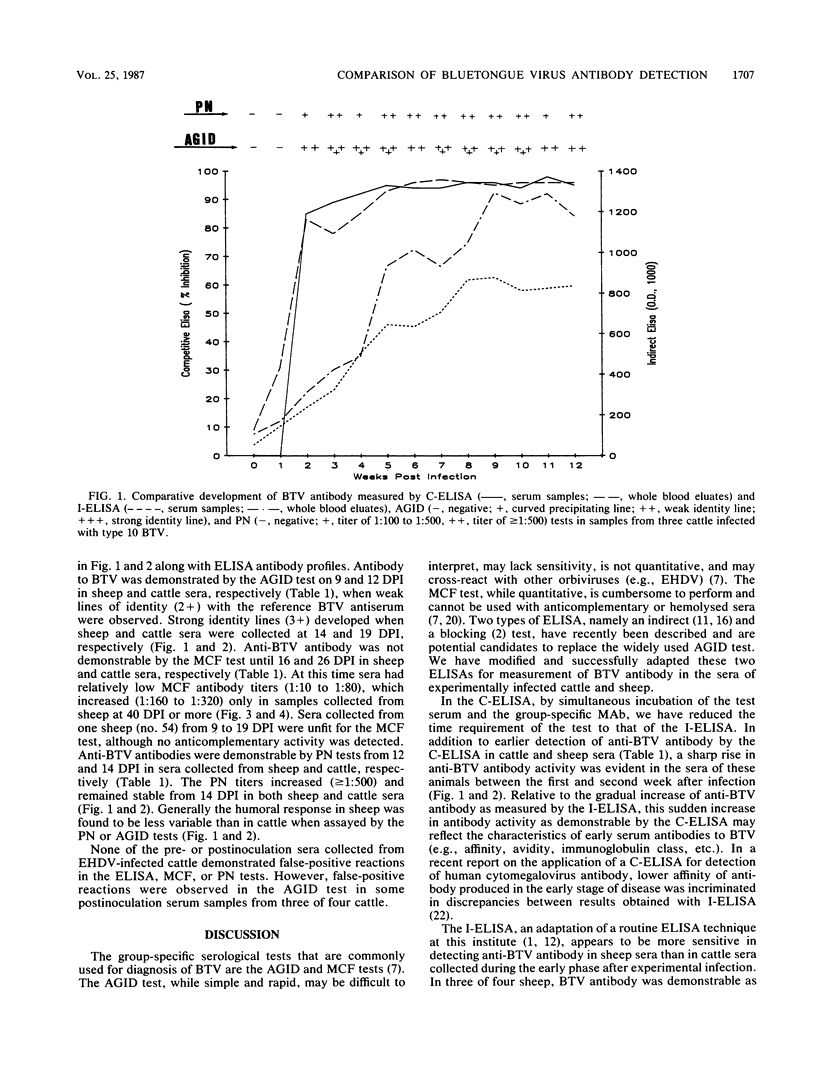
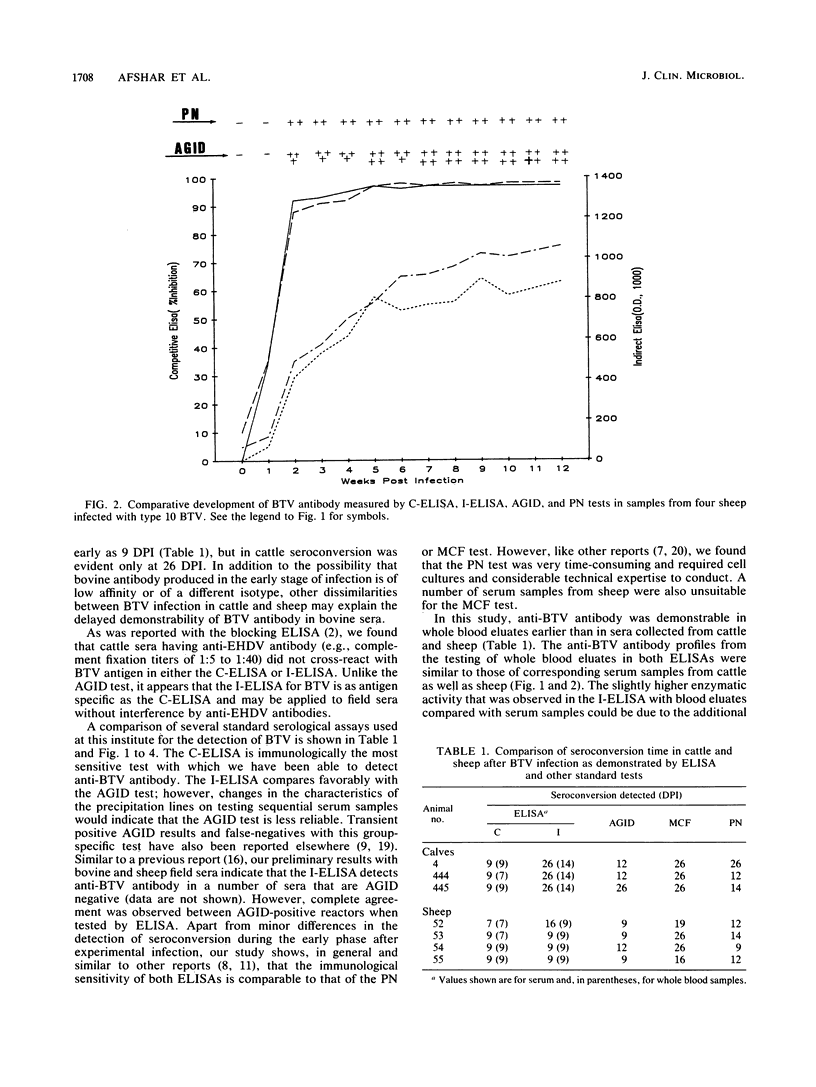
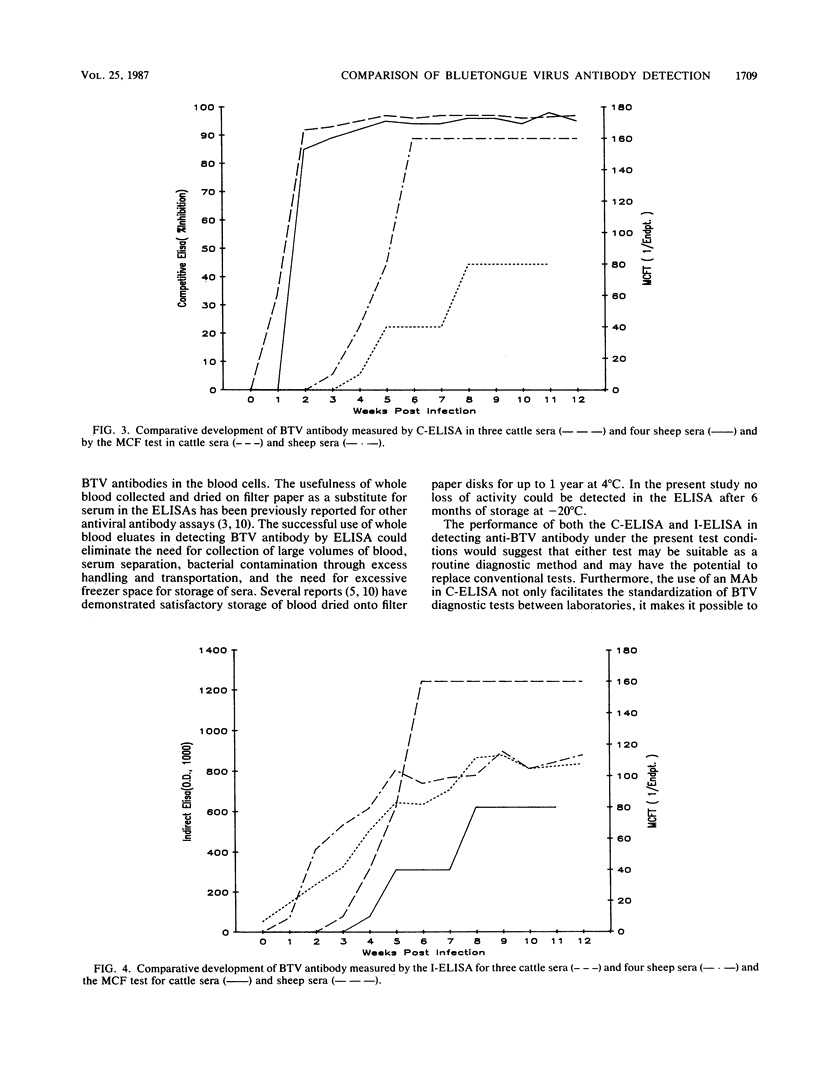
Abstract
An indirect (I) enzyme-linked immunosorbent assay (ELISA) and a competitive (C) ELISA, using a group-specific monoclonal antibody against bluetongue virus (BTV), are described for the detection of antibodies to BTV in cattle and sheep sera. The performance of these assays in detecting anti-BTV antibody in sequential serum samples and eluates from whole blood (WB) dried on filter paper from three calves and four sheep experimentally infected with type 10 BTV was evaluated. The C-ELISA was superior to the I-ELISA in the detection of anti-BTV antibody in the sera and WB samples from both cattle and sheep early after infection with BTV. BTV antibodies were demonstrable by C-ELISA in all the bovine and ovine sera and WB eluates by 9 days postinfection; whereas the I-ELISA results for sheep sera and WB eluates were similar, anti-BTV antibody was not detected in bovine serum and WB eluates until 26 and 14 days postinfection, respectively. While both ELISAs proved reliable, under the present test conditions involving detection of early postinfection reactions of experimentally infected animals, the C-ELISA was always as sensitive or more sensitive than the standard agar gel immunodiffusion test, the modified complement fixation test, and the plaque neutralization tests in the detection of anti-BTV antibodies. Unlike observations with the immunodiffusion test, no reaction was seen between BTV antigen and bovine epizootic hemorrhagic disease virus antiserum in either ELISA. The results suggest that either ELISA may be suitable for routine diagnostic testing and may have the potential to replace other tests for detection of anti-BTV group-specific antibodies and that the C-ELISA may have the most potential.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afshar A., Wright P. F., Dulac G. C. Development and evaluation of an indirect enzyme immunoassay for detection of porcine antibodies to pseudorabies virus. Can J Vet Res. 1986 Jul;50(3):422–426. [PMC free article] [PubMed] [Google Scholar]
- Anderson J. Use of monoclonal antibody in a blocking ELISA to detect group specific antibodies to bluetongue virus. J Immunol Methods. 1984 Nov 16;74(1):139–149. doi: 10.1016/0022-1759(84)90375-2. [DOI] [PubMed] [Google Scholar]
- Banks M. Detection of antibodies to Aujeszky's disease virus in whole blood by Elisadisc. J Virol Methods. 1985 Oct;12(1-2):41–45. doi: 10.1016/0166-0934(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Ruckerbauer G. M., Bannister G. L., Gray D. P., Girard A. Studies on bluetongue. 3. Comparison of two complement-fixation methods. Can J Comp Med Vet Sci. 1967 Jul;31(7):166–170. [PMC free article] [PubMed] [Google Scholar]
- Brugh M., Beard C. W. Collection and processing of blood samples dried on paper for microassay of Newcastle disease virus and avian influenza virus antibodies. Am J Vet Res. 1980 Sep;41(9):1495–1498. [PubMed] [Google Scholar]
- Campbell C. H., Grubman M. J. Current knowledge on the biochemistry and immunology of bluetongue. Prog Vet Microbiol Immunol. 1985;1:58–79. [PubMed] [Google Scholar]
- Della-Porta A. J., Parsonson I. M., McPhee D. A. Problems in the interpretation of diagnostic tests due to cross-reactions between orbiviruses and broad serological responses in animals. Prog Clin Biol Res. 1985;178:445–453. [PubMed] [Google Scholar]
- Hübschle O. J., Lorenz R. J., Matheka H. D. Enzyme-linked immunosorbent assay for detection of bluetongue virus antibodies. Am J Vet Res. 1981 Jan;42(1):61–65. [PubMed] [Google Scholar]
- Jochim M. M. An overview of diagnostics for bluetongue. Prog Clin Biol Res. 1985;178:423–433. [PubMed] [Google Scholar]
- Lana D. P., Marquardt W. W., Snyder D. B. Comparison of whole blood dried on filter paper and serum for measurement of the temporal antibody response to avian infectious bronchitis virus by enzyme-linked immunosorbent assay. Avian Dis. 1983 Jul-Sep;27(3):813–821. [PubMed] [Google Scholar]
- Oberst R. D., Squire K. R., Stott J. L., Chuang R. Y., Osburn B. I. The coexistence of multiple bluetongue virus electropherotypes in individual cattle during natural infection. J Gen Virol. 1985 Sep;66(Pt 9):1901–1909. doi: 10.1099/0022-1317-66-9-1901. [DOI] [PubMed] [Google Scholar]
- Pearson J. E., Carbrey E. A., Gustafson G. A. Bluetongue and related orbivirus diagnosis in the United States. Prog Clin Biol Res. 1985;178:469–475. [PubMed] [Google Scholar]
- Poli G., Stott J., Liu Y. S., Manning J. S. Bluetongue virus: comparative evaluation of enzyme-linked immunosorbent assay, immunodiffusion, and serum neutralization for detection of viral antibodies. J Clin Microbiol. 1982 Jan;15(1):159–162. doi: 10.1128/jcm.15.1.159-162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B. W., Buckley D. J., St Amour G., Lin C. S., Duncan J. R. A computer-interfaced photometer and systematic spacing of duplicates to control within-plate enzyme-immunoassay variation. J Immunol Methods. 1983 Jul 29;61(3):367–375. doi: 10.1016/0022-1759(83)90233-8. [DOI] [PubMed] [Google Scholar]
- Thomas F. C., Girard A., Boulanger P., Ruckerbauer G. A comparison of some serological tests for bluetongue virus infection. Can J Comp Med. 1976 Jul;40(3):291–297. [PMC free article] [PubMed] [Google Scholar]
- Wreghitt T. G., Hicks J., Gray J. J., O'Connor C. Development of a competitive enzyme-linked immunosorbent assay for detecting cytomegalovirus antibody. J Med Virol. 1986 Feb;18(2):119–129. doi: 10.1002/jmv.1890180204. [DOI] [PubMed] [Google Scholar]


