Abstract
Extracellular carboxylic ester hydrolases are produced by many bacterial pathogens and have been shown recently to be important for virulence of some pathogens. However, these hydrolases are poorly characterized in enzymatic activity. This study prepared and characterized the secreted ester hydrolase of Streptococcus equi ssp. equi (designated SeE for S. equi esterase). SeE hydrolyzes ethyl acetate, acetylsalicylic acid, and tributyrin but not ethyl butyrate. This substrate specificity pattern does not match those of the three conventional types of non-specific carboxylic ester hydrolases (carboxylesterases, arylesterases, and acetylesterases). To determine whether SeE has lipase activity, a number of triglycerides and vinyl esters were tested in SeE-catalyzed hydrolysis. SeE does not hydrolyze triglycerides and vinyl esters of long chain carboxylic acids nor display interfacial activation, indicating that SeE is not a lipase. Like the conventional carboxylesterases, SeE is inhibited by diisopropylfluorophosphate. These findings indicate that SeE is a novel non-specific carboxylic ester hydrolase that has broader substrate specificity than the conventional carboxylesterases.
Keywords: Streptococcus equi, esterase, lipase, carboxylic ester hydrolase, carboxylesterase, Sse, SeE
Introduction
Carboxylic ester hydrolases are a diverse group of enzymes that hydrolyze carboxylic esters. They can be divided into non-specific and specific carboxylic ester hydrolases, and the former can be divided into lipases and esterases. Lipases catalyze hydrolysis of both short-chain (water-soluble) and long-chain (water-insoluble) triglycerides and are usually interfacially activated with an abrupt increase in activity when substrates form emulsions. On the other hand, esterases act on only short-chain triglycerides in solution and are not interfacially activated in substrate emulsions. Besides triglycerides, vinyl esters have also been used to distinguish esterases from lipases. Unlike lipases, esterases are inactive against long-chain vinyl esters in solution or emulsion (Chahinian et al., 2002).
Non-specific carboxylic ester hydrolases can also be classified into carboxylesterase, arylesterase, and acetylesterase based on substrate specificity using ethyl acetate, ethyl butyrate, tributyrin, and phenyl acetate as well as their sensitivity to diisopropylfluorophosphate (DFP) (Whitaker 1972). Phenyl acetate is hydrolyzed by all the three types of non-specific carboxylic ester hydrolases, while ethyl acetate is hydrolyzed by carboxylesterases and acetylesterases but not by arylesterases. Ethyl butyrate and tributyrin are hydrolyzed only by carboxylesterases, and only carboxylesterases and some arylesterases are inhibited by DFP (Whitaker 1972, Fenster et al., 2003).
It has been known for long time that esterases are widespread in bacterial pathogens such as Group A Streptococcus (Stock et al., 1961). However, the roles of esterases in virulence and pathogenesis of pathogenic bacteria are largely unknown. Active and passive immunizations with the secreted esterase of Group A Streptococcus protect mice against subcutaneous infection of Group A Streptococci (Liu et al., 2007). A cell wall-anchored carboxylesterase is required for virulence in Mycobacterium tuberculosis (Lun & Bishai, 2007), and a putative esterase was among the genes required for lung infection in mice caused by Streptococcus pneumoniae in a large scale screen (Hava & Camilli, 2002). Nevertheless, the enzymatic activities of these extracellular esterases have not been characterized in details, though an intracellular esterase, Rv1399c, of Mycobacterium tuberculosis has been characterized (Canaan et al., 2004). The horse pathogen Streptococcus equi ssp. equi, which causes equine strangles (Harrington et al., 2002), has a homologue of the secreted esterase of Group A Streptococcus (designated SeE for S. equi esterase). We prepared recombinant SeE and characterized its enzymatic activity. Our results indicate that SeE is a novel non-specific carboxylic ester hydrolase.
Materials and methods
Materials and bacterial strain
Tripropionin was purchased from TCI America (Portland, OR, USA). Vinyl propionin, vinyl butyrate, vinyl laurate, tributyrin, trioctanoin and lipase from Mucor meihei (5350 units/mg solid) were purchased from Sigma (St. Louis, MO, USA). Acetylsalicylic acid, triacetin and ethyl butyrate were purchased from Fisher Scientific (Fair Lawn, NJ, USA). S. equi strain SEM1 has been described (Liu et al., 2008).
Gene cloning
The see gene encoding the secreted esterase of S. equi was PCR cloned from strain SEM1 using primers 5′-ACCATGGGCACGCGATCCTGGAAAAGCTG-3′ and 5′-dCGAATTCTTATTTTTGGGGTTCGTACTC -3′. The PCR product was digested with EcoRI and NcoI and was ligated into pET-His (Lei et al., 2003) at the EcoRI and NcoI sites to yield the plasmid pSEE. Recombinant SeE made from this construct had 12 amino acid residues, MHHHHHHLETMG, fused to the second amino acid residue, 34Thr, of mature SeE. The cloned gene was sequenced to rule out spurious mutations.
Purification of recombinant SeE
Recombinant SeE was expressed and purified from Escherichia coli strain BL21 containing pSEE. The bacteria were grown in 6 liters of Luria-Bertani broth supplemented with 100 mg ampicillin/liter at 37°C. When the optical density at 600 nm (OD600) of the culture was about 0.5, 0.5 mM isopropyl-β-D-thiogalactopyronoside was added to induce SeE production. After 10 h of induction, bacteria were harvested by centrifugation.
The bacterial pellet obtained was suspended in 80 mL of 20 mM Tris-HCl, pH 8.0, and sonicated on ice for 20 min and centrifuged. The lysate was adjusted to 0.5 M NaCl and loaded onto a Ni-nitrilotriacetic acid agarose column (2.5 × 3 cm). The column was washed with 50 ml of 20 mM Tris-HCl containing 0.5 M NaCl and eluted with a 100-ml gradient of 0-75 mM imidazole. Fractions containing SeE were pooled and dialyzed against 3 L of 20 mM Tris-HCl, and the dialyzed sample was loaded onto a DEAE sepharose column (2.5 × 3 cm) that was eluted with a 110-ml gradient of 0-50 mM NaCl. Fractions containing SeE with >95% purity were pooled and dialyzed against 20 mM Tris-HCl.
SeE activity assay
The enzymatic activity of SeE was determined with potentiometric titration using a pH-stat (Model 360, Denver Instrument, USA), as described previously with minor modifications (Chahinian et al., 2002). SeE was stirred together with each substrate at specified concentrations at 25°C in 25 ml of 2.0 mM Tris-HCl, pH 7.6, and released acid was titrated in real time with 0.02 M NaOH to keep pH at 7.6 for 3 min. Hydrolysis rate was measured from the slope of the plot of the volume of NaOH added versus time. Enzyme activity was defined as μmoles of acid formed per minute per mg SeE protein.
Inhibition of SeE activity by DFP
SeE at 0.26 mg/mL in 100 μl of 2 mM Tris-HCl, pH 7.4, was incubated with DFP at concentrations from 0.0 to 100 μM at 37°C for 30 min, and the remaining activity of the treated SeE was determined using 100 mM vinyl propionate as described above.
pH dependence of SeE activity
SeE at 0.26 mg/ml was incubated at 25°C for 1 h in the buffers at pH from 4 to 9 that were same as those Canaan et al. used (Canaan et al., 2004), and the remaining activity of the treated SeE was determined using 100 mM vinyl propionate as described above.
Effect of temperature on SeE activity and stability
To evaluate the effects of temperature on SeE activity and stability, SeE activity was determined at 10, 20, 30, 40, 50 and 60°C for 5 min using 100 mM vinyl propionate as described above. Thermostability of SeE was also studied by measuring the remaining activity after incubation of SeE at 40°C for 10, 20, 30, 45 and 60 min.
Nucleotide sequence accession number
The GenBank accession number for the nucleotide sequence of the S. equi see gene is EU938321.
Results
SeE gene and protein
A blast search of available S. equi genome (http://www.sanger.ac.uk/Projects/S_equi) with the sse gene of Group A Streptococcus identified the esterase gene of S. equi, see, which encodes a 345-amino-acid protein with an inferred molecular mass of 39,358 Da. The inferred protein has a putative 32-amino-acid secretion signal sequence. The Sse and SeE proteins share 62% sequence identity (Liu et al., 2007). To obtain recombinant SeE, the see gene was cloned, and recombinant SeE protein with a molecular weight of 39 kDa was overexpressed in E. coli and purified to > 95% purity as determined by SDS-PAGE analysis (Picture not shown). SeE was detected in the culture supernatant of all 5 S. equi strains tested and had specific antibody in convalescent sera of all 5 horses with strangles (data not shown), indicating that SeE was produced in vitro and in vivo.
SeE does not belong to the conventional three types of non-specific carboxylic ester hydrolases
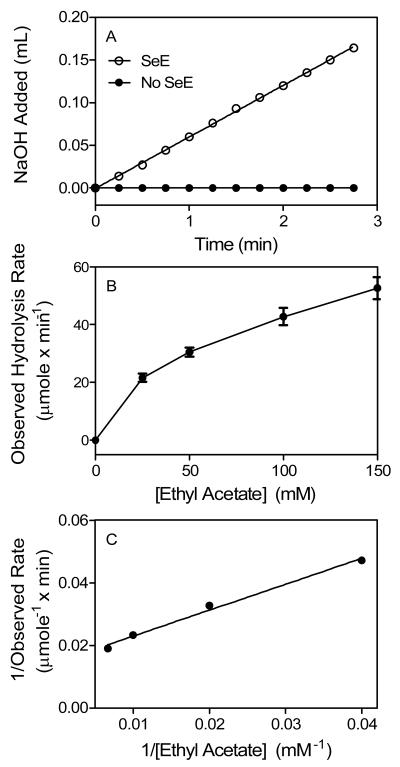
Differential specificities of esterases for substrates ethyl acetate, phenyl acetate, ethyl butyrate, and tributyrin have been used to divide non-specific carboxylic ester hydrolases into groups of carboxylesterases, arylesterases, and acetylesterases. We used acetylsalicylic acid, a derivative of phenyl acetate, and the other substrates to determine whether SeE is a member of one of these esterase families. SeE-catalyzed ester hydrolysis was monitored with potentiometric titration using a pH-stat in which NaOH was automatically pumped in to neutralize released acid and keep pH of the reaction solution constant. After SeE protein was mixed with 25 mM ethyl acetate, 0.02 M NaOH was pumped in at a constant rate of 0.06 ml/min, the slope of the plot of the accumulative NaOH volume versus time. In contrast, no NaOH was added in a control reaction without SeE (Fig. 1A). These results indicate that SeE can catalyze hydrolysis of ethyl acetate. From the rate of NaOH addition, an observed reaction rate was calculated to be that 23 μmole ethyl acetate was hydrolyzed per min by 1 mg SeE. The reaction was repeated at other ethyl acetate concentrations, and the observed rate increased hyperbolically with increasing substrate concentration (Fig. 1B). Double-reciprocal plotting analysis of the data in Fig. 3B indicates that the reaction catalyzed by SeE follows the Michaelis-Menten model (Fig. 1C). According to the slope and intercept on the y axis in Fig. 3C, the Michaelis constant (Km) and specific activity of SeE were calculated to be 56 mM and 68 μmole min−1 mg−1, respectively (Table 1). Because arylesterases do not hydrolyze ethyl acetate, the ability of SeE to hydrolyze ethyl acetate indicates that it is not an arylesterase.
Fig. 1.
SeE-catalyzed hydrolysis of ethyl acetate. (A) Titration of the released acetic acid with 0.02 N NaOH in a reaction of 25 mM ethyl acetate in the absence (solid circles) or presence (open circles) of 52 μg SeE in 25 ml of 2 mM Tris-HCl buffer pH 7.6 at 25°C using a pH-stat. Presented is the accumulative volume of NaOH added as a function of time. (B) Plotting of hydrolysis rate versus ethyl acetate concentration. The rates were calculated from the slope of the curve in panel A and similar experiments at the other ethyl acetate concentrations. (C) Double reciprocal plotting of the data in panel B.
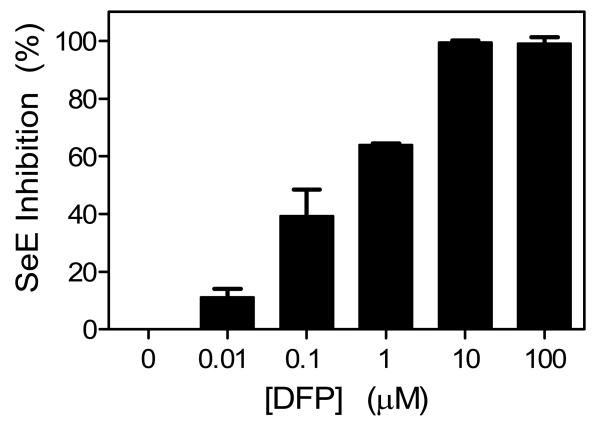
Fig. 3.
Inhibition of SeE activity by DFP. SeE was incubated with DFP at 37°C for 30 min, and the remaining activity of the treated SeE was determined using 100 mM vinyl propionate. Presented are the percentages of inhibition at the indicated DFP concentrations.
Table 1.
Specific activities and Km of SeE and Mucor meihei lipase against various substrates.
| SeE |
Lipase |
||
|---|---|---|---|
| Substrates | Specific activity μmole min−1mg−1 |
Km mM |
Specific activity μmole min−1mg−1 |
| Ethyl acetate | 68a | 56 | 2b |
| Ethyl butyrate | 0 | 13c | |
| Tributyrin | 3b | 133b | |
| Acetylsalicylic acid | 44a | 3 | 1b |
| Triacetin | 513a | 203 | 2a |
| Tripropionin | 73b | 22c | |
| Trioctanoin | 0 | 111c | |
| Vinyl propionate | 815a | 68 | 573b |
| Vinyl butyrate | 24b | 412c | |
| Vinyl laurate | 0 | 13c | |
| Acetylcholine | 0 | 0 | |
The value was obtained by using double-reciprocal analysis of enzyme activity.
The activity was measured at optimal substrate concentration.
The activity was determined at 100 mM substrate.
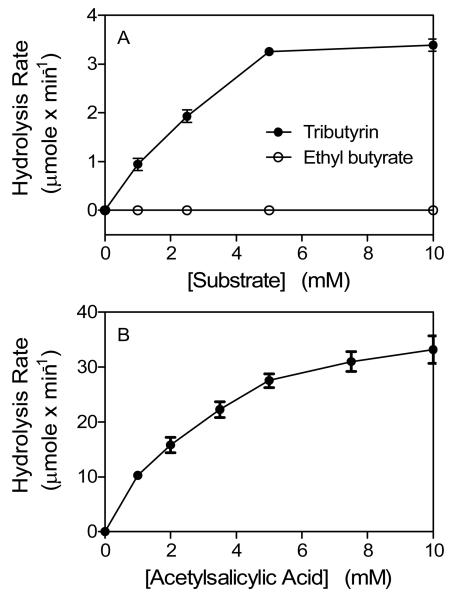
We next tested the activity of SeE on the other substrates. SeE cannot hydrolyze ethyl butyrate (Fig. 2A). Since carboxylesterases, but not acetylesterases, hydrolyze ethyl butyrate, the inability of SeE to hydrolyze ethyl butyrate indicates that SeE is not a member of the conventional carboxylesterase. This result also suggests that SeE may be an acetylesterase, which cannot hydrolyze tributyrin. However, SeE hydrolyzes tributyrin with a specific activity of 3.4 μmole min−1 mg−1 using an optimal substrate concentration (Fig. 2A). These results indicate that SeE is not an acetylesterase. Thus, SeE does not belong to any of the three conventional non-specific carboxylic ester hydrolases.
Fig. 2.
SeE hydrolyzes tributyrin and acetylsalicylic acid but not ethyl butyrate. The hydrolysis assays were performed as in Fig. 2, and presented are the hydrolysis rates of the indicated reactions as a function of substrate concentration.
Ability to hydrolyze phenyl acetate appears to be a common feature of non-specific carboxylic ester hydrolases since all the three types of non-specific carboxylic ester hydrolases can hydrolyze phenyl acetate. Therefore, we tested whether SeE also hydrolyzes phenyl acetate-type ester. SeE can hydrolyze acetylsalicylic acid, a derivative of phenyl acetate. The reaction shows a hyperbolic relationship between the observed rate of hydrolysis and substrate concentration (Fig. 2B) and follows the Michaelis-Menten model with a Km value of 3.3 mM and a specific activity of 44 μmole min−1 mg−1. This result supports that SeE is a member of non-specific carboxylic ester hydrolases.
Inhibition of SeE activity by DFP
DFP is a potent inhibitor of carboxylesterases but not acetylesterases. Whether SeE is inhibited by DFP was tested to further confirm that SeE is not an acetylesterase. SeE was incubated with DFP from 0 to 100 μM for 30 min, and the SeE activity was determined using vinyl propionate as a substrate. The activity of SeE was inhibited in a dose-dependent manner by DFP at < 10 μM, and SeE was completely inhibited by DFP at 10 μM (Fig. 3). These results confirm that SeE is not an acetylesterase and suggest that SeE is more like a carboxylesterase.
SeE does not have lipase activity
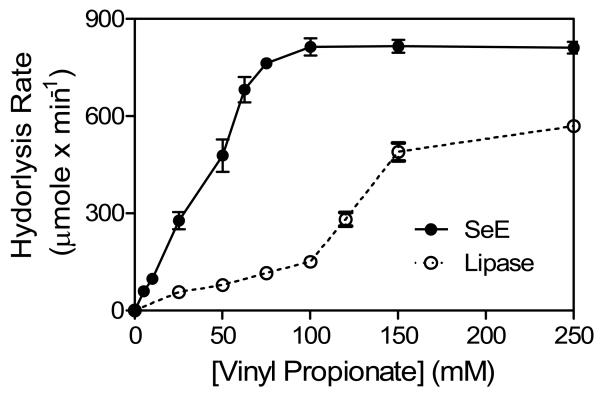
Non-specific carboxylic ester hydrolases can be either esterases or lipases. One distinction between esterases and lipases is that lipases, but not esterases, display interfacial activation when substrates turn from a solution to emulsion. To use this criterion to test whether SeE is a lipase, we compared the activities of SeE and M. meihei lipase on tripropionin, which has the solubility of 12.5 mM in water and forms emulsion at > 12.5 mM. M. meihei lipase had low activity to hydrolyze tripropionin at up to 12.5 mM but much higher activity when vinyl propionate was at > 12.5 mM (Fig. 4). In contrast, SeE activity increased hyperbolically with increasing substrate concentration and reached its maximal value at concentration slightly beyond 12.5 mM without obvious interfacial activation. These results suggest that SeE is not a lipase.
Fig. 4.
Lack of interfacial activation in SeE-catalyzed hydrolysis of vinyl propionate. The hydrolysis reactions of vinyl propionate at various concentrations in the presence of 220 μg SeE (solid circles) or 375 μg Mucor meihei lipase (open circles) were formed as described in Fig. 2. Presented are the hydrolysis rates as a function of substrate concentration.
Another distinction between esterases and lipases is that esterases hydrolyze short-chain triglycerides and vinyl esters, whereas lipases are active for both short- and long-chain triglycerides and vinyl esters. We tested the activities of SeE and M. meihei lipase for the following substrates: Triacetin, tripropionin, trioctanoin, vinyl propionate, vinyl butyrate, and vinyl laurate. SeE is active for the short chain substrates triacetin, tripropionin, tributyrin, vinyl propionate, and vinyl butyrate but inactive for the long chain substrates trioctanoin and vinyl laurate (Table 1). In contrast, M. meihei lipase is active for all these substrates. These results further confirm that SeE is not a lipase.
Specific carboxylic ester hydrolases include phopholipases and acetylcholinesterases. Since SeE has no activity for the long-chain esters, SeE is not expected to be phospholipases. SeE was found to be inactive for acetylcholine (Table 1). This result further support that SeE is a non-specific carboxylic ester hydrolase.
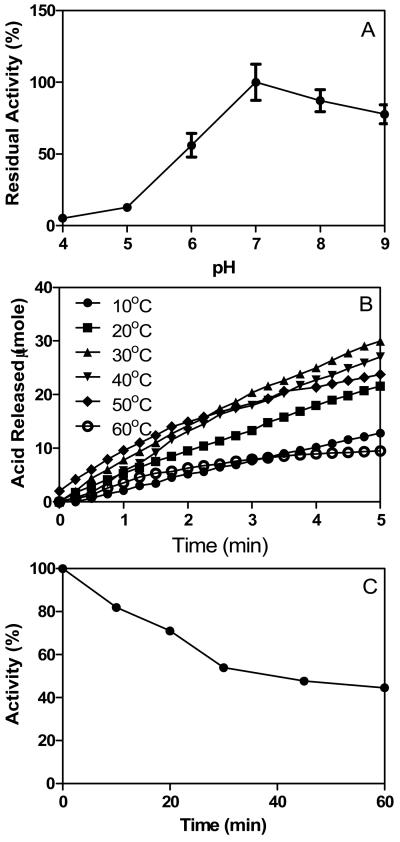
Effect of pH and temperature on SeE activity and stability
SeE is sensitive to the pH as little activity was observed after 1 h incubation in acetate buffer with pH 4 and 5 (Fig. 5A), which was similar to a carboxylesterase identified from Mycobacterium tuberculosis. It appeared that optimal pH for seE activity was around 7 since largest activity was observed at pH=7.
Fig. 5.
(A) pH dependence activity. (B) Effect of temperature on SeE activity. (C) Thermostability of SeE. SeE was incubated at 40°C for up to 60 min, and the remaining activity of SeE was determined using 100 mM vinyl propionate. Presented are the percentages of enzyme activity.
SeE exhibited highest activity at 30°C, which appeared to be the optimal temperature for its activity; whereas SeE lost >50% activity at 10 or 60°C (Fig. 5B). Plotting of acid released during hydrolysis at 10 to 30°C versus time appeared to be linear, indicating that seE was quite stable at these temperatures. On the other hand, the hydrolysis rate of SeE at 40 to 50°C was not constant, and dropped during reaction timecourse, suggesting that SeE was inactivated under these temperatures. To further characterize thermostability of SeE, we measured the remaining activity of after incubation of SeE at 40°C for up to 60 min. SeE lost about 20 and 50% activity after 10 and 60 min, respectively (Fig. 5C).
Discussion
Many bacterial pathogens produce extracellular carboxylic ester hydrolases. A couple of recent publications report that these proteins are important for virulence (Liu et al., 2007; Lun & Bishai, 2007; Hava & Camilli, 2002). These proteins are poorly characterized in enzymatic activity, and whether these proteins are different from other characterized carboxylic ester hydrolases is not known. We have characterized the enzymatic activity of carboxylic ester hydrolase secreted by S. equi. We found that SeE does not belong to any of the three conventional families of nonspecific carboxylic ester hydrolases nor has lipase activity. Our results thus identify SeE as a secreted novel non-specific carboxylic esterase.
SeE is ruled out as a member of arylesterases or acetylesterases by the following convincing evidences. Substrate specificity pattern of SeE using ethyl acetate, ethyl butyrate, and tributyrin does not match those of arylesterases and acetylesterases (Whitaker, 1972). Even though SeE is inhibited by DFP like carboxylesterases, unlike these conventional carboxylesterases, SeE is not active to hydrolyze ethyl butyrate. SeE hydrolyzes many carboxylic esters of aliphatic and aromatic alcohols like conventional carboxylesterases, suggesting that SeE is similar to carboxylesterases. However, SeE is apparently different from conventional carboxylesterases in that SeE has broader substrate specificity. SeE is not activated interfacially and cannot hydrolyze esters of long chain carboxylic acids, indicating that SeE does not have lipase activity. Thus, SeE is a novel carboxylic ester hydrolase with broad substrate specificity.
Since SeE is inhibited by DFP, a serine-reacting compound (Whitaker, 1972), SeE must have a seryl hydroxyl group in its active site, a possibility supported by the fact that SeE shares high sequence identity with the serine esterase Sse of group A Streptococcus (Liu et al., 2007). Both SeE and Sse contains the GXSXG motif of serine esterases (Okazaki et al., 2006), and the serine residue of the GXSXG motif in Sse is indeed critical for its esterase activity (Liu et al., 2007).
Esterases are widely used in biochemical, pharmaceutical and food industry. Esterases show high stereospecificity, which makes them attractive biocatalysts for the production of optically pure compounds (Bornscheuer 2002). Interestingly, these enzymes do not require cofactors, and are usually very stable in organic solvents. Esterases are also capable of catalyzing interesterification, aminolysis, and peracid formation (Mnisi et al., 2005). Therefore, the ability of SeE to hydrolyze a variety of ester compounds suggests that the protein may have its potential for industrial applications.
In summary, SeE is a novel carboxylesterase that hydrolyzes various carboxylic esters of aliphatic and aromatic alcohols, suggesting that it may play important roles in nutrient utilization and tissue invasion. The findings will facilitate the identification of the function of of SeE in physiology and pathogenesis of S. equi.
Acknowledgements
This work was supported by USDA NRI/CSREES grant 2007-35204-18306 and NIH grant P20 RR-020185, USDA Formula Funds and the Montana State University Agricultural Experimental Station.
References
- Bornscheuer UT. Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev. 2002;26:73–81. doi: 10.1111/j.1574-6976.2002.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Canaan S, Maurin D, Chahinian H, Pouilly B, Durousseau C, Frassinetti F, Scappuccini-Calvo L, Cambillau C, Bourne Y. Expression and characterization of the protein Rv1399c from Mycobacterium tuberculosis. A novel carboxyl esterase structurally related to the HSL family. Eur J Biochem. 2004;271:3953–61. doi: 10.1111/j.1432-1033.2004.04335.x. [DOI] [PubMed] [Google Scholar]
- Chahinian H, Nini L, Boitard E, Dubès JP, Comeau LC, Sarda L. Distinction between esterases and lipases: a kinetic study with vinyl esters and TAG. Lipids. 2002;37:653–62. doi: 10.1007/s11745-002-0946-7. [DOI] [PubMed] [Google Scholar]
- Fenster KM, Parkin KL, Steele JL. Nucleotide sequencing, purification, and biochemical properties of an arylesterase from Lactobacillus casei LILA. J Dairy Sci. 2003;86:2547–57. doi: 10.3168/jds.S0022-0302(03)73849-1. [DOI] [PubMed] [Google Scholar]
- Harrington DJ, Sutcliffe IC, Chanter N. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 2002;4:501–510. doi: 10.1016/s1286-4579(02)01565-4. [DOI] [PubMed] [Google Scholar]
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–406. [PMC free article] [PubMed] [Google Scholar]
- Lei B, Mackie S, Lukomski S, Musser JM. Identification and immunogenicity of group A Streptococcus culture supernatant proteins. Infect Immun. 2000;68:6807–6818. doi: 10.1128/iai.68.12.6807-6818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Liu M, Voyich JM, Prater CI, Kala SV, DeLeo FR, Musser JM. Identification and characterization of HtsA, a second heme-binding protein made by Streptococcus pyogenes. Infect Immun. 2003;71:5962–5969. doi: 10.1128/IAI.71.10.5962-5969.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhu H, Zhang J, Lei B. Active and passive immunizations with streptococcal esterase Sse protect mice against subcutaneous infection with Group A Streptococci. Infect Immun. 2007;75:3651–3657. doi: 10.1128/IAI.00038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, McClure MJ, Zhu H, Xie G, Lei B. The two-component regulatory system VicRK is important to virulence of Streptococcus equi subspecies equi. Open Microbiol J. 2008;2:94–99. doi: 10.2174/1874285800802010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnisi SM, Louw ME, Theron J. Cloning and characterization of a carboxylesterase from Bacillus coagulans 81-11. Curr Microbiol. 2005;50:196–201. doi: 10.1007/s00284-004-4423-3. [DOI] [PubMed] [Google Scholar]
- Okazaki H, Igarashi M, Nishi M, Tajima M, Sekiya M, Okazaki S, Yahagi N, Ohashi K, Tsukamoto K, Amemiya-Kudo M, Matsuzaka T, Shimano H, Yamada N, Aoki J, Morikawa R, Takanezawa Y, Arai H, Nagai R, Kadowaki T, Osuga J, Ishibashi S. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes. 2006;55:2091–2097. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]
- Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S, Harel M, Remington SJ, Silman I. The α/β hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Stock AH, Uriel J, Grabar P. Esterase in extracellular concentrates of group A streptococci and the homologous antibody. Nature. 1961;192:434–435. doi: 10.1038/192434a0. [DOI] [PubMed] [Google Scholar]
- Whitaker JR. Principles of enzymology for the food sciences. Marcel Dekker, Inc.; New York, NY: 1972. pp. 481–501. [Google Scholar]