Abstract
Bimodal neurons in the rattlesnake tectum, which receive sensory input from the retina and from the infrared-sensing pit organ, exhibit novel, highly nonlinear cross-modality interactions. Some units respond only to simultaneous bimodal stimulation. Others respond to only one of the two modalities, but show greatly enhanced or depressed responses when stimulated simultaneously in the second modality. These cross-modality interactions may play an important role in recognizing and orienting toward biologically important objects.
The optic tectum (1) is an important integrative center of sensory information. Besides receiving a projection from the retina, the tecta of many species receive somatosensory and auditory inputs (2-4). These are often organized in spatiotopic maps that are, to a degree, in register with the more precise retinotectal map of the visual system (2, 3, 5). The organization of these inputs, along with evidence obtained from behavioral studies (6), suggests that the tectum aids in the control of orientation movements and the spatial shift of attention.
Many tectal neurons receive inputs from two or more sensory modalities. In the mouse, hamster, and rabbit, visualtactile bimodal cells and visual-tactile-auditory trimodal cells have been reported (5, 7). Other studies have described visual-auditory cells in the cat and monkey (4, 8). In most of these investigations, tectal multimodal responses were tested through the use of unimodal stimuli exclusively (9). Interactions between modalities were not studied.
We now report an investigation of cross-modality interactions in tectal neurons of the rattlesnake. The rattlesnake tectum receives a major input from a specialized infrared (IR) sense as well as a normal retinotopically organized visual projection (10, 11). The pit organ of rattlesnakes and other pit vipers is sensitive to IR radiation, and receives a crude IR image of the world with its pinhole-camera optics (12). The IR projection onto the tectum is organized spatiotopically and is roughly in register with the visual tectal map (13).
Hartline et al. (13) showed that many tectal cells of the rattlesnake receive input from both the visual and IR systems. They described two types of multimodal neurons: or units, which are reliably driven by a unimodal stimulus of either modality, and and units, which do not respond well to unimodal stimuli but which are reliably driven by simultaneous visual-IR stimulation. In addition to these two types, we now describe neurons showing other unusual kinds of cross-modality interactions. These cells display highly nonlinear summation characteristics, including cross-modality enhancement and depression, properties that indicate a complexity of multimodal integration not previously described (to our knowledge) in tectal neurons of any species.
We used NaCl-filled micropipettes to record the electrical activity of single units from the exposed tectum of the southern Pacific rattlesnake (Crotalus viridis). During recording, the snakes were lightly anesthetized with Metofane (methoxyflurane). Visual and IR stimuli were rigorously segregated through the use of visible and IR filters and mirrors positioned in front of the contralateral eye and pit organ. Visual stimuli (white spots, 0.1° to 15° in diameter projected onto a rear-projection screen) were flashed on or off or moved at controlled velocities. The IR stimuli (wavelengths > 850 nm, with an unattenuated intensity of 3.3 mW/cm2 at the pit organ) were stationary on flashes of an incandescent bulb ∼ 3° in diameter. Visual and IR stimuli were adjusted to obtain maximal responses for each unit characterized. Stimuli were positioned near receptive field centers in both modalities. The diameter, velocity, and trajectory of visual stimuli were also adjusted for maximal responses; when white spots proved ineffective, bars and black spots and bars were tested.
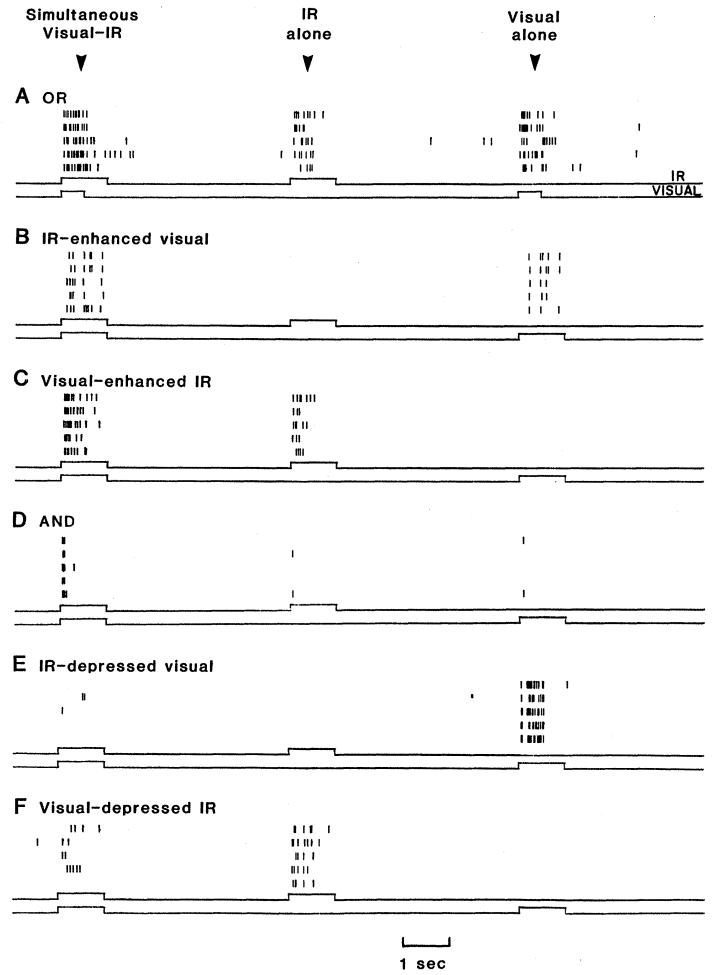
Of the 196 tectal units we characterized, 103 showed some degree of cross-modality interaction. We categorized these 103 units into six groups according to response properties (Table 1 and Fig. 1). (A few units shared properties of two or more groups.)
Table 1.
Classification of 196 rattlesnake tectal neurons
| Neurons | N | Approximate depth beneath tectal surface (μm) |
|---|---|---|
| Bimodal | ||
| or | 25 | 350 to 1300 |
| IR-enhanced visual | 15 | 150 to 800 |
| Visual-enhanced IR | 27 | 500 to 1300 |
| and | 3 | 300 to 1000 |
| IR-depressed visual | 17 | 250 to 700 |
| Visual-depressed IR | 16 | 500 to 900 |
| Unimodal | ||
| Visual | 66 | 50 to 1000 |
| IR | 27 | 400 to 1500 |
Fig. 1.
Raster displays of six units representing the six classes of visual-infrared bimodal neurons of the rattlesnake tectum. Each vertical line represents an action potential (closely spaced lines are not always distinguishable). Five successive 15-second trials are repeated in each raster display. The time courses of the IR and visual stimuli are shown in the upper and lower traces below each display. (A) Per presentation, the OR unit gives a moderate IR response of 6.0 spikes (average of five trials), a visual response of 12.4 spikes, and a visual-IR bimodal response of 17.6 spikes, a 4 percent occlusion. (B) A visual response of 3.8 spikes per presentation is enhanced 63 percent by simultaneous IR stimulation. Unimodal IR stimulation produces no response. (C) An IR response of 5.6 spikes per presentation is enhanced 100 percent by simultaneous visual stimulation. Unimodal visual stimulation gives no response. (D) Both IR and visual unimodal stimuli produce unreliable single spikes, while simultaneous bimodal stimulation produces a brief but strong response of 4.2 spikes per presentation, a 425 percent facilitation. (E) A strong visual response of 15.6 spikes per presentation is depressed 96 percent by simultaneous IR stimulation. (F) An IR response of 6.4 spikes per presentation is depressed 56 percent by simultaneous visual stimulation. IR stimuli: 1 second on flashes. Visual stimuli: (A) 1°, 0.5-second off flash; (B) 10° spot moved at 40° per second for 1 second; (C) 5° spot moved at 40° per second for 1 second; (D) 3°, 1-second on flash; (E) 0.5° spot moved at 20° per second for 1 second; and (F) 15°, 1-second off flash.
The or units responded well to both visual and IR unimodal stimuli and gave combined responses to simultaneous visual-IR stimulation. Some or units displayed greater than linear summation [cross-modality facilitation (14)]; responses (total number of spikes) to simultaneously presented visual-IR stimuli were larger than the sum of the two unimodal stimulus responses. Other or units summed less than linearly [cross-modality occlusion (14)], in extreme cases giving bimodal responses equal to only the greater of the two unimodal responses. Not surprisingly, summation characteristics often varied with stimulus intensity. When visual and IR stimuli were attenuated to produce smaller unimodal responses, simultaneous visual-IR stimulation produced either greater cross-modality facilitation or less cross-modality occlusion.
The IR-enhanced visual and visual-enhanced IR units were reliably driven by only one of the two stimulus modalities (the primary stimulus). The secondary stimulus, in the other modality, was ineffective in driving the unit alone but enhanced the response to the primary stimulus when presented simultaneously with it. The IR-enhanced visual unit illustrated in Fig. 1B, for instance, gave an average response of 3.8 spikes per presentation to unimodal visual stimulation, no response to IR stimulation, and 6.2 spikes per presentation to simultaneous visual-IR stimulation, an enhancement of 63 percent over the unimodal visual response (14).
Enhancement differed widely among different units. Cells with strong responses to unimodal primary stimulation were not enhanced greatly by simultaneous secondary stimulation. When primary responses were reduced by attenuating the primary source, however, the same secondary stimulus became more effective in enhancing the response. Enhancement ranged from ∼ 10 percent to ∼ 300 percent under conditions where the unimodal primary stimulus produced a moderate response.
The and units responded poorly or not at all to visual or IR unimodal stimuli, but responded reliably when stimuli were presented simultaneously. We encountered few of these units. They habituated rapidly to stimuli of either modality, making characterization difficult. and units gave relatively brief but strong responses to bimodal stimulation (Fig. 1D).
The IR-depressed visual units were driven reliably by visual stimulation (the primary stimulus), gave no response to IR stimulation (the secondary stimulus), and showed depressed responses to simultaneously presented visual-IR stimuli. Visual-depressed IR units responded similarly; their IR-evoked responses were depressed by simultaneous visual stimulation.
Depression (14) in these two types of units varied considerably. In some cases the response to primary stimulation was abolished completely by simultaneous presentation of the secondary stimulus. In other cases the primary response was depressed only 5 to 10 percent. In general, the weaker the primary response, the greater was the degree of depression produced by secondary stimulation.
Tectal depths at which the six classes of units are recorded are listed in Table 1. Kass et al. (11), using depth measurements and lesioning techniques in C. viridis, found that visual units (which included our IR-enhanced and IR-depressed visual classes) were located in the stratum fibrosum et griseum superficiale (SFGS) and the superficial region of the stratum griseum centrale (SGC), while IR units (including our visual-enhanced and visual-depressed IR classes) were located in the SGC [nomenclature of Huber and Crosby (15)]. Our depth measurements indicate that IR-enhanced visual and IR-depressed visual cells are located in the superficial SGC and extend into the SFGS, that or and and units are located throughout the SGC, and that visual-enhanced IR and visual-depressed IR units are located in the deep SGC. Unimodal visual units are the only cells found in the superficial SFGS.
The multimodal interactions reported here are equal in complexity to those seen in cortical cells of mammalian species (16, 17). Visual-auditory or, and, and depressed units are seen, for example, in monkey orbital and temporal cortex (16). There are no similar descriptions of multimodal interactions in tectal cells however. Previous reports of multimodal cells in the tectum have described unimodal response properties but have failed to investigate cross-modality interactions. The enhancing and depressing interactions described here are, to our knowledge, the first complex cross-modality interactions to be reported in the tectum of any species.
These interactions could play an important role in tectal function and suggest some forms of neural processing not previously attributed to the tectum. A few examples are sketched below.
Stimulation and ablation experiments (6) indicate that, in many species, the tectum is involved in the control of orienting movements and spatial direction of attention. or units may play a role in such an attentional system since they signal the occurrence of events in particular regions of space, regardless of whether the events are of a visual or IR nature; Hartline et al. (13) have shown that, in the anterior half of the tectum, the visual and IR receptive fields of single rattlesnake or units have similar spatial locations (18). Enhancing neurons might also play a role in an attentional system by priming areas of the tectum for further sensory stimulation. The IR input arising from a portion of the external world, for instance, would make IR-enhanced visual cells more likely to respond to visual stimuli arising from the same area.
Cross-modality interactions could generate multimodal “feature detectors.” For instance, and units are driven reliably only by objects that simultaneously stimulate the visual and IR systems, for example, a warm-blooded, moving animal. On the other hand, IR-depressed visual units respond better to thermoneutral, visual objects. Natural stimuli having different visual-IR characteristics could be distinguished by these bimodal units. Such units could aid in the initiation of behavioral responses appropriate to the stimuli encountered.
Excitatory cross-modality interactions increase the sensitivity of enhancing and or tectal cells to warm visual objects, eliciting responses in these cells under conditions when visual and IR unimodal units might remain silent. These interactions could lead to better localization of dual-modality objects in space at times when visibility is poor.
The use of a unimodal-bimodal stimulus procedure and the rigorous comparison of response magnitudes permitted us to identify subtle multimodality interactions in many units we otherwise would have classified as unimodal. (Only ∼ 10 percent of deep tectal units of the rattlesnake were identified as bimodal by Hartline et al. (12), who used more qualitative methods.) Some visual-auditory and visual-somatosensory tectal neurons in mammals might also show subtle interactions if examined with appropriate techniques. We anticipate that multimodal neurons, having modality combining properties similar to those described here, will be found in the tecta of many species.
Acknowledgments
We thank S. Raymond, E. Gruberg, and J. Gepner for their helpful criticisms of the text. Supported by the Charles A. King Trust, Boston, NIH grants EY 07028 and EY 02491, and NSF grants BNS 7824162 and BNS 7817084.
References and Notes
- 1.We use the term “tectum” in referring to the optic tecta of nonmammalian vertebrates as well as the superior colliculi of mammals.
- 2.Stein BE, Magalhaes-Castro B, Kruger L. J. Neurophysiol. 1976;39:401. doi: 10.1152/jn.1976.39.2.401. [DOI] [PubMed] [Google Scholar]
- 3.Drager UC, Hubel DH. ibid. 1975;38:690. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]; Gruberg ER. thesis. University of Illinois; 1969. [Google Scholar]; Gaither NS, Stein BE. Science. 1979;205:595. doi: 10.1126/science.451623. [DOI] [PubMed] [Google Scholar]; Chalupa LM, Rhoades RW. J. Physiol. (London) 1977;270:595. doi: 10.1113/jphysiol.1977.sp011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon B. J. Neurophysiol. 1973;36:157. doi: 10.1152/jn.1973.36.2.157. [DOI] [PubMed] [Google Scholar]
- 5.Drager UC, Hubel DH. ibid. 1976;39:91. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Schiller PH, Stryker M. ibid. 1972;35:915. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]; Wurtz RH, Goldberg ME. ibid. :587. [Google Scholar]; Stein BE, Goldberg SJ, Clamann HP. Brain Res. 1976;118:469. doi: 10.1016/0006-8993(76)90314-0. [DOI] [PubMed] [Google Scholar]; Keating EG. ibid. 1974;67:538. [Google Scholar]; Sprague JM, Meikle TH., Jr. Exp. Neurol. 1965;11:115. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]; Schneider GE. Science. 1969;163:895. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- 7.Finlay BL, Schneps SE, Wilson KG, Schneider GE. Brain Res. 1978;142:223. doi: 10.1016/0006-8993(78)90632-7. [DOI] [PubMed] [Google Scholar]; Horn G, Hill RM. Exp. Neurol. 1966;14:199. doi: 10.1016/0014-4886(66)90007-0. [DOI] [PubMed] [Google Scholar]
- 8.Wickelgren BG. Science. 1971;173:69. doi: 10.1126/science.173.3991.69. [DOI] [PubMed] [Google Scholar]; Allon N, Wollberg Z. Brain Res. 1978;159:321. doi: 10.1016/0006-8993(78)90538-3. [DOI] [PubMed] [Google Scholar]
- 9.Stein et al. (2) found no somatosensory modulation of visual activity in ten cat tectal neurons tested with bimodal stimuli.Schaefer KP. Brain Behav. Evol. 1970;3:222. doi: 10.1159/000125475.observed inhibition of tectal neurons caused by head movements in the rabbit, andBisti S, Maffei L, Piccolino M. J. Neurophysiol. 1974;37:146. doi: 10.1152/jn.1974.37.1.146.found that body tilt modified visual activity in the monkey tectum.
- 10.Goris RC, Terashima S. J. Exp. Biol. 1973;58:59. doi: 10.1242/jeb.58.1.59. [DOI] [PubMed] [Google Scholar]; Hartline PH. In: Handbook of Sensory Physiology. part 3. Fessard A, editor. Vol. 3. Springer-Verlag; New York: 1974. pp. 297–312. Electroreceptors and Other Specialized Receptors in Lower Vertebrates. [Google Scholar]; Gruberg ER, Kicliter E, Newman EA, Kass L, Hartline PH. J. Comp. Neurol. 1979;188:31. doi: 10.1002/cne.901880104. [DOI] [PubMed] [Google Scholar]; Newman EA, Gruberg ER, Hartline PH. ibid. 1980;191:465. doi: 10.1002/cne.901910309. [DOI] [PubMed] [Google Scholar]
- 11.Kass L, Loop MS, Hartline PH. J. Comp. Neurol. 1978;182:811. doi: 10.1002/cne.901820505. [DOI] [PubMed] [Google Scholar]
- 12.Noble GK, Schmidt A. Proc. Am. Philos. Soc. 1937;77:263. [Google Scholar]; Bullock TH, Diecke FDJ. J. Physiol. (London) 1956;134:47. doi: 10.1113/jphysiol.1956.sp005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartline PH, Kass L, Loop MS. Science. 1978;199:1225. doi: 10.1126/science.628839. [DOI] [PubMed] [Google Scholar]
-
14.We define percent facilitation and occlusion as
where Rvis+IR is the number of spikes evoked by simultaneous visual-IR stimulation, Rvis is the number of spikes evoked by visual stimulation, and RIR is the number of spikes evoked by IR stimulation. The equation represents facilitation if Rvis+IR ≥ Rvis + RIR and occlusion if Rvis+IR ≤ Rvis + RIR. We define percent enhancement and depression as
where Rp+s is the number of spikes evoked by simultaneous primary-secondary stimulation and Rp is the number of spikes produced by unimodal primary stimulation. The equation represents enhancement if Rp+s ≥ Rp and depression if Rp+s ≤ Rp. - 15.Huber GC, Crosby EC. J. Comp. Neurol. 1933;57:57. [Google Scholar]
- 16.Benevento LA, Fallon J, Davis BJ, Rezak M. Exp. Neurol. 1977;57:849. doi: 10.1016/0014-4886(77)90112-1. [DOI] [PubMed] [Google Scholar]
- 17.Loe PR, Benevento LA. Electroencephalogr. Clin. Neurophysiol. 1969;26:395. doi: 10.1016/0013-4694(69)90089-3. [DOI] [PubMed] [Google Scholar]; Robertson RT, et al. J. Neurophysiol. 1975;38:780. doi: 10.1152/jn.1975.38.4.780. [DOI] [PubMed] [Google Scholar]
- 18.The magnification factor of the IR tectal map is ∼×1.3 that of the visual map. The registration of the two maps is good in the anterior half of the tectum, but becomes progressively worse more posteriorly. The or units have visual and IR receptive field locations similar to those predicted from the two maps; the fields are nearly concentric in the anterior tectum and progressively less so at more posterior locations.