Abstract
Objective
Brainstem gliomas are common in children and have the worst prognosis of any brain tumor in children. On the other hand, brainstem gliomas are rare in adult, and clinical studies have suggested different biological behavior between young and adult. The present study was designed to develop an orthotropic C6 brainstem glioma model in young and adult rats, and to investigate the tumor biological behavior in the two age groups.
Methods
C6 glioma cells were stereotactically implanted into the pons of young or adult male rats. Neurological presentation and survival time were recorded. Tumor proliferation and apoptosis in brainstem gliomas of young and adult rats were determined by immunohistochemical staining of Ki-67 and TUNEL assay, respectively.
Results
Striking difference were found between young and adult brainstem glioma in the onset of neurological signs, duration of symptoms, survival time, tumor growth pattern, as well as tumor proliferation and apoptosis. Relatively focal tumors were observed in adult rats harboring brainstem glioma, while diffusive tumors were found in young rats. Furthermore, brainstem gliomas in adult rats were less proliferative and had more apoptosis than those in young rats.
Conclusion
The present study demonstrated that C6 brainstem glioma model in young and adult rats closely imitates human brainstem glioma in neurological findings and histopathology. Our findings suggest that the different growth pattern and invasiveness of brainstem glioma between children and adult could be due to the different host cellular environments between the two age groups, hence, warrant further investigation of the different host-response between childhood and adult brainstem glioma in human.
Keywords: Brainstem, glioma, progression, animal model
INTRODUCTION
Brainstem gliomas compose up to 20% of total childhood brain tumors, of which 80% are diffuse intrinsic brainstem gliomas and has the worst prognosis of any brain tumor in children [15,19,22,23,31]. On the other hand, brainstem gliomas in adults are much less common, accounting for less than 2% of gliomas [20,29]. Increasing evidence indicates that adult brainstem gliomas are different from the childhood subtypes. Overall, brainstem gliomas in adults are less aggressive than those in children, which in part results in the much better prognosis compared with children [20]. Median survival of adults with intrinsic low-grade brainstem gliomas is 7.3 years, which is similar to patients with low-grade supratentorial gliomas. While, the median survival of children with intrinsic brainstem glioma is less than 1 year after diagnosis [22].
Different classification of brainstem gliomas have been made by site, imaging, and pathology. By location, brainstem gliomas have been subclassified into midbrain glioma, tectal glioma, pontine glioma, medullary glioma, and cervicomedullary gliomas. By neuroimaging, brainstem gliomas have been categorized into diffuse glioma, focal glioma, pencil glioma, dorsal exophytic brainstem glioma, and cystic glioma. By histopathology, brainstem gliomas have been divided into low grade (WHO Grades1 and 2) and high grade (WHO Grades 3 and 4) gliomas. More recently, brainstem gliomas have been separated into two predominant classes as diffusely infiltrative brainstem gliomas known for their relentless growth and bleak outcome, and focal discrete brainstem gliomas normally associate with a favorable prognosis [17,19,23,28,32]. For diffuse brainstem tumor, surgery has been limited because even partial resection may lead to severe functional deficits. So far chemotherapy has not shown any benefit, therefore, radiation remains the standard treatment for brainstem tumor. Diffuse brainstem tumor has 2-year survival rates of 10-15%. On the other hand, patients harboring focal brainstem tumors may benefit from surgery. Such lesions can undergo radical surgery with a good outcome and prognosis [22,38].
Generally, it is considered that low-grade brainstem gliomas have a characteristic growth pattern of growing focally, while, high-grade brainstem gliomas grow diffusely and aggressively [32,37]. However, this is not always the case. Very few histological data is available for diffuse brainstem glioma since biopsy results will not alter its treatment strategies [1,19]. Further, low grade glioma as fibrillary astrocytoma has been found to associate also with diffuse brainstem gliomas [17]. Diffuse brainstem gliomas comprise over 80% of brainstem gliomas in children, while majority of brainstem gliomas in adults are focal glioma [19,22]. It is now appreciated that the genetic abnormalities associated with high grade gliomas in children are different than those in adults, which could contribute to the different growth pattern of brainstem glioma between children and adults. In addition, factors as anatomical, histological, and immunological diversity between children and adults could also contribute to the different biological behavior between diffuse and focal brainstem glioma. The present study was designed to investigate the different biological behavior of brainstem gliomas between children and adults using a rat orthotropic C6 cell brainstem glioma model.
MATERIALS AND METHODS
Experimental Animals
Sprague-Dawley young male rats (3 weeks, body weight 40g to 50g with a mean of 45.6g) and the same strain adult male rats (10 weeks, body weight 325g to 350g with of mean of 337g) were purchased from Charles Rivers, and maintained in a vivarium in 48 × 26.7 × 20 cm polycarbonate cages (2 rats per cage). The colony room was maintained at 23 ± 1°C under a 12-hour light-dark cycle beginning at 1800 h. All rats had access to food and water, ad libitum. All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Cell culture and surgical procedures for brainstem glioma model
The C6 cell line, originally cloned from an N-nitrosomethylurea transformed rat astrocyte cell line, was obtained from American Type Culture Collection (ATCC, Manassas, VA.) [3]. The C6 cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) and 100 μg/ml of penicillin and streptomycin at 37°C with 5% CO2 in a humidified incubator. The cells were prepared for injection using the standard cell preparation protocol.
The brainstem glioma model was modified from the one as previously described [27,40]. The animals were anesthetized with ketamine (40mg/Kg) in combination with xylazine (10mg/Kg) administered intraperitoneally. With the head secured in a stereotactic frame, a midline incision was made and lambda was identified. A burrhole (1 mm in diameter) was drilled at the coordinates of 0.5 mm right and 1 mm posterior to lambda. C6 cells were trypsinized, washed twice, resuspended and diluted in DMEM (non FBS) to a concentration of 2.0 × 105 /15 μl. Using a Hamilton syringe with 27-gauge needle (Hamilton Co., Reno, NV), C6 cells (2.0 × 105 in 15 μl) were then slowly implanted into pons (10 mm depth from the dura mater in adult rats and 7 mm depth in young rats). To avoid back flow of the cells, the injection was made over a 5-minute period, after which the syringe was left in site for 5 minutes, and then slowly withdrawn. The scalp was closed with 4.0 vicryl sutures in a standard fashion. The rats were returned to animal facility after recovery from anesthesia. Each rat was weighed every other day in the first two weeks and every day after, and was evaluated for neurological deficits started at 1 week after the implantation.
Neurological function assessment
Abnormal behaviors and neurological signs associated with brainstem tumor were observed everyday started at 1 week after C6 cells implantation, and onset of paresis was recorded. A modified rotarod test was used to assess coordinated motor function [21]. The accelerating rotarod is a motor-driven treadmill (Omnitech Electronics, Omnirotor treadmill, Columbus, OH) used to measure running coordination. The treadmill was set to accelerate gradually from 0 to 75 rpm for each trial. The starting speed was 0 rpm, and the total time of each trial was 150 seconds. The rotor consisted of a nylon cylinder with a 7-cm in diameter mounted horizontally. The cylinder rotated (by means of a microprocessor-controlled motor) with an acceleration of 0.5 rpm per second. In a given trial, four rats were placed on the cylinder, one rat in each compartment. The cylinder was made to rotate and a timer switch was simultaneously activated. Acceleration continued until either a speed of 75 rpm was reached or the last animal was unable to perform the running response and had fallen to the padded surface. When rat landed on the surface, a photosensitive switch was tripped, and the timer for that compartment stopped. The elapsed time was recorded in tenths of a second as the measure of performance on each trial. The rotarod test was performed started at 1 week after implantation of C6 cells. Rats received two sessions daily. Each session consisted of four trials with an interval of 10 min between successive trials and a minimum of 2 hr between the two successive daily sessions.
Tissue preparation and histological studies
Immediately after death, the animals were perfused transcardially with 0.9% saline, followed by 4% formalin. The brains were harvested and postfixed in 4% formalin. The hindbrain was embedded in paraffin and 7μm coronal sections were cut by a microtome (Leica, Bannockbum). The slides were then stained with hematoxylin and eosin. The presence of tumor and the pattern of infiltration were assessed.
For immunohistochemistry, the slides were deparaffinized, rehydrated, and boiled in 10mM citrate buffer (pH 6.0) for 10 min to expose the antigen epitope. Nonspecific binding sites were blocked by incubation of the slices with 10% normal goat serum in PBS-Tween (0.2%) for one hour at room temperature. The sections were incubated with primary antibody (rabbit anti-Ki-67 at 1:50 dilution, ABcam Inc) at 4°C overnight. Then the sections were washed with PBS and incubated with a secondary antibody at room temperature for 1 hour. After three times washing with PBS, a drop of Anti-Fade solution with DAPI was added, glass coverslips were mounted, and then the slides were viewed under a fluorescent microscope. For proliferation index, Ki-67 positive cells in brainstem gliomas of young and adult rat were counted, the percentage of Ki-67 positive cells in 1×103 tumor cells were calculated and statistically compared.
Fluorometric TUNEL assay
The terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay was used to assess apoptosis of tumor cells by examining DNA fragmentation. Briefly, the slides were deparaffinized, rehydrated, fixed with 4% methanol-free formaldehyde solution in PBS, and incubated with 100μl of 20μg/ml proteinase K for 10 min at room temperature. A second fixation was performed after washing by immersing the slides in 4% methanol-free formaldehyde solution. Slides were washed again with PBS, and fragmented DNA in the apoptotic tumor cells was detected by adding fluorescein 12-dUTP to nicked ends of DNA (DeadEnd Fluorometric TUNEL System, Promega, Madison, WI, USA). Slides were incubated for 1 hr at 37°C, and the reaction was terminated with 2× SSC (Promega). Then a drop of Anti-Fade solution with DAPI was added to the slides, glass coverslips were mounted, and the slides were visualized under a fluorescent microscope, with green fluorescence correlating with DNA fragmentation. The percentage of TUNEL-positive cells was determined and statistically compared.
Statistical Analysis
All data were expressed as mean ± SEM. Kaplan-Meier survival curves for the two groups were generated, and the survival of the two groups was compared by log-rank test. The proliferation-index and TUNEL-positive cells were analyzed by student t test using Prism software (GraphPad Software, San Diego, CA, USA). The differences for each comparison were considered significant at p < 0.05 level.
RESULTS
Progression of neurological function in young and adult rats harboring brainstem gliomas
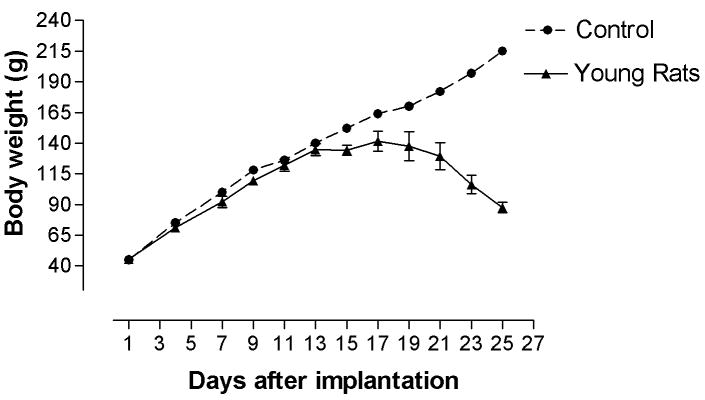
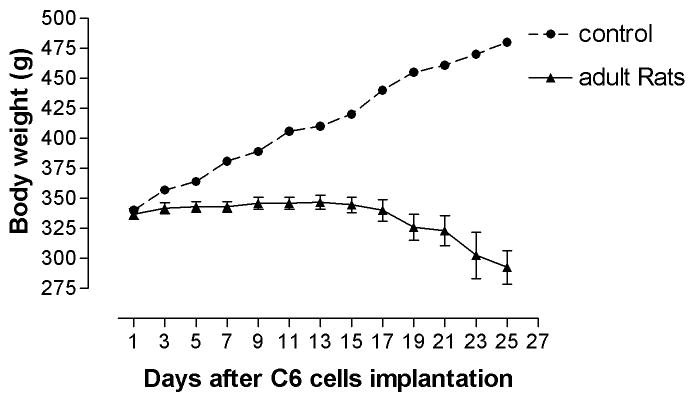
The body weights of young rats continued to increase in the first two weeks after tumor cell implantation, although the increase rate was not as fast as that of age-matched control. As compared with age-matched controls, the body weights of adult rats stopped increasing as soon as tumor cells were implanted. The body weight of young and adult rats began to decrease at 14 and 17 days after glioma implantation, respectively, which is consistent with their rotarod performance. Further, the body weights of young rats with brainstem gliomas decreased more sharply than that of adult in the last week (Figure 1. A, B).
Figure 1. Body weight in young (A) and adult rats (B) after tumor cells implantation.
Body weight of young rats stopped increasing at around 14 days after implantation, and started to decrease at 17 days after implantation. Body weight of adult rats stopped increasing immediately after implantation and began to decrease at 17 days after implantation.
All adult rats presented a focal symptom as left forelimb paresis with a mean onset time at 14 days after implantation. The mild ipsilateral forelimb weakness gradually progressed to severe paralysis usually within 5 to 7 days. On the other hand, all young rats presented with alternative symptom of ataxia, multiple cranial nerve deficits, urinary and fecal incontinence, with no evident focal neurological symptoms or signs in the early stage. All symptoms were observed at the late stage (18 days after implantation) and deteriorated rapidly within 2 to 3 days, indicating rapid tumor proliferation.
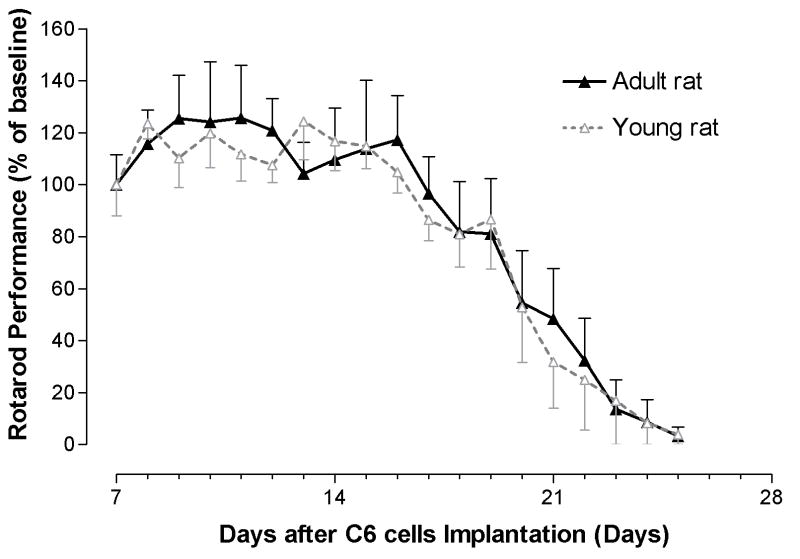
The latency of each rat fall from rotarod apparatus began to stabilize after a brief process of learning. Adult rats began to show diminished performance by day 17, whereas young rats began to show diminished performance by day 14. The latency to fall in both young and adult rats gradually decreased thereafter (Figure. 2).
Figure 2. Rotarod performance in young and adult rats harboring brainstem gliomas.
Young rats began to show impairment at 14 days after implantation, whereas, adult rats began at 17 days. The rotarod performance of both young and rats gradually decreased over time.
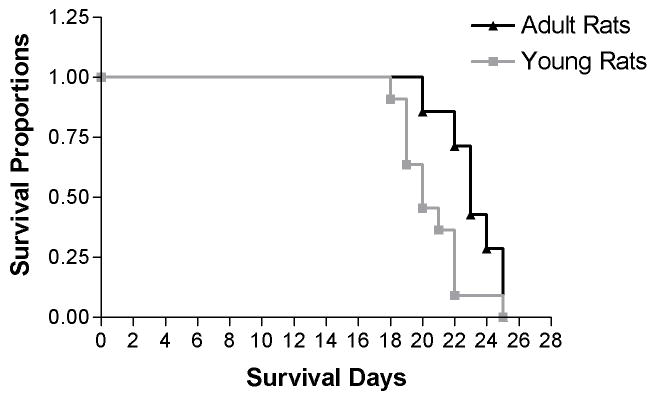
Kaplan-Meier survival analysis suggests a different survival cure between young and adult rats with brainstem gliomas. The median survival time of young and adult rats was 20 and 23 days after glioma implantation, respectively. A statistically significant difference was noted between the median survival times between the two groups (Figure. 3).
Figure 3. Kaplan-Meier survival curves of young and adult rats harboring brainstem gliomas.
The log-rank test indicates a significant difference between survival cure of the two groups (p<0.05). The median survival time of young and adult rats was 20 and 23 days, respectively.
Histopathology of brainstem gliomas in young and adult rats
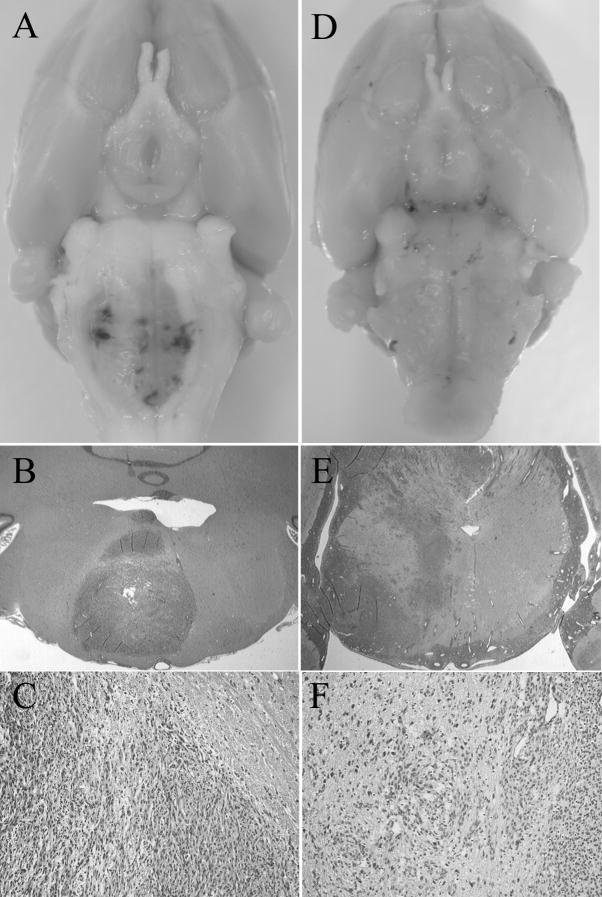
Brainstem gliomas were observed in all young and adult rats implanted with C6 under gross anatomy. As shown in Figure 4A, relatively focal lesions were evident in all adult rats harboring brainstem tumors. In all young rats harboring brainstem tumors, whereas, no focal lesion but enlargement of entire brainstem was observed (Figure. 4D). Intratumoral hemorrhage was found in 28.6% (2/7) of adult rats and 60% (6/10) of young rats. All adult rats and 60% (6/10) of young rats presented with obstructive hydrocephalus. Tumor location, boundary, incidence of intratumoral hemorrhage, and presentation of hydrocephalus in young and adult rats were summarized in Table 1.
Figure 4. Representative photographs and microscopies of brainstem gliomas in adult (A, B, and C) and young (D, E, and F) rats.
A. Depicted is the relatively focal lesion with a displacement of long tract in an adult rat harboring brainstem tumor. B and C: Hematoxylin and eosin stain of brainstem cross section of an adult rat revealed highly cellular lesions invading the white matter with a relatively clear boundary. Abundant necrosis was consistently observed (B, magnification ×1; C, magnification ×10). D. Depicted is the enlargement of the entire brainstem without focal lesion in a young rat harboring brainstem tumor. E and F: Hematoxylin and eosin stain of brainstem cross section of a young rat revealed more infiltrative and diffuse lesion, with a high degree of brainstem parenchyma invasion. Endothelial proliferation was evident and angiogenesis were frequently observed (E, magnification ×1; F, magnification, ×10).
Table 1.
Tumor location, boundary, incidence of hemorrhage, and hydrocephalus in adult and young rats harboring brainstem gliomas.
| Adult Rats (n.) | Young Rats (n.) | ||
|---|---|---|---|
| Tumor Location | Midbrain-Pons | 2 (7) | 3 (10) |
| Pons-Medulla | 5 (7) | 7 (10) | |
| Tumor Boundary | 7 (7) | 0 (10) | |
| Intratumoral Hemorrhage | 2 (7) | 6 (10) | |
| Hydrocephalus | 7 (7) | 6 (10) | |
| Brainstem Distortion | 0 (7) | 10 (10) | |
The histological examination of adult rats revealed relatively demarcated lesions invading white matter. Abundant necrosis surrounded by palisading nuclei was consistently observed in the center of tumors. Endothelial proliferation was scarce with limited angiogenesis (Figure 4B, C). While, brainstem gliomas in young rats revealed more invasive, infiltrative, and diffusive lesions. Endothelial proliferation was evident and alternating areas of angiogenesis were frequently observed (Figure 4E, F).
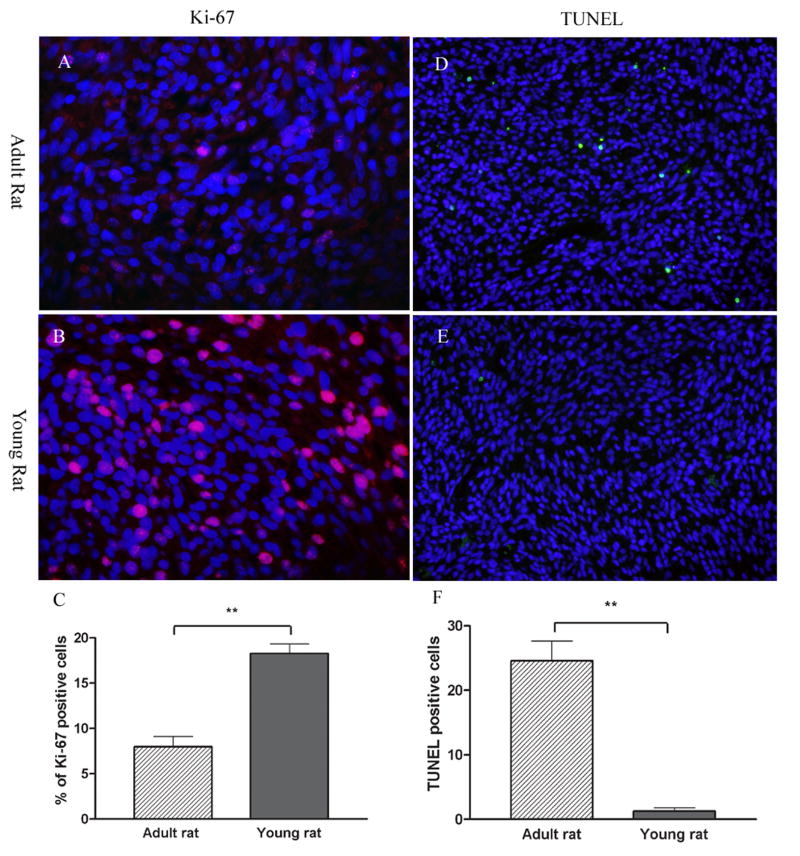
Ki-67 expression was used to evaluate the degree of tumor proliferation in young and adult rats. As shown in figure 5A, B, C, the proliferation-index of brainstem gliomas in young rats, calculated from the percentage Ki-67 positive cells of 1×103 tumor cells (DAPI), was significantly higher than that of adult rats (P<0.001). TUNEL assay was performed to further assess apoptosis of tumor cell in young and adult rats with brainstem gliomas. As depicted in Figure 5D, a number of tumor cells were going through apoptosis in adult brainstem glioma. On the other hand, very few apoptosis was found in brainstem gliomas of young rats (Figure 5E, F. P<0.001).
Figure 5. Tumor proliferation and apoptosis in adult (A, B and C) and young (D, E and F) brainstem gliomas.
Tumor cell proliferation was determined by immunohistochemistry of Ki-67 protein in red (A, B). Nuclei were counter stained by DAPI in blue. Ki-67 index of brainstem gliomas in young rats were significant higher than that of adult rats (C). Apoptosis was determined by TUNEL staining in green (D, E). Nuclei were counter stained by DAPI in blue. Few TUNEL positive cells were observed in the brainstem gliomas of young rats. While, a number of tumor cell underwent apoptosis in adult rats (F). Significant differences between the two groups were indicated by ** P<0.001.
Discussion
Brainstem gliomas were used to be regarded as a single pathological entity as malignant tumors since their location in itself renders them inoperable [23]. As the development of modern technologies in neuroimage and micro-neurosurgery, favorable surgical outcomes for certain types of brainstem gliomas have been reported [2,11,13,38]. However, surgical intervention has only been beneficial for focal brainstem gliomas. Little progress has been made in increasing the survival rates of patients with diffuse brainstem gliomas, which comprise over 80% of brainstem gliomas in children [6,36]. There has been significant progress in understanding the molecular pathogenesis of glioma, hence leading to discovery of novel therapy for malignant glioma. Temozolomide, which has been proven to be effective for adult cerebral glioblastoma multiform, did not alter the poor prognosis associated with newly diagnosed diffuse brainstem glioma in children when combined with radiotherapy [7]. No effective therapeutic intervention available for diffuse brainstem glioma, our understanding of the biological behavior of brainstem gliomas and the impact of hosts on the progression and invasion of brainstem glioma has been lagged behind some other tumors, partly because of no appropriate model available [39]. Currently, diffuse brainstem gliomas have the worst prognosis of any brain tumor in children [16]. The development of a satisfactory model for brainstem glioma is critical for understanding the biological behavior of this tumor and discovery of novel therapies.
The C6 orthotropic glioma model is simple, reliable, and easily reproducible, which has been extensively used to study growth kinetics, mechanisms of angiogenesis, as well as the expression of growth factors and receptors involved in the progression of astrocytic tumor [10,26]. Many studies indicate that the C6 glioma model best mimics the cell biological stages of early glioma progression in human. Jallo et al. have recently reported an experimental brainstem tumor model in neonatal rat pups and adult rats using 9L and F98 tumor cell suspensions [24,25]. In the present study, we modified the model by changing the coordinates from 1.4 mm right and 1.0 mm anterior to lambda to 0.5 mm right and 1 mm posterior to lambda in order to ensure that the lesion would be mainly in pons. To investigate the difference between childhood and adult brainstem glioma, we used 3 weeks old young rats and 10 weeks old adult rats. C6 glioma grew in all rats with a consistent location.
It has been suggested that the duration of preoperative history, as well as prognosis, is influenced by patient age, tumor localization, growth pattern, and histopathology [20] [29]. Consistently, adult rats had more focal neurological symptoms with a longer duration than that of young rats, suggesting that C6 cells in young brainstem progresses more aggressively than in adult brainstem. In pediatric patients with brainstem glioma, the median duration of history was 6 months, whereas, adult patients had a median history of 17 months [17]. In present study, we found that adult rats harboring brainstem tumor survived longer than young rats, even though the flexibility of brainstem and skull structure in young rats made the distortion of medulla and spinal cord junction possible, which could potentially extend the survival time.
The difference of biological behavior between young and adults brainstem gliomas was also indicated by histopathology. In young rats, brainstem gliomas demonstrated a high degree of brainstem parenchyma invasion. In contrast, brainstem tumors in adult rats grew relatively focally and displaced long tract of brainstem. Clinically, many of intrinsic focal brainstem glioma tumors have an area of gliotic tissue as focal gliomas tend to displace rather than infiltrate neural elements, which provide a safety margin enabling aggressive resection [12,14]. Our findings confirmed this characteristic of focal brainstem glioma in histopathology, and suggested that anatomical, histological, or immunological diversity of brainstems between young and adult may affect the growth pattern and invasiveness of brainstem glioma.
Although intratumoral hemorrhage is uncommon, symptomatic intratumoral hemorrhage may occur in nearly 20% of children after the diagnosis of diffuse brainstem glioma. The striking association between intratumoral hemorrhage and necrotic tumor areas suggests that bleeding is more likely in the areas in which the blood-brain barrier is disrupted [8]. In the present study, the intratumoral hemorrhage was more common in young rats than that in adult rats, indicating that this phenomenon is associated with the inherent biologic characteristics of this type of tumor.
Tumor cell invasion is defined as translocation of neoplastic cells through host cellular and extracellular matrix barriers [9]. Invading glioma cells seem to follow distinct anatomic structures within the central nervous system. Tumor cell dissemination may occur along structures such as basement membranes of blood vessels or glial external limitans, which contain extracellular matrix proteins [4]. Occasionally, invasive glioma cells are also found to migrate along myelinated fiber tracts of white matter. This behavior is most likely a consequence of constitutive extracellular ligands expression along the pathways of preferred dissemination, for glioma cells may be able to use a multiplicity of matrix ligands as activating separate mechanisms for invasion. Our findings indicate that glioma cells in young rat brainstem may have or create a more permissive cellular environment for tumor to spread into the adjacent brain tissue.
The ability of tumor progression is determined both by the rate of cell proliferation and the rate of cell death, at which apoptosis represents a major source. Ki-67 antigen is a prototypic cell cycle related nuclear protein, expressed by proliferating cells in all phases of active cell cycle. Ki-67 is routinely used as a proliferation marker in brain tumors. In astrocytomas, Ki-67 expression is up-regulated and correlates with tumor grade and clinical prognosis [33]. We found that tumors in young rats had a higher proliferation rate than those in adult rats. Further, few tumor cells underwent apoptosis in young rats harboring brainstem gliomas, while, a fair amount of apoptotic tumor cells were found in brainstem glioma of adult rats. Therefore, the high proliferative rate and low apoptosis could contribute to the faster tumor progression and more aggressive biological behavior in young brainstem gliomas.
The present study was designed to reproduce the major characteristics of human brainstem glioma in a rat orthotropic C6 cell brainstem glioma model. Our findings demonstrated that this model simulates the major differences between young and adult brainstem glioma of human in the duration of symptoms, tumor growth pattern, and prognosis. However, there are limitations for the current study. The C6 glioma cell line was derived from nitrosomethylurea-induced brain tumor in outbred rats, and intracranial growth of C6 cells results in the formation of morphologically glioblastoma after injection of cells in Wistar and Sprague-Dawley rats [18]. We could not rule out the role of immune response in this model as C6 cells are not syngeneic in outbred rats [5,30]. In fact, immunogenicity has also been found in syngeneic glioma models, such as 9L cells in Fisher 344 rats and Gl261 cells in C57BL/6 mice [34,35]. Nonetheless, our findings warrant further investigation of the different host-response between childhood and adult brainstem glioma in human, including angiogenesis, extracellular matrix, and reactive gliosis.
CONCLUSIONS
In conclusion, our modified brainstem tumor model in young and adult rat imitates clinical brainstem glioma in neurological findings and histopathology. Brainstem glioma in young rats is different from those in adult rats in the onset of neurological signs, duration of symptoms, survival time, tumor growth pattern, as well as tumor infiltration, proliferation, and apoptosis. Our study indicates that the growth pattern and invasiveness of brainstem glioma could depend not only on the malignancy of tumor cell, but also the host cellular environment. The different biological behavior of brainstem glioma in young and adult could attribute to the interaction of tumor cells and the host cellular environment. As no model currently available simulates human gliomas exactly, our findings in this rat brainstem glioma model need to be verified in future clinical studies. In addition, the establishment of this brainstem glioma model in young and adult rats could facilitate future studies for discovery of novel therapeutic paradigms for the treatment of diffuse brainstem glioma.
References
- 1.Allen J, Siffert J, Donahue B, Nirenberg A, Jakacki R, Robertson P, et al. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999;86:1064–1069. doi: 10.1002/(sici)1097-0142(19990915)86:6<1064::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Alvisi C, Cerisoli M, Maccheroni ME. Long-term results of surgically treated brainstem gliomas. Acta Neurochir (Wien) 1985;76:12–17. doi: 10.1007/BF01403823. [DOI] [PubMed] [Google Scholar]
- 3.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 4.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Beutler AS, Banck MS, Wedekind D, Hedrich HJ. Tumor gene therapy made easy: allogeneic major histocompatibility complex in the C6 rat glioma model. Hum Gene Ther. 1999;10:95–101. doi: 10.1089/10430349950019228. [DOI] [PubMed] [Google Scholar]
- 6.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9:197–206. doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 7.Broniscer A, Iacono L, Chintagumpala M, Fouladi M, Wallace D, Bowers DC, et al. Role of temozolomide after radiotherapy for newly diagnosed diffuse brainstem glioma in children: results of a multiinstitutional study (SJHG-98) Cancer. 2005;103:133–139. doi: 10.1002/cncr.20741. [DOI] [PubMed] [Google Scholar]
- 8.Broniscer A, Laningham FH, Kocak M, Krasin MJ, Fouladi M, Merchant TE, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer. 2006;106:1364–1371. doi: 10.1002/cncr.21749. [DOI] [PubMed] [Google Scholar]
- 9.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 10.Engelhard HH, Duncan HA, Kim S, Criswell PS, Van Eldik L. Therapeutic effects of sodium butyrate on glioma cells in vitro and in the rat C6 glioma model. Neurosurgery. 2001;48:616–624. doi: 10.1097/00006123-200103000-00035. discussion 624-615. [DOI] [PubMed] [Google Scholar]
- 11.Epstein F, McCleary EL. Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg. 1986;64:11–15. doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- 12.Epstein F, Wisoff J. Intra-axial tumors of the cervicomedullary junction. J Neurosurg. 1987;67:483–487. doi: 10.3171/jns.1987.67.4.0483. [DOI] [PubMed] [Google Scholar]
- 13.Epstein F, Wisoff JH. Intrinsic brainstem tumors in childhood: surgical indications. J Neurooncol. 1988;6:309–317. doi: 10.1007/BF00177425. [DOI] [PubMed] [Google Scholar]
- 14.Epstein F, Wisoff JH. Surgical management of brain stem tumors of childhood and adolescence. Neurosurg Clin N Am. 1990;1:111–121. [PubMed] [Google Scholar]
- 15.Farmer JP, Montes JL, Freeman CR, Meagher-Villemure K, Bond MC, O’Gorman AM. Brainstem Gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34:206–214. doi: 10.1159/000056021. [DOI] [PubMed] [Google Scholar]
- 16.Finlay JL, Zacharoulis S. The treatment of high grade gliomas and diffuse intrinsic pontine tumors of childhood and adolescence: a historical - and futuristic - perspective. J Neurooncol. 2005;75:253–266. doi: 10.1007/s11060-005-6747-7. [DOI] [PubMed] [Google Scholar]
- 17.Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569–1576. doi: 10.1002/1097-0142(20001001)89:7<1569::aid-cncr22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002;310:257–270. doi: 10.1007/s00441-002-0651-7. [DOI] [PubMed] [Google Scholar]
- 19.Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Curr Opin Neurol. 2001;14:711–715. doi: 10.1097/00019052-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 21.Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 22.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 23.Jallo GI, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Childs Nerv Syst. 2004;20:143–153. doi: 10.1007/s00381-003-0870-6. [DOI] [PubMed] [Google Scholar]
- 24.Jallo GI, Penno M, Sukay L, Liu JY, Tyler B, Lee J, et al. Experimental models of brainstem tumors: development of a neonatal rat model. Childs Nerv Syst. 2005;21:399–403. doi: 10.1007/s00381-004-1100-6. [DOI] [PubMed] [Google Scholar]
- 25.Jallo GI, Volkov A, Wong C, Carson BS, Sr, Penno MB. A novel brainstem tumor model: functional and histopathological characterization. Childs Nerv Syst. 2006;22:1519–1525. doi: 10.1007/s00381-006-0174-8. [DOI] [PubMed] [Google Scholar]
- 26.Lampson LA. New animal models to probe brain tumor biology, therapy, and immunotherapy: advantages and remaining concerns. J Neurooncol. 2001;53:275–287. doi: 10.1023/a:1012230113527. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Jallo GI, Guarnieri M, Carson BS, Sr, Penno MB. A novel brainstem tumor model: guide screw technology with functional, radiological, and histopathological characterization. Neurosurg Focus. 2005;18:E11. [PubMed] [Google Scholar]
- 28.Mauffrey C. Paediatric brainstem gliomas: prognostic factors and management. J Clin Neurosci. 2006;13:431–437. doi: 10.1016/j.jocn.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Mursch K, Halatsch ME, Markakis E, Behnke-Mursch J. Intrinsic brainstem tumours in adults: results of microneurosurgical treatment of 16 consecutive patients. Br J Neurosurg. 2005;19:128–136. doi: 10.1080/02688690500145530. [DOI] [PubMed] [Google Scholar]
- 30.Parsa AT, Chakrabarti I, Hurley PT, Chi JH, Hall JS, Kaiser MG, et al. Limitations of the C6/Wistar rat intracerebral glioma model: implications for evaluating immunotherapy. Neurosurgery. 2000;47:993–999. doi: 10.1097/00006123-200010000-00050. discussion 999-1000. [DOI] [PubMed] [Google Scholar]
- 31.Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: where are we today? Pediatr Neurosurg. 2007;43:192–201. doi: 10.1159/000098831. [DOI] [PubMed] [Google Scholar]
- 32.Sandri A, Sardi N, Genitori L, Giordano F, Peretta P, Basso ME, et al. Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome. Experience in a single institution. Childs Nerv Syst. 2006;22:1127–1135. doi: 10.1007/s00381-006-0083-x. [DOI] [PubMed] [Google Scholar]
- 33.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Smilowitz HM, Joel DD, Slatkin DN, Micca PL, Nawrocky MM, Youngs K, et al. Long-term immunological memory in the resistance of rats to transplanted intracerebral 9L gliosarcoma (9LGS) following subcutaneous immunization with 9LGS cells. J Neurooncol. 2000;46:193–203. doi: 10.1023/a:1006488301412. [DOI] [PubMed] [Google Scholar]
- 35.Szatmari T, Lumniczky K, Desaknai S, Trajcevski S, Hidvegi EJ, Hamada H, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner S, Warmuth-Metz M, Emser A, Gnekow AK, Strater R, Rutkowski S, et al. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79:281–287. doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 37.Walker DA, Punt JA, Sokal M. Clinical management of brain stem glioma. Arch Dis Child. 1999;80:558–564. doi: 10.1136/adc.80.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang CC, Zhang JT, Liu AL. Surgical management of brain-stem gliomas: a retrospective analysis of 311 cases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005;27:7–12. [PubMed] [Google Scholar]
- 39.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q, Tyler B, Sukay L, Rhines L, DiMeco F, Clatterbuck RE, et al. Experimental rodent models of brainstem tumors. Vet Pathol. 2002;39:293–299. doi: 10.1354/vp.39-3-293. [DOI] [PubMed] [Google Scholar]