figure 3).
Figure 3.
Designs of two randomized, placebo-controlled, double-blind trials of L-dopa/carbidopa for cocaine dependence. Participants completed a 3-10 day intake evaluation phase prior to randomization to a treatment condition. Following randomization, participants underwent 7 (Study 2) or 14 day (Study 1) stabilization phase that involved a dose run-up (Study 1). Subsequently, participants began either a 28 day (Study 1) or 64 day (Study 2) treatment phase. After the treatment phase, those in Study 1 discontinued medication while those in Study 2 were switched to placebo for a 1-week run-down.
2.3.1. Study 1
One half of the subjects remained on placebo throughout the study while those assigned to active medication were dosed first at 200/50 mg of L-dopa/carbidopa and then maintained at 400/100 mg L-dopa/carbidopa until the final dose run-down (see Figure 3).
2.3.2. Study 2
In this subsequent study, those randomized to placebo received it throughout, while those assigned to either of the two active doses received first 200/50 mg or 400/200 mg of L-dopa/carbidopa, then doubled to 400/100 mg or 800/200 mg L-dopa/carbidopa for the treatment period of 8 weeks followed by a one week run-down (see Figure 3).
2.4 Analyses
2.4.1 Sample size and randomization
Sample size for Study 1 was based on available resources. Sample size for Study 2 was calculated to detect medium effect sizes (Cohen, 1988) for retention and cocaine-free urines (Bavry, 1993). Study 1 employed an adaptive enrollment strategy in which subject enrollment continued in each condition until 10 subjects completed the 5-week trial, however data from all enrolled subjects were analyzed. For Study 2, the goal was to randomize 40 subjects to each of three conditions.
2.4.2 Assumptions
All analyses were conducted using the Statistical Analysis System, Version 9.1 (SAS Institute Inc., 2005). Values of p < .05 were considered statistically significant, with the exception of side effects. In side effects analyses, Type I error rate was controlled within study for side effects not a priori expected to occur more often in L-dopa treated subjects (i.e., nausea, vomiting, anxiety/nervousness, depression, dizziness, drowsiness, problems concentrating, and twitching), using a Bonferroni adjustment (i.e., α = .05/22 = .002). Due to participant attrition and frequent missing data, the number of subjects or data points available for statistical analysis varied.
2.4.3 Techniques
Descriptive and univariate statistics were calculated to test for baseline group differences. Kaplan-Meier survival analysis with right censoring was used to test for differences in length of time in treatment as a function of condition. In the case of repeated measures analyses, we employed procedures robust to missing data, including generalized estimating equations and multilevel mixed models. Continuous measures of weight, blood pressure, heart rate, semi-quantitative cocaine levels, cocaine craving, and mood were evaluated using repeated measures ANCOVA. Side effects, cocaine use, and medication adherence proportions were analyzed using repeated-measures logistic regression, under an events/trials format. For cocaine use and medication adherence, in a given week, the proportion was expressed as the number of events (e.g., cocaine positive tests, medication positive tests) to the number of tests (e.g., cocaine urine tests, urine riboflavin fluorescence tests). Side effects were collapsed over the duration of the trial (i.e., the effect of time was disregarded) due to low rates of endorsement at the weekly level. In all repeated measures analyses, the effects of medication, time, and their interaction were evaluated. All repeated measures analyses focused on the treatment period (see Figure 3), i.e., 4 weeks (Study 1) or 8 weeks (Study 2). In repeated measures analyses, the value or average value of the dependent measure during the stabilization phase was used as covariate. One exception was cocaine use analyses in which self-reported cocaine use in the 30 days preceding treatment was employed as the covariate (Carroll et al., 2004a; McLellan et al., 1992).
3. Results
3.1. Study 1
3.1.1. Sample Description
The demographic, substance use, and psychosocial characteristics of participants at randomization are presented in Table 1.
Table 1.
Sample Characteristics
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
| Condition | 0 mg | 400 mg | 0 mg | 400 mg | 800 mg |
| (n = 36) | (n = 31) | (n = 40) | (n = 43) | (n = 39) | |
| Demographic | |||||
| Age | 34.1 (7) | 36.4 (7.1) | 37.7 (7.6) | 40.7 (6.9) | 40.7 (6.2) |
| % Female | 33 (12) | 32 (10) | 10 (4) | 19 (8) | 18 (7) |
| Education | 13.5 (2.8) | 12.9 (1.7) | 12.3 (2) | 13.3 (2.2) | 13.0 (2.6) |
| Race | |||||
| %White | 48.6 (17) | 41.9 (13) | 18.4 (7) | 26.2 (11) | 41 (16) |
| %Black | 37.1 (13) | 58.1 (18) | 76.3 (29) | 73.8 (31) | 46.2 (18) |
| %Hispanic | 14.3 (5) | 0.0 (0) | 5.3 (2) | 0.0 (0)*a | 12.8 (5) |
| % Married | 45 (16) | 27 (8) | 23 (9) | 14 (6) | 28 (11) |
| % Employed | 52 (19) | 47 (14) | 54 (22) | 62 (27) | 64 (25) |
| Drug Use | |||||
| %Intake Cocaine | 61 (22) | 68 (21) | 73 (29) | 67 (29) | 72 (28) |
| Cocaine (Yrs.) | 8.5 (4.5) | 8.6 (5.8) | 10 (4.9) | 10 (6.3) | 12.1 (7.1) |
| Alcohol (Yrs.) | 11.6 (7.4) | 13.8 (9.7) | 17.1 (8.5) | 17.8 (9.3) | 17.2 (10.2) |
| Marijuana (Yrs.) | 12 (8) | 11.1 (9.1) | 9.8 (9.6) | 11.9 (10.3) | 10.6 (9.8) |
| ASI Scores | |||||
| Medical | 0.05 (0.16) | 0.05 (0.19) | 0.05 (0.2) | 0.07 (0.17) | 0.04 (0.16) |
| Employment | 0.43 (0.3) | 0.49 (0.29) | 0.58 (0.28) | 0.49 (0.3) | 0.48 (0.26) |
| Alcohol | 0.19 (0.19) | 0.14 (0.17) | 0.23 (0.21) | 0.21 (0.17) | 0.19 (0.18) |
| Drug | 0.28 (0.08) | 0.26 (0.08) | 0.27 (0.09)*b | 0.23 (0.08) | 0.23 (0.07) |
| Legal | 0.05 (0.13) | 0.09 (0.16) | 0.07 (0.17) | 0.05 (0.1) | 0.05 (0.11) |
| Family/Social | 0.21 (0.24) | 0.26 (0.26) | 0.16 (0.23) | 0.12 (0.16) | 0.24 (0.26) |
| Psychiatric | 0.12 (0.16) | 0.1 (0.15) | 0.17 (0.22) | 0.03 (0.09) | 0.08 (0.14) |
Note.
p < .01.
Significantly fewer Hispanics in 400 mg condition.
Significantly higher ASI Drug Composite score in 0 mg condition.
Most subjects were young males who presented at their initial clinic visit with toxicological evidence of recent cocaine use. The majority of participants were “crack” cocaine users (74.0%).
3.1.2. Retention
In order to assess participant retention we evaluated subjects during the stabilization and maintenance phases.
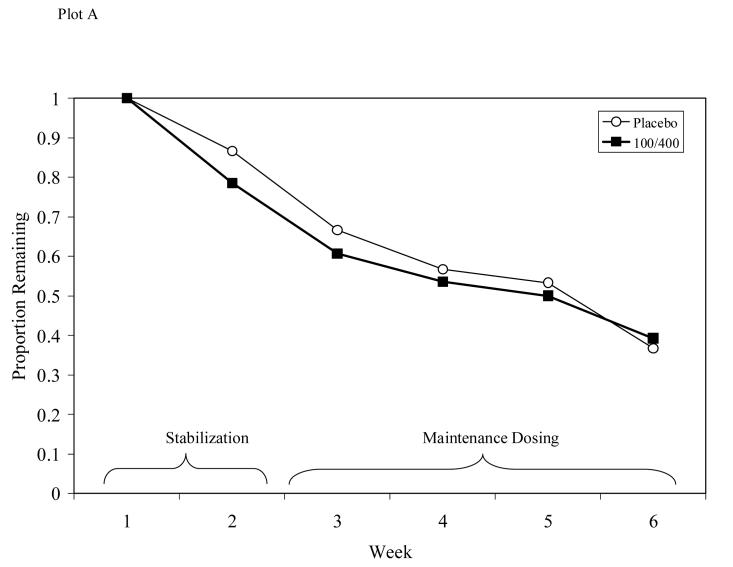
Survival analysis indicated no difference in dropout rates between those receiving L-dopa/carbidopa and those receiving placebo (see Figure 4, Plot A; Log Rank Statistic = .004, d.f. = 1, p = .95), with 38% of participants completing treatment through the maintenance phase. The two medication groups did not differ in the number of weeks completed (M = 3.9, SD = 2.2).
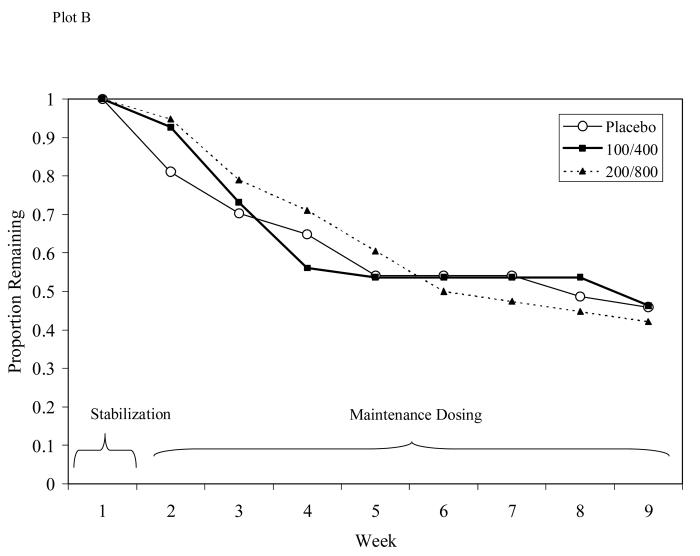
Figure 4.
Participant retention for Study 1 (Plot A) or Study 2 (Plot B). No significant differences in retention seen between groups in either study.
3.1.3. Side Effects
The average rates of thirty-three self-reported side effects (collapsing across time due to low weekly endorsement) were compared across treatment conditions during the maintenance phase. The side effect rates reflect the average proportion of subjects endorsing that symptom throughout treatment (controlling for length in treatment using an events/trials format and accounting for baseline endorsement of a given symptom by including it as a covariate). In this small initial trial, no treatment differences were observed (see Table 2).
3.1.4. Adverse Events
No expected side effects with unexpected severity or unexpected side effects occurred warranting discontinuation of treatment. Two subjects (1 placebo, 1 400/100 mg L-dopa/carbidopa) discontinued medications due to intolerance of medication.
3.1.5. Adherence
Medication adherence was evaluated using riboflavin fluorescence levels. Each urine test was classified as being presumptively medication positive if the level exceeded 35 fluorescence units. The placebo group had a significantly higher rate of medication taking over the 4-week treatment period (80.5%) than the group receiving L-dopa/carbidopa (63.7%), χ2(1) = 5.00, p = .0253. No effects of time or medication × time were seen.
3.1.6. Vital Signs
Weight, diastolic blood pressure, systolic blood pressure, and heart rate were evaluated during the treatment period on a weekly basis. Baseline values were included as covariates. Subject weight, diastolic blood pressure, systolic blood pressure, and heart rate did not differ as a function of medication assignment, time or their interaction.
3.1.7 Cocaine Use – Categorical
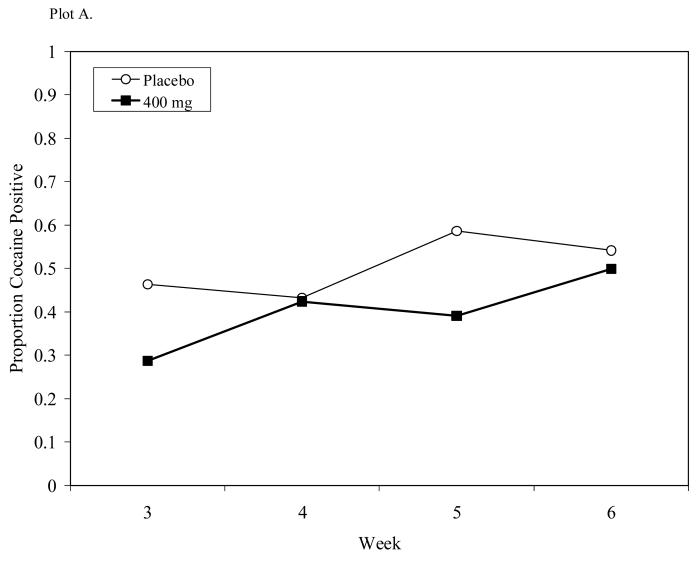
Observed cocaine use proportions were evaluated as a function of medication, time (biweekly intervals), and medication × time, with cocaine use in the preceding 30 days included as covariate (c.f., Carroll et al., 2004a). Percentages of cocaine use during treatment were for placebo (51.0%) and 400/100 mg L-dopa/carbidopa (40.0%). No medication or time effects were observed while a significant medication × time effect was seen, F(3, 78) = 2.95, p = .0378 (see Figure 5, Plot A). This interaction reflected variable proportions of abstinence in the placebo condition.
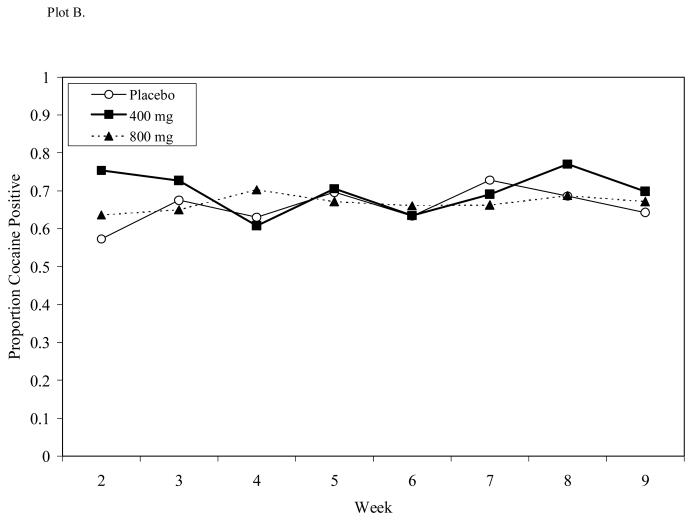
Figure 5.
Cocaine-use proportions for Study 1 (Plot A) or Study 2 (Plot B) during respective 4- and 8-week dosing periods. A significant medication × time effect was seen in Study 1 while no treatment effects were observed in Study 2.
3.1.8 Cocaine Use – Continuous
Benzoylecognine levels, adjusted for urine concentration with creatinine, were evaluated during the treatment period. Due to non-normality, quantities were logarithmically transformed prior to analysis (e.g., Batki et al., 1996). No differences in benzoylecognine levels were observed by medication treatment, time, or their interaction.
3.1.9. Cocaine Craving
Weekly craving assessed on 100 mm VAS did not differ by medication assignment, time in treatment, or their interaction.
3.1.10. Mood
No differences between treatment groups were seen in total BDI Score. Scores did not differ over the treatment course, nor was this course modified by medication assignment.
3.2. Study 2
3.2.1. Sample Description
The demographic, substance use, and psychosocial characteristics of participants in Study 2 at randomization are presented in Table 1. As with Study 1, most subjects were young males who presented at their initial clinic visit with toxicological evidence of recent cocaine use. Again, the majority of participants were “crack” cocaine users (85.0%).
3.2.2. Retention
Survival analysis indicated no significant differences in dropout rates between those receiving L-dopa/carbidopa and those receiving placebo (see Figure 4, Plot B; Log Rank Statistic = .023, d.f. = 1, p = .99), with 45% of participants completing treatment through the maintenance phase. The three medication groups did not differ in the number of weeks completed (M = 5.8, SD = 3.3).
3.2.3. Side Effects
Side effects for Study 2 are presented in Table 2. Patients receiving L-dopa showed dose-dependently higher rates of “nausea”, χ2(2) = 9.95, p = .0069, which followed a linear trend, χ2(1) = 6.86, p = .0088. Both the 400/100 mg, χ2(1) = 9.87, p = .0017, and 800/200 mg, χ2(1) = 16.6, p <.0001, doses had significantly higher rates of nausea than placebo. Dizziness increased dose-dependently, χ2(2) = 7.69, p = .0214, linear trend, χ2(1) = 7.83, p = .0051. The 800/200 mg L-dopa/carbidopa condition had a significantly higher rate of dizziness than both placebo, χ2(1) = 11.93, p = .0006, and 400/100 mg L-dopa/carbidopa conditions, 400/100 mg, χ2(1) = 7.63, p = .0057.
3.2.4. Adverse Events
No expected side effects with unexpected severity or unexpected side effects occurred warranting discontinuation of treatment. In Study 2, no participants ended treatment expressly due to side effects.
3.2.5. Adherence
No effects of medication, time, or medication × time were found. Rates of medication taking by dose were as follows: placebo (80.7%), 400/100 mg L-dopa/carbidopa (77.5%), and 800/200 mg L-dopa/carbidopa (71.5%).
3.2.6. Vital Signs
As with Study 1, subject weight and heart rate did not differ as a function of medication assignment, time, or their interaction. In the case of systolic blood pressure, a significant effect of time, F(7, 41.4) = 3.69, p = 0.0035, was present, reflecting small variations in this measure over treatment. Diastolic blood pressure showed a significant dose-response to medication, F(2, 56.6) = 5.50, p =.0065, linear trend, F(1, 56.6) = 10.3, p =.0022, placebo (M = 77.8 mmHg), 400/100 mg L-dopa/carbidopa (M = 75.1 mmHg), and 800/200 mg L-dopa/carbidopa (M = 70.0 mmHg). The 800/200 mg L-dopa/carbidopa condition had significantly lower diastolic blood pressure than both placebo, t(56.6) = 3.21, p = 0.0022 and 400/100 mg L-dopa/carbidopa conditions, t(59.0) = 2.12, p = 0.0383.
3.2.7 Cocaine Use – Categorical
Percentages of cocaine use during treatment were for placebo (66%), 400/100 mg L-dopa/carbidopa (70%), and 800/200 mg L-dopa/carbidopa (67%). No medication, time, or medication × time effects were observed (see Figure 5, Plot B).
3.2.8 Cocaine Use – Continuous
Benzoylecognine levels did not differ by medication condition, time in treatment, or the interaction of these variables.
3.2.9. Cocaine Craving
Craving for cocaine in the preceding 24 hours did not differ by medication assignment, time in treatment, or their interaction.
3.2.10. Mood
All three treatment groups showed a similar improvement in self-reported mood during treatment, with a decline in BDI scores over time, F(7, 51.4) = 4.32, p =.0008.
4. Discussion
In two randomized, placebo-controlled, double-blind trials, L-dopa/carbidopa was well tolerated and generally did not differ significantly from placebo in most reported side effects in cocaine-dependent patients. While dose-dependent increases in nausea and dizziness were seen for L-dopa/carbidopa, these side effects were never severe enough to constitute adverse events. L-dopa/carbidopa produced a dose-dependent reduction in diastolic or resting blood pressure. Further, there were no differences in treatment completion and little evidence of inequalities in medication adherence. Neither study provided evidence of direct medication effects on cocaine use, cocaine craving, or mood.
Safety and tolerability of medications for substance dependence have been relatively neglected in part because many clinical trials in the field have examined extant approved medications for which the primary actions and side effects have been well characterized. Still, safety studies in this target population, especially cocaine-medication interaction investigations, are likely warranted and are increasingly expected to be conducted prior to larger clinical trials (e.g., Dackis et al., 2003). At the same time, in light of the severe and even life-threatening consequences of cocaine use, it is understandable that researchers have foregone phase I/II testing in preference for the immediate collection of efficacy data. Further, it can be argued that this is justified when existing data about a medication are abundant and putative mechanisms are unlikely to produce additive risk. Unfortunately, as we learned in our own work with the atypical antipsychotic, risperidone, some medications, at times at unexpectedly low doses, are not well tolerated by cocaine-dependent patients (Grabowski et al., 2000). In the current trials, we established that patients were generally as well-retained on L-dopa/carbidopa as placebo. While the smaller trial (Study 1) showed poorer medication adherence for a dose of 400/100 mg L-dopa/carbidopa compared to placebo, a larger trial showed no differential adherence. Adherence, ideally accompanied by no or nominal side effects, is critical for both rigorous efficacy evaluation and eventual clinical use if effectiveness can be demonstrated.
Considering the broad range of potential side effects for L-dopa/carbidopa, that only two side effects were more common in the active treatment than placebo was a positive outcome. In a supplemental analysis looking at ever-occurrence of side effects, fewer than half of those receiving active treatment ever experienced nausea or dizziness. Moreover, at any given time point, fewer than a quarter could expect to have either side effect. Most vital signs were unchanged by L-dopa/carbidopa therapy. However, diastolic or resting blood pressure was dose-dependently decreased by L-dopa/carbidopa. This objective finding is consistent with reports of dizziness, mostly reflecting orthostatic hypotension. However, no falls or episodes of syncope were seen in either trial. This side effect may be potentially problematic for the actively or very recently cocaine-using population. Lethargy and fatigue associated with hypotension in the aftermath of cocaine use could either prompt use or at minimum attenuate adherence in standard clinical practice. However, other data from our group (Schmitz et al., 2006) indicate that the L-dopa/carbidopa preparation is most likely to be effective in combination with behavior therapy. For these individuals, return to relatively stable normal cardiovascular function might minimize hypotension-related noncompliance.
These studies gave no support to the prospect that the L-dopa preparation would reduce cocaine in active users. No changes were evident in cocaine-use rates or semi-quantitative cocaine use levels. The current study provided a modest, though previously validated, cognitive behavioral therapy (Schmitz et al., 2001). The ideal psychotherapy platform would increase retention, medication adherence, and psychosocial adjustment, permitting the medication to work, but not be so potent as to surmount or overwhelm the effects of the pharmacotherapy (Carroll et al., 2004b). However, the intensity and kind of psychosocial intervention needed as a platform to evaluate a pharmacotherapy for cocaine dependence is not clear. For example research on efficacy of desipramine has been equivocal, but Kosten et al., (2003) reported that desipramine facilitated cocaine abstinence in buprenorphine-maintained cocaine-opioid patients, but only when patients were provided abstinence-contingent reinforcement. Similarly, after several negative studies of fluoxetine by our group, one implemented by Schmitz et al., (1998) demonstrated efficacy of a fluoxetine–contingency management combination. Finally, Poling and colleagues (2006) recently showed that bupropion combined with an abstinence-contingent monetary reward was superior to bupropion treatment alone for reducing cocaine use in jointly cocaine-opioid dependent patients. Thus, several medications, previously dismissed as ineffective treatment agents on their own, have shown efficacy when combined with the behavioral intervention of contingency management. Currently we are investigating this possibility in a study of L-dopa/carbidopa pharmacotherapy with and without abstinence-based contingency management procedures. Thus, while Studies 1 and 2 were negative for efficacy, based on our experience (Schmitz et al., 1998), we decided that we could not definitively reject L-dopa/carbidopa without a test involving a stronger psychotherapy platform, involving contingency management. The next study in our series suggests that this is the case (Schmitz et al., 2006).
Acknowledging that behavioral therapies may interact favorably with medication, medication combinations may also confer added benefit. Ideally, two medications with somewhat different actions might be used in combination at lower doses than either alone, thus not only providing potential benefit but reduced likelihood of adverse events. One potentially effective combination might be L-dopa/carbidopa with an agonist-like treatment such as dextroamphetamine (Grabowski et al., 2004b) or modafinil (Dackis et al., 2005); this would also counter emergent hypotension associated with L-dopa administration in this population. When confronted with dysphoria and even episodes of major depression among cocaine users (Rounsaville, 2004), L-dopa might be used in combination with SSRIs which would alter mood while the L-dopa preparation plus behavior therapy reduced cocaine use.
The current study has several limitations. No direct or surrogate measure of L-dopa exposure was obtained and these data might be informative for dosing. In future studies, homovanillic acid (HVA) levels should be evaluated to get direct evidence of enhancement of dopamine levels. Relatedly, while a range of doses was examined, higher doses may produce direct efficacy even in non-abstinent subjects. Despite our best efforts to maximize retention, overall rates of treatment completion were low, with fewer than half of subjects receiving the full course of treatment. While not a result of medication side effects, since the rates were similar across all groups, the lack of larger sample sizes certainly limited the statistical power of our studies. Finally, though substantial effort was made to have equal numbers of males and females in the reported studies, the current disparity limits the opportunity to make sex-based comparisons.
In conclusion, L-dopa/carbidopa was safe and well-tolerated in chronic cocaine users. However, no evidence of efficacy was observed for cocaine use, craving, or mood. Additional research further exploring dosing, medication combinations and behavior therapy medication combinations are recommended, based on finding on several other trials (Kosten et al., 2003; Poling et al., 2006; Schmitz et al., 1998).
Acknowledgements
The first author is supported by National Institute of Drug Abuse (NIDA) grant K01-DA-019446. This research was supported by the NIDA grants R01-DA-6143 and P50-DA-9262. The current studies were presented at the annual meeting of the College of Problems on Drug Dependence in 2004 by Howard Rhoades, Ph.D. (Rhoades et al., 2004). The authors wish to thank Peter B. Silverman Ph.D., J.D. for his important early contributions to our examination of L-dopa/carbidopa as a candidate medication for the treatment of cocaine dependence. We express particular appreciation to Shelly Sayre, Clayton Dreyer, Patricia Hokanson, and Howard Rhoades for their contributions to the conduct of these trials. We are indebted to the participants for taking part in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batki SL, Washburn AM, Delucchi K, Jones RT. A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend. 1996;41:137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- Bavry JL. STAT-POWER. Scientific Software International, Incorporated; Chicago, IL: 1993. [Google Scholar]
- Beck A, Ward C, Mendelson M, Mack J, Erbaugh J. An inventory of measuring depression. Archives of General Psychiatry. 1961;49:599–608. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004a;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004b;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1988. [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, Rowan A, Poole S, White L, O'Brien CP. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97:931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- Eiler K, Schaefer MR, Salstrom D, Lowery R. Double-blind comparison of bromocriptine and placebo in cocaine withdrawal. Am J Drug Alcohol Abuse. 1995;21:65–79. doi: 10.3109/00952999509095230. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-VI Axis I Disorders- Patient Edition (SCID -I / P, Version 2.0) Biometric Research Department; NY: 1995. [Google Scholar]
- Giannini AJ, Folts DJ, Feather JN, Sullivan BS. Bromocriptine and amantadine in cocaine detoxification. Psychiatry Res. 1989;29:11–16. doi: 10.1016/0165-1781(89)90182-0. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Arnoni G, Elk R, Rhoades H, Schmitz J. Baseline assessment, study entry, and stabilization: double-blind clinical trials in drug dependence. NIDA Res Monogr. 1997;175:158–181. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Halikas JA, Kuhn KL, Crosby R, Carlson G, Crea F. The measurement of craving in cocaine patients using the Minnesota Cocaine Craving Scale. Compr Psychiatry. 1991;32:22–27. doi: 10.1016/0010-440x(91)90066-l. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Rosenblum A, Palij M, Magura S, Foote J, Lovejoy M, Stimmel B. Bromocriptine for cocaine dependence. A controlled clinical trial. Am J Addict. 1997;6:54–64. [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le HT, Creese I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett. 1987;79:315–320. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten TR, Avants SK, Wilkins J, Ling W, Beckson M, Arndt IO, Cornish J, Ascher JA, Li SH, et al. A multicenter trial of bupropion for cocaine dependence in methadone- maintained patients. Drug Alcohol Depend. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Liu S, Blanchette H, Blundell P, Madras BK. Design and synthesis of an irreversible dopamine-sparing cocaine antagonist. Bioorg Med Chem. 2002;10:3583–3591. doi: 10.1016/s0968-0896(02)00244-4. [DOI] [PubMed] [Google Scholar]
- Mezinskis J, Dyrenforth S, Goldsmith RJ, Cohen M, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatric Annals. 1998;28:577. + [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology (Berl) 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Reid MS, Casadonte P, Baker S, Sanfilipo M, Braunstein D, Hitzemann R, Montgomery A, Majewska D, Robinson J, Rotrosen J. A placebo-controlled screening trial of olanzapine, valproate, and coenzyme Q10/L-carnitine for the treatment of cocaine dependence. Addiction. 2005;100(Suppl 1):43–57. doi: 10.1111/j.1360-0443.2005.00990.x. [DOI] [PubMed] [Google Scholar]
- Rhoades H, Moeller FG, Cowan K, Stotts A, Schmitz J, Grabowski J. Dose-ranging trial of l-dopa/carbidopa for cocaine dependence: Randomized, double-blind placebo controlled trial. In: Martell BA, Schottenfeld RS, editors. New Vistas in Treating Cocaine Dependence; Symposium conducted at the meeting of the College of Problems on Drug Dependence; San Juan, Puerto Rico. 2004. Chair. Co-chair. [Google Scholar]
- Rosen H, Flemenbaum A, Slater VL. Clinical trial of carbidopa-L-dopa combination for cocaine abuse. Am J Psychiatry. 1986;143:1493. doi: 10.1176/ajp.143.11.1493a. [DOI] [PubMed] [Google Scholar]
- Rothman RB. High affinity dopamine reuptake inhibitors as potential cocaine antagonists: a strategy for drug development. Life Sci. 1990;46:PL17–21. doi: 10.1016/0024-3205(90)90466-5. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ. Treatment of cocaine dependence and depression. Biol Psychiatry. 2004;56:803–809. doi: 10.1016/j.biopsych.2004.05.009. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. SAS Institute Inc.; Cary, NC: 2005. [Google Scholar]
- Schmitz JM, Mooney M, Stotts A, Moeller FG, Grabowski J. Randomized placebo-controlled trial of levodopa-carbidopa and behavior therapy in cocaine-dependent outpatients. College of Problems on Drug Dependence; Scottsdale, AZ: 2006. [Google Scholar]
- Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Experimental and Clinical Psychopharmacology. 1998;6:162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, Ling W. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100(Suppl 1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Soares BG, Lima MS, Reisser AA, Farrell M. Dopamine agonists for cocaine dependence. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003352. CD003352. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- Tennant FS, Jr., Sagherian AA. Double-blind comparison of amantadine and bromocriptine for ambulatory withdrawal from cocaine dependence. Arch Intern Med. 1987;147:109–112. [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr., Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JN. Quantitative urine levels of cocaine and other substances of abuse. NIDA Res Monogr. 1997;175:235–252. [PubMed] [Google Scholar]
- Wolfsohn R, Angrist B. A pilot trial of levodopa/carbidopa in early cocaine abstinence. J Clin Psychopharmacol. 1990;10:440–442. [PubMed] [Google Scholar]
- Wolfsohn R, Sanfilipo M, Angrist B. A placebo-controlled trial of L-dopa/carbidopa in early cocaine abstinence. Neuropsychopharmacology. 1993;9:49–53. doi: 10.1038/npp.1993.42. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr., Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]