Abstract
The β2 subunit of a class Ia or Ib ribonucleotide reductase (RNR) is activated when its carboxylate-bridged Fe2II/II cluster reacts with O2 to oxidize a nearby tyrosine (Y) residue to a stable radical (Y•). During turnover, the Y• in β2 is thought to reversibly oxidize a cysteine (C) in the α2 subunit to a thiyl radical (C•) by a long-distance (~35 Å) proton-coupled electron-transfer (PCET) step. The C• in α2 then initiates reduction of the 2' position of the ribonucleoside-5'-diphosphate substrate by abstracting the hydrogen atom from C3'. The class I RNR from Chlamydia trachomatis (Ct) is the prototype of a newly recognized subclass (Ic), which is characterized by the presence of a phenylalanine (F) residue at the site of β2 where the essential radical-harboring Y is normally found. We recently demonstrated that Ct RNR employs a heterobinuclear MnIV/FeIII cluster for radical initiation. In essence, the MnIV ion of the cluster functionally replaces the Y• of the conventional class I RNR. The Ct β2 protein also auto-activates by reaction of its reduced (MnII/FeII) metal cluster with O2. In this reaction, an unprecedented MnIV/FeIV intermediate accumulates almost stoichiometrically and decays by one-electron reduction of the FeIV site. This reduction is mediated by the near-surface residue, Y222, a residue with no functional counterpart in the well-studied conventional class I RNRs. In this review, we recount the discovery of the novel Mn/Fe redox cofactor in Ct RNR and summarize our current understanding of how it assembles and initiates nucleotide reduction.
Ribonucleotide reductases (RNRs) catalyze the reduction of ribonucleotides to deoxyribonucleotides, the building blocks for DNA. They provide the only de novo pathway for deoxyribonucleotide synthesis and are thus essential to all known life. Their central role in nucleotide metabolism makes them important targets for anticancer and antiviral therapeutics (1). It is thought that the evolution of the first RNR initiated the transition from the use of RNA as the primary information-encoding molecule (in the “RNA world”) to the use of DNA (in the “DNA world”) (2, 3).
The RNR reaction involves replacement by hydrogen of the hydroxyl group on the 2'-carbon of the nucleoside di- or tri-phosphate (NDP or NTP) substrate. This chemically difficult replacement occurs by a free-radical mechanism (4). Evidence suggests that all known RNRs share a common fundamental strategy for catalysis, in which a transient cysteine thiyl radical (C•) in the active site (5–8) initiates the reduction by abstracting the 3'-hydrogen atom (9). Following replacement of the 2'-hydroxyl group by a hydrogen, the H• is returned to C3', regenerating the C•.
Although all RNRs apparently use this catalytic strategy, none possesses a C• in its resting state. Rather, the 3'-H-abstracting radical is produced at the beginning of each turnover and reduced back to the resting cysteine at the end (10–12). RNRs from different organisms have been divided into three classes primarily on the basis of the cofactor and mechanism that each employs for reversible C• production (2, 4). The enzymes from aerobically growing Escherichia coli (Ec), most eukaryotes (including all mammals), and herpes simplex I virus belong to class I. The C•-generating cofactor in each of these RNRs is a stable tyrosyl radical (Y•) in close proximity to a (µ-oxo)-Fe2III/III cluster (12, 13). The cofactor is found in the enzyme's homodimeric β2 subunit. It is generated when the Fe2II/II form of the cluster reacts with O2 to oxidize the Y residue by one electron to the Y• (13) (vide infra). The active holoenzyme is a dissociable 1:1 complex2(14) of the homodimeric β2 subunit and the homodimeric catalytic subunit, α2, which contains the active site for substrate reduction and the binding sites for allosteric effectors (2). To initiate turnover, the Y• in β2 oxidizes the cysteine residue in α2 to the C• by a long-distance, inter-subunit, proton-coupled electron transfer (PCET) reaction (12) (vide infra).
Class I RNRs have been further sub-classified as a–c. The Ia (e.g., from mammals) and Ib (e.g., from Salmonella typhimurium) enzymes both use the FeIII/III-Y• cofactor but differ from each other in primary structure, allosteric regulatory behavior, and preference for the protein source of electrons for nucleotide reduction (3). These differences are functionally less profound than their shared difference from the Ic RNR(s). This third and most recently recognized sub-class, of which the enzyme from the human pathogen, Chlamydia trachomatis (Ct), is the founding member, is defined by the replacement of the radical-harboring Y in the β2 subunit with the redox incompetent F (15, 16). We recently solved the seven-year mystery of how a class I RNR can be active without the initiating Y• by demonstrating that Ct RNR employs a stable, heterobinuclear, MnIV/FeIII cofactor to initiate catalysis (17). Although the novel cofactor and radical-initiation strategy were just discovered, extensive analogy to the more well-characterized class Ia Ec enzyme has served to focus initial studies, so that considerable insight has rapidly emerged and detailed working hypotheses can already be advanced. For proper context, we must briefly summarize what is known about the formation and function of the Ec RNR Fe2III/III-Y• cofactor before reviewing these studies on the novel Ct enzyme.
Activation of Ec β2
In assembly of the Fe2III/III-Y• cofactor, the Fe2II/II cluster reacts with O2 to oxidize the buried tyrosine residue (Y122) by one electron (18, 19). The O2-reactive complex forms spontaneously in vitro upon addition of FeII to apo β2, but it is possible that accessory factors (e.g., an iron chaperone) are involved in vivo. The reduced protein can also form by in situ reduction of the Fe2III/III cluster in the “met” protein (lacking the Y•). Indeed, reactivation of β2 that has lost its Y• occurs by this mechanism in vivo and has been termed the “maintenance pathway” (20). The Fe2S2-containing ferredoxin, YfaE, and the flavodoxin, NrdI, have been identified as proteins that effect the Fe2III/III →Fe2II/II conversion for the class Ia and Ib β2s, respectively (21–23).
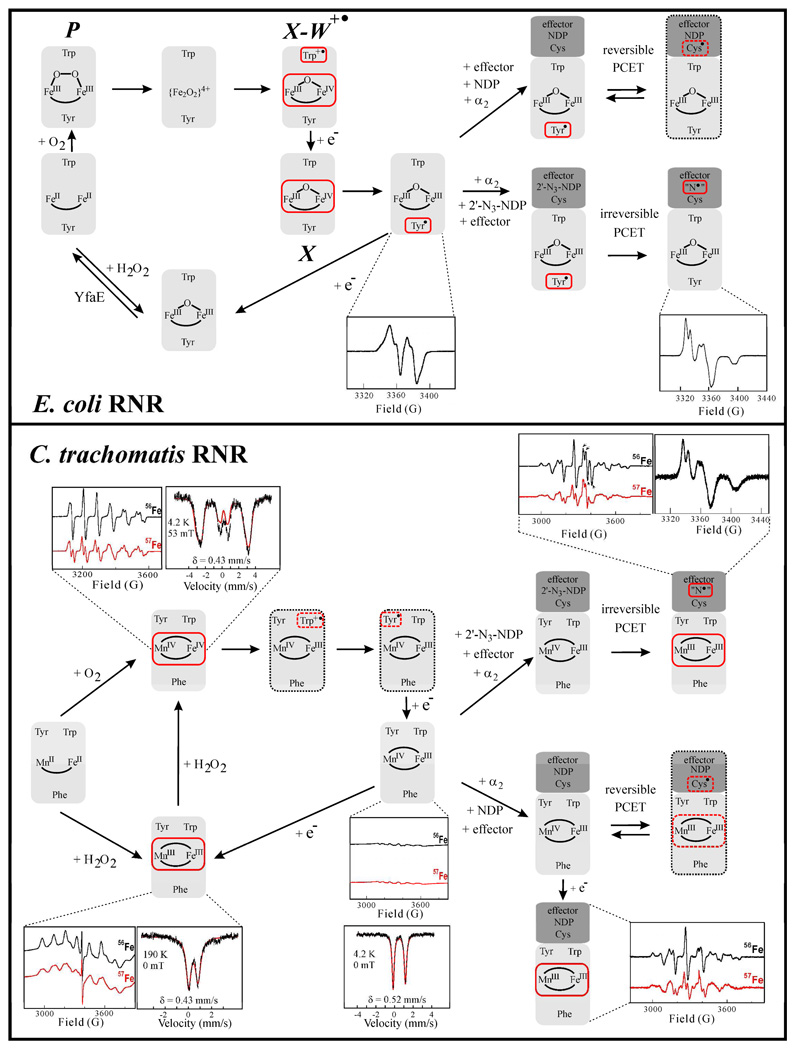
The mechanism of activation of wild-type (wt) Ec β2 was investigated by a combination of rapid kinetic and spectroscopic methods (Figure 1, top) (12). An intermediate, termed X, was shown to be kinetically competent to oxidize Y122 in the last, slowest step of the reaction carried out in the presence of reductant [e.g., ascorbate] (19, 24). X has an S = 1/2 ground state that gives rise to a sharp, nearly isotropic g = 2.0 signal in the X-band EPR spectrum. Mössbauer and 57Fe-ENDOR spectroscopies revealed that the S = 1/2 ground state is a consequence of antiferromagnetic coupling between high-spin FeIII (S = 5/2) and FeIV (S = 2) sites (25). X has been subjected to extensive spectroscopic (X-ray absorption, 1H-, 2H-, and 17O-ENDOR, and MCD) and computational analysis (26–29).
Figure 1.
Assembly, maintenance, and role in catalysis of the Fe2III/III-Y• and MnIV/FeIII cofactors of Ec β2 (top) and Ct β2 (bottom). The red boxes indicate EPR active states. States encircled with a grey dotted line have not been directly detected. Selected EPR and Mössbauer spectra of various states are also shown. The 3'-H-cleaving C• in α2 has not been detected in either enzyme. However, copious indirect evidence, including structural analogy to the class II enzyme (12) in which the C• was directly detected (7), indicates that it forms in the Ec enzyme and by analogy the Ct enzyme. The spectrum of the N• in Ec RNR was adapted from (4).
The complete reduction of O2 at the Fe2 cluster requires four electrons, of which three are provided by the oxidation of the reduced Fe2II/II cofactor to generate the Fe2III/IV intermediate, X. The transfer of the forth (“extra”) electron to the diiron site is mediated by the near-surface residue, W48, which is transiently oxidized to a tryptophan cation radical (W48+•) (30, 31). In vitro, the W48+• is subsequently reduced by an exogenous reductant (e.g., ascorbate, a thiol, or FeIIaq) (31). The in vivo reductant for the W48+• remains unclear, but the ferrodoxin YfaE is a candidate (21).
Intermediates occurring before the X-W48+• state barely accumulate during activation of wt Ec β2 (31, 32) but have been observed in variants of the Ec protein and in the wt β2 from mouse [Mus musculus (Mm)]. A (μ-1,2-peroxo)-Fe2III/III intermediate, which exhibits spectroscopic properties similar to those of the (μ-1,2-peroxo)-Fe2III/III intermediate, Hperoxo (or P), in the reaction of soluble methane monooxygenase (33), was observed in Ec β2 variants with the ligand substitution D84E (34, 35) and, more importantly, in wt Mm β2 (36). Insight into the immediate precursor to the X-W48+• state was provided by studies on the Ec β2 W48A/Y122F variant. 3-Methylindole was used as an analogue of the truncated W48 sidechain to chemically trigger the ET step needed to form X (37). This precursor state comprises at least two distinct antiferromagnetically coupled Fe2III/III clusters, which may be protonated successors to the (μ-1,2-peroxo)-Fe2III/III intermediate.
PCET between α2 and β2
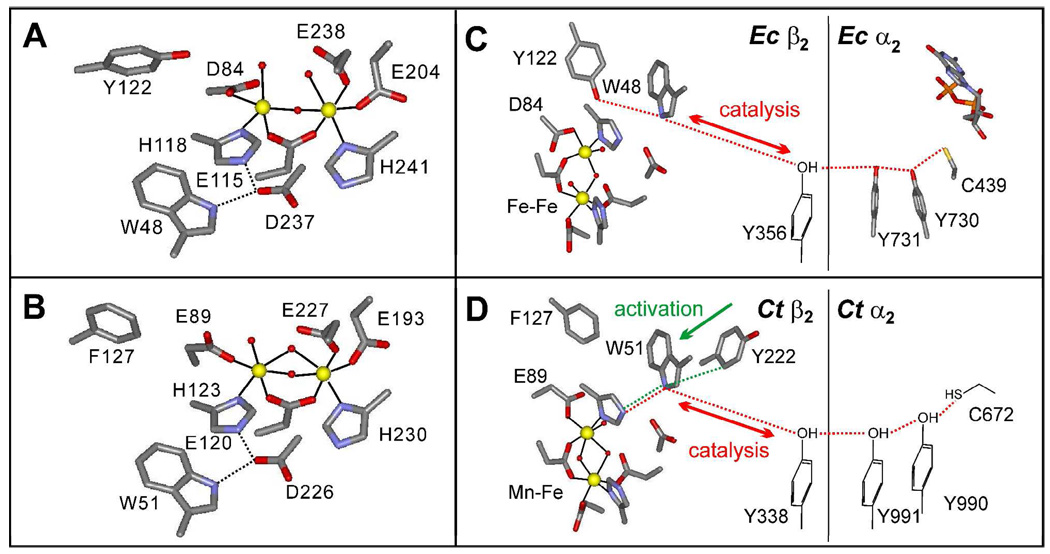
The distance in the Ec RNR holoenzyme between the Y• in β2 and the C•-forming cysteine (C439) in α2 is unknown but is ~ 35 Å in a “docking” model generated from the structures of the individual subunits (8). This model has been validated by pulsed electron-electron double resonance (PELDOR) and double quantum coherence (DQC) measurements (38, 39). The rate constant for ET by a single electron-tunneling step over this long distance would be 10−4 – 10−9 s−1, which is far too slow to account for the single-turnover and steady-state rate constant of ~ 10 s−1 (25 °C) for Ec RNR (12). Rather, the ET is mediated by a chain of conserved, hydrogen-bonded, aromatic amino acids, including W48 and Y356 in Ec β2 and Y731 and Y730 in Ec α2 (Figure 2C), which form transient radicals in an “electron relay” mechanism (12). These residues are conserved in all known class I RNRs, and substitution of any of them by F leads to drastic diminution of activity (40–43). Further, the ET step is believed to be coupled to proton transfer (proton-coupled electron transfer or PCET). In the absence of environmental effects, pure ET (not proton-coupled) from a neutral C to a neutral Y• to give a Y–/C+• state should be unfavorable. However, PCET to give a state with neutral constituents (Y/C•) is expected to be thermodynamically feasible (12). Thus, it is thought that the cysteine loses its proton and the tyrosine obtains a proton in the PCET step.
Figure 2.
Structural models of the Fe2III/III clusters in (A) Ec and (B) Ct β2s (pdb codes 1MXR and 1SYY, respectively). Schematic representations of the proposed PCET pathways in (C) Ec and (D) Ct RNR. (C) and (D) are adapted from (12) and (65), respectively.
Two obstacles long thwarted the direct interrogation of the PCET step. First, the forward PCET to generate the C• is preceded by a slow physical step, which is gated by the binding of a substrate and allosteric effector (PCET is thus said to be “conformationally gated”) (10). Consequently, intermediates are “kinetically masked.” Second, although it is possible that the proposed pathway radical intermediates might still accumulate to low levels, their spectroscopic properties (in particular EPR) are expected to be similar to those of Y122•, potentially preventing their detection. For these reasons, the studies of Ec β2 activation stood for some time as the best evidence for a radical-hopping mechanism for ET to the cofactor. In addition to establishing the role of W48 in relaying the “extra” electron, they also showed that W48+• can (under the proper conditions) oxidize Y122 (30, 31), which would be analogous to the last step in the reverse inter-subunit PCET from Y122 to the C•, and that a rapid redox equilibrium between W48 and Y356 is engaged in the presence of Mg2+ at concentrations similar to the 15 mM employed in the RNR activity assay (44).
Stubbe and co-workers have at last provided definitive evidence for the proposed electron-relay PCET mechanism. First, by replacing the subunit-interfacial pathway tyrosine in the β2 subunit of the Ec enzyme (Y356) with mono- and poly-fluorinated tyrosines, they systematically varied the radical-reduction potential and pKa of this pathway position. The catalytic activities (and their dependence on pH) of these variants established both the narrow range of reduction potential required for functional PCET and that the Y356 phenolic proton is not obligatorily transferred (45). Second, by replacing any of the three pathway tyrosines (Y356 in β2; Y730 and Y731 in α2) with a more easily oxidized analogue, they engineered depressions in the free-energy profile for the radical-hopping process, causing the radical to reside on the unnatural residue upon engagement of the ready holoenzyme complex (45–48). Importantly, the Y730/Y731→3-aminotyrosine (NH2Y) α2s retain significant catalytic activity, and the NH2Y radicals detected in these variants are kinetically competent to be on the catalytic pathway (48).
Discovery of the Y•-less class I (Ic) RNR(s)
McClarty and co-workers isolated and sequenced the genes encoding the subunits of a class I RNR from the pathogenic intracellular parasite, Chlamydia trachomatis (Ct) (15). Comparison of the predicted protein sequences to those of other RNR subunits revealed conservation of all but one of the β2 cofactor ligands and all but one of the α2 and β2 PCET pathways residues (Figure 2). The important exceptions are the replacements of the radical-harboring Y by a redox-inert phenylalanine (F127) and the Fe1 aspartate ligand (D84 in Ec β2) by glutamate (E89 in Ct β2). The crystal structure of Ct β2 confirmed that F127 is located at the site where the radical harboring tyrosine is normally found (16). Despite the absence of the radical-harboring Y, the Ct RNR produced in and isolated from Ec was found to be catalytically active, suggesting a fundamentally different strategy to generate the C• in the α2 subunit (15). The Y→F and D→E non-identities are also found in (hypothetical) β2 proteins from other organisms, including the human pathogens Mycobacterium tuberculosis and Tropheryma whipplei (16). A third sub-class, Ic, was founded to comprise these Y•-less RNRs.
Nordlund, Gräslund, McClarty and co-workers proposed that the Fe2III/IV cluster, X, which they detected in the Ct β2 protein by EPR spectroscopy, functionally replaces the Y• of the conventional class I RNRs (16, 49). In support of this hypothesis, the stability of X was shown to be enhanced by the presence of α2 (49). We recently confirmed an essential aspect of this innovative hypothesis: Ct RNR does indeed use a high-valent metal ion in place of the conventional Y• as radical initiator. However, the Fe2III/IV complex of β2 is not its active form. Rather, Ct RNR employs a MnIV/FeIII cofactor (50) to initiate catalysis (17).
Discovery of the Mn-requirement of class Ic RNRs
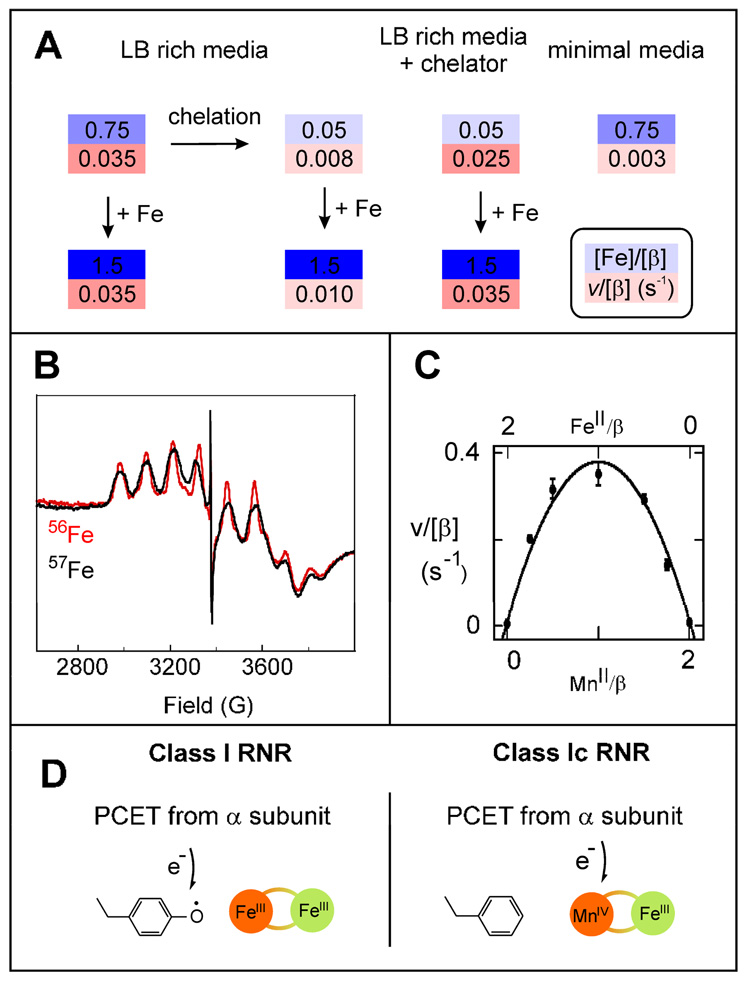
We began our study of Ct RNR by measuring the catalytic activities of preparations of β2 that had been isolated from different media and subjected to different metal-chelation and reconstitution procedures (17). We expected on the basis of the published hypothesis and previous studies on the Ec enzyme (51) to find a tight correlation between activity and iron content (Figure 3A). Ct β2 emerged from the over-producing Ec cells grown in rich medium with 0.75 Fe/β and a turnover number of 0.035 s−1/β, which is less than the value reported by the Gräslund group [0.05 s−1 (49)] and < 1% the optimal activity of the Ec protein (12). Addition of FeII in the presence of O2 to reconstitute these preparations to full cofactor content (theoretically 2 Fe/β) failed to increase activity. A reductive chelation procedure removed almost all the iron (leaving < 0.05/β) and – as expected – diminished activity (to ≤ 0.008 s−1), but subsequent reconstitution of these preparations with excess FeII and O2 increased activity only marginally, if at all (to ≤ 0.010 s−1). Ct β2 purified from cells grown in the presence of the FeII-chelator, 1,10-phenanthroline, had very little iron (< 0.05), but greater than expected activity (0.025 s−1). Addition of excess FeII to this protein in the presence of excess O2 increased its activity only slightly (to ≤ 0.035 s−1). Finally, preparations from cells grown on minimal medium supplemented with iron had the same Fe content (~ 0.75 Fe/β) as protein from rich medium but less than one-tenth its activity (~ 0.003 s−1). The absence of the expected correlation between iron content and enzyme activity suggested the possibility of an additional cofactor component (17).
Figure 3.
(A) Comparison of Fe/β (top, on blue shaded part) and activity/β (s−1) (bottom, on red shaded part) for Ct β2 prepared and treated in different ways. (B) EPR spectra of Ct β2 that was isolated from Ec cells grown on rich medium, depleted of its Fe, reconstituted with 1.5 FeII/β (either natural abundance or >95% enriched 57Fe), dialyzed to remove excess Fe, and reduced with 20 mM dithionite for 2 min at 22 °C. Spectrometer conditions: T = 4 K, ν = 9.45 GHz, power = 20 mW, modeulation amplitude = 10 G modulation amplitude, scan time = 167 s, and time constant = 167 ms. (C) Dependence of the catalytic activity of Ct β on the equivalencies of MnII and FeII at a constant total metal equivalency of 2/β; adapted from (17). (D) Schematic representation of the radical-generating cofactors of class Ia/b (top) and class Ic (bottom) RNRs; adapted from (50).
In parallel, we examined the Ct β2 preparations subjected to the above and other treatments by a combination of spectroscopic methods. A multi-line, g ~ 2 EPR spectrum (Figure 3B) exhibited by preparations reconstituted with FeII and then briefly treated with an excess of the strong reductant, dithionite (17), was the crucial clue. Use of 57FeII (with nuclear spin quantum number, I, of 1/2) in this treatment led to the broadening of several lines, indicating that the associated complex contains Fe. The multiple lines suggested hyperfine coupling to an additional transition metal with a high value of I, e.g., 55Mn with I = 5/2. Aware of an earlier report of a similar EPR spectrum from a MnIII/FeIII complex (52), we considered that the dithionite-reduced Ct β2 might harbor such a cluster. Following this spectroscopic clue, we examined the dependence of catalytic acitivity on the Mn/Fe ratio. Two equiv of total metal ions was added to the metal-depleted protein with varying mole-fractions of Mn and Fe, and the activity was monitored in the presence of O2 (Figure 3C) (17). Addition of either FeII or MnII alone resulted in no significant increase over the residual activity (0.005–0.008 s−1/β) of the metal-depleted protein (a key point discussed further below), whereas addition of one equiv of each MII activated by a factor of more than 50 (17). These results strongly suggested that Ct β2 uses a Mn/Fe cofactor rather than a Fe2 cofactor.
A MnIV/FeIII product from reaction of the MnII/FeII-β2 complex with O2
Reasoning by analogy to the conventional class I RNRs that the function of the Ct β2 cofactor should be to oxidize the conserved cysteine in Ctα2 (C672) to the C•, we anticipated that the reduced MnII/FeII-β2 complex would be inactive and would be activated by reaction with O2. We verified this expectation by showing that addition of the O2-free MnII/FeII-β2 complex to an RNR reaction solution also lacking O2 did not result in turnover, whereas prior exposure of the complex to O2 gave maximal activity, irrespective of the presence of O2 in the subsequent RNR reaction (17). The stable (half-life of at least hours at 5 °C) product of the O2 reaction is, by contrast to the active, Y•-containing forms of the conventional β2 proteins, EPR-silent.3 It exhibits a quadrupole doublet in the 4.2-K/zero-field Mössbauer spectrum (Figure 1). The isomer shift, δ= 0.52 mm/s, indicates the presence of a high-spin FeIII site. Its Mössbauer spectra in varying magnetic field show that it has a STotal = 1 ground state, which could most simply arise from antiferromagnetic coupling of the SFe = 5/2 FeIII ion with an SMn = 3/2 MnIV site (50). The demonstration that the aforementioned dithionite treatment giving the 55Mn- and 57Fe-coupled g ~ 2 EPR signal does not change the oxidation state of the FeIII site confirmed this assignment by establishing that the STotal = 1/2 ground state of the dithionite-treated form arises from antiferromagnetic coupling of SFe = 5/2 FeIII and SMn = 2 MnIII ions (17). Thus, the product of the O2 reaction in Ct β2 (before its reduction by dithonite) is a MnIV/FeIII complex. Like the product of the cognate reaction in a conventional β2, it is more oxidized than the M2II/II reactant by three electrons (Figure 1).
The MnIV site functionally replaces the Y• of a conventional class I RNR
The next question was whether this stable product is the active form of Ct β2. The inactivity of the dithionite-reduced MnIII/FeIII form was consistent with this possibility. The substrate analog, 2'-azido-2'-deoxyadenosine-5'-diphosphate (N3-ADP), was used to confirm that the MnIV/FeIII complex is the active form (17). In previous studies on Ec RNR, it had been shown that treatment with the 2'-azido-substituted nucleotide causes irreversible reduction of the Y• (53) along with formation of a meta-stable, nitrogen-centered radical (N•) (54) in which a cysteine of the enzyme is covalently linked to C3' via its sulfur and an N3-derived nitrogen (55). Our expectation was that N3-ADP would cause irreversible one-electron reduction of the Ct RNR radical initiator, resulting in conversion of the EPR-silent MnIV/FeIII state to the EPR-active MnIII/FeIII state, together with formation of the N•. The spectrum of the N• was indeed observed. In addition, a spectrum with hyperfine coupling to both 55Mn and 57Fe was seen to develop in the N3-ADP reaction but not in the reaction with the normal substrate, CDP. Although much sharper and more featured than the spectrum produced by dithionite treatment of β2 in isolation, this spectrum was also seen upon dithionite treatment of the holoenzyme under turnover conditions.4(56) Parameters (g, AMn, and AFe) extracted by simulation of the spectrum, as well as more recent (unpublished) variable-field Mössbauer spectroscopic characterization of the N3-ADP-generated species, establish that its cluster is in the MnIII/FeIII oxidation state. The reduction of the cluster by one-electron concomitantly with accumulation of the N• establish that the EPR-silent, MnIV/FeIII cluster is the radical initiator in Ct RNR. The observations also indicate that the EPR spectrum, and thus the structure, of the MnIII/FeIII cluster are sensitive to whether it is generated in isolated β2 or the functioning holoenzyme. Presumably, binding of β2 to α2(•CDP•ATP) causes a conformational change that is communicated to the cluster site. This change is likely to reflect the conformational gate for the PCET step (57).
After we had reported the activity of the MnIV/FeIII form of Ct β2, the Gräslund group published a study also recognizing this fact (56). However, they interpreted their data to indicate that the Fe2III/IV form is also active (albeit less so). Our data are inconsistent with this view (17). Addition of FeII alone (in the presence of O2) to metal-depleted (< 0.05 Fe/β) β2 results in no significant increase in its RNR activity (< 50 % increase), even though the protein takes up the added FeII and accumulates the Fe2III/IV state, X. By contrast, the optimized (MnII + FeII) reconstitution procedure activates by ~ 100-fold (10,000 %). Moreover, treatment of the holoenzyme containing X in β2 with N3-ADP does not accelerate the very slow decay of X nor cause accumulation of the N•. We view these observations as compelling evidence that the Fe2III/IV β2 is inactive (17) (but confess to being surprised by this fact).
A MnIV/FeIV intermediate during activation of Ct β2
Further analysis of the activation reaction revealed accumulation of a novel MnIV/FeIV intermediate followed by the one-electron reduction of the FeIV site to give the active form (58). The MnIV/FeIV intermediate exhibits a broad optical feature centered near 390 nm and a sharp g ~ 2 EPR signal with six lines (from hyperfine coupling to a single 55Mn nucleus) separated by ~ 80 G. When the intermediate contains 57Fe, the sextet signal also shows hyperfine coupling to this I = 1/2 nucleus (Figure 1). The AMn tensor extracted from EPR simulation analysis is nearly isotropic (247, 216, 243 MHz), similar to AMn for the MnIV site in catalase (59). The Mössbauer isomer shift of the iron site (δ= 0.17 ± 0.06 mm/s) is indicative of the +IV oxidation state and is similar to that observed for the Fe2IV/IV complex, Q, in the reaction of sMMOH (33, 60). Field-dependent Mössbauer spectra show that the FeIV site is in the high spin (SFe = 2) configuration. Antiferromagnetic coupling between the FeIV (SFe = 2) and the MnIV (SMn = 3/2) ions results in the S = 1/2 ground state (58). To our knowledge, the MnIV/FeIV complex has no precedent in either inorganic chemistry or biochemistry. It is the cognate of the Fe2IV/IV intermediate Q from sMMO (33, 60, 61). It is likely to have a bis-μ-oxo-MnIV/FeIV “diamond core” structure, as was suggested for Q (62). The crystal structure of the Fe2III/III form of β2 revealed the presence of two solvent (water or hydroxo) bridges (16) (Figure 2A), which contrasts with the single oxo-bridge found in the class Ia (63) (Figure 2B) and Ib β2s (64). This difference suggests that the Ct β2 site could be adapted to stabilize the diamond core in the MnIV/FeIV and MnIV/FeIII states. Formation of the MnIV/FeIV intermediate is first order in [O2] (kform = 13 ± 3 mM−1s−1 at 5 °C) (58). Thus, intermediates preceding the MnIV/FeIV state [e.g., a peroxo-MnIII/FeIII analogue of the µ-1,2-peroxo-Fe2III/III complex detected during activation of the Mm (36) and Ec (32) proteins] do not accumulate. The EPR-active, S = 1/2 ground state of the MnIV/FeIV intermediate makes it amenable to structural characterization by multinuclear paramagnetic resonance methods (27), an opportunity not afforded by the homobinuclear homolog, Q, with its diamagnetic ground state.
Evidence for branched electron-relay pathways in Ct β2
The MnIV/FeIV state decays to the stable MnIV/FeIII state by transfer of the “extra” electron to the FeIV site (58). The “intrinsic” decay rate constant is 0.02 ± 0.005 s−1 (5 °C), and the process is accelerated by the reductant, ascorbate, with a second order rate constant of 1.3 ± 0.3 mM−1s−1 (58). In activation of Ec β2, the “extra” electron is relayed to the cluster by W48, which is transiently oxidized to the W48+• (31). In Ct β2, the surface residue Y222 plays a key role in the electron relay (65). The rate constants for intrinsic decay and ascorbate reduction are diminished by 10-fold and 65-fold (respectively) in the Y222F variant. The same product (the MnIV/FeIII cluster) still forms, and the Y222F protein is then as active as the wt protein, establishing that Y222 is not part of the inter-subunit PCET pathway (Figure 2D). Conversely, substitution of Y338, the counterpart of the subunit-interfacial PCET-pathway residue (Y356) in Ec β2 (47), with F does not affect the kinetics of the activation reaction but diminishes catalytic activity to an undetectable level. Substitution of W51, the counterpart of Ec β2 W48, with F compromises both activation and catalysis, marking this residue as the branch point for the activation- and catalysis-specific pathways (65).
Reaction of the MnIII/FeIII and MnII/FeII forms with H2O2
Before our identification of its unique cofactor, it had been suggested that the Y•-less class Ic β2s might have evolved to tolerate oxidative stress imposed by the host’s immune response (16). Our examination of the reactivities of the MnIV/FeIII, MnIII/FeIII, and MnII/FeII forms of Ct β2 toward hydrogen peroxide, an important host-generated reactive oxygen species (ROS), is consistent with this notion (66). Activity of the MnIV/FeIII form is completely stable in the presence of H2O2. The inactive, one-electron-reduced MnIII/FeIII form is rapidly (8 ± 1 M−1s−1 at 5 °C) and quantitatively (> 90%) reactivated by H2O2 via the MnIV/FeIV intermediate. The fully-reduced MnII/FeII form is also efficiently activated in a three-step reaction through MnIII/FeIII and MnIV/FeIV intermediates (see Figure 1). The propensity of Ct β2 to become fully active upon exposure to H2O2 essentially irrespective of its initial redox state represents a potentially relevant contrast to the behavior of the Ec protein. Although its fully-reduced (Fe2II/II) state reacts readily with H2O2 to generate the “met” (Y•-less) Fe2III/III form, this form further reacts only very inefficiently to generate the active Y•-containing protein (67).
Outlook
Our recent work has demonstrated that Nature chose a decidedly bioinorganic solution to replacement of the radical-initiating Y•-Fe2III/III cofactor of a conventional class I RNR: the use of a high-valent MnIV site in a MnIV/FeIII cluster. To our knowledge, Ct RNR provides the first example of a heterobinuclear Mn/Fe redox cofactor in biology and the only uncontradicted evidence for a Mn-containing RNR.5 (68, 69) Its use of the Mn/Fe cofactor affords unique opportunities to dissect the conformationally-gated, inter-subunit, long-distance PCET. The absence of an EPR signal from the resting Ct enzyme could permit detection of even trace levels of pathway radicals that in the conventional RNRs might be obscured by the spectrum of the initiating Y•. In addition, by contrast to the best-studied conventional class I RNR from Ec, the reduced form of the cofactor (MnIII/FeIII) in Ct RNR is EPR-active, and its structure is apparently sensitive to remote binding events. Efforts to capitalize on these opportunities are underway.
Acknowledgment
We thank our colleagues whose work is reviewed herein.
Abbreviations
- ATP
adenosine-5'-triphosphate
- C•
cysteinyl radical
- CDP
cytidine-5'-diphosphate
- Ct
Chlamydia trachomatis
- dCDP
2'-deoxycytidine-5'-diphosphate
- Ec
Escherichia coli
- EPR
electron paramagnetic resonance
- NH2Y
3-aminotyrosine
- N3-ADP
2'-azido-2'-deoxyadenosine-5'-diphosphate
- NDP
nucleoside-5'-diphosphate
- dNDP
2'-deoxynucleoside-5'-diphosphate
- NTP
nucleoside-5'-triphosphate
- PCET
proton-coupled electron transfer
- RNR
ribonucleotide reductase
- Y•
tyrosyl radical
Footnotes
This work was supported by the National Institutes of Health (GM-55365 to JMB and CK), the Beckman Foundation (Young Investigator Award to CK), and the Dreyfus Foundation (Teacher Scholar Award to CK).
Some class I RNRs, e.g., the enzyme from Homo sapiens, can also have α4β4 and α6β6 composition under certain conditions (14).
The absence of an EPR signal from the active form was at least partly responsible for the ~ 7-year interval between the discovery of the Y•-less Ct β2 and the correct identification of its functional cofactor.
After we reported the spectrum of the MnIII/FeIII cluster generated by treatment of the holoenzyme with either dithionite or N3-ADP, Gräslund and co-workers reported that treatment with hydroxyurea can generate the same spectrum (56).
It was initially reported that the class I RNR from Corynebacterium ammoniagenes (formerly called Brevibacterium ammoniagenes) uses a Mn-containing cofactor of unknown composition (68). Recent studies suggest that this RNR can utilize the conventional Y•-Fe2III/III cofactor (69). This controversy is one of several, recent, stark illustrations of the importance of correlating catalytic activity with metal (cluster) content of a redox metalloenzyme in identifying its functional cofactor.
Contributor Information
Wei Jiang, Email: wuj101@psu.edu.
J. Martin Bollinger, Jr., Email: jmb21@psu.edu.
Carsten Krebs, Email: ckrebs@psu.edu.
REFERENCES
- 1.Licht S, Stubbe J. Mechanistic investigations of ribonucleotide reductases. In: Poulter CD, editor. Comprehensive natural products chemistry. New York: Elsevier; 1999. pp. 163–203. [Google Scholar]
- 2.Nordlund P, Reichard P. Ribonucleotide reductases. Annu. Rev. Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 3.Reichard P. The evolution of ribonucleotide reduction. Trends Biochem Sci. 1997;22:81–85. doi: 10.1016/s0968-0004(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 4.Stubbe J, van der Donk WA. Protein radicals in enzyme catalysis. Chem. Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 5.Mao SS, Yu GX, Chalfoun D, Stubbe J. Characterization of C439SR1, a mutant of Escherichia coli ribonucleotide diphosphate reductase: evidence that C439 is a residue essential for nucleotide reduction and C439SR1 is a protein possessing novel thioredoxin-like activity. Biochemistry. 1992;31:9752–9759. doi: 10.1021/bi00155a031. [DOI] [PubMed] [Google Scholar]
- 6.Mao SS, Holler TP, Yu GX, Bollinger JM, Jr, Booker S, Johnston MI, Stubbe J. A model for the role of multiple cysteine residues involved in ribonucleotide reduction: amazing and still confusing. Biochemistry. 1992;31:9733–9743. doi: 10.1021/bi00155a029. [DOI] [PubMed] [Google Scholar]
- 7.Licht S, Gerfen GJ, Stubbe J. Thiyl radicals in ribonucleotide reductases. Science. 1996;271:477–481. doi: 10.1126/science.271.5248.477. [DOI] [PubMed] [Google Scholar]
- 8.Uhlin U, Eklund H. Structure of ribonucleotide reductase protein R1. Nature. 1994;370:533–539. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 9.Stubbe J, Ackles D. On the mechanism of ribonucleoside diphosphate reductase from Escherichia coli. J. Biol. Chem. 1980;255:8027–8030. [PubMed] [Google Scholar]
- 10.Ge J, Yu G, Ator MA, Stubbe J. Pre-steady-state and steady-state kinetic analysis of E.coli class I ribonucleotide reductase. Biochemistry. 2003;42:10017–10083. doi: 10.1021/bi034374r. [DOI] [PubMed] [Google Scholar]
- 11.Licht SS, Lawrence CC, Stubbe J. Class II ribonucleotide reductases catalyze carbon-cobalt bond reformation on every turnover. J. Am. Chem. Soc. 1999;121:7463–7468. [Google Scholar]
- 12.Stubbe J, Nocera DG, Yee CS, Chang MCY. Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer? Chem. Rev. 2003;103:2167–2202. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- 13.Stubbe J. Di-iron-tyrosyl radical ribonucleotide reductases. Curr. Opin. Chem. Biol. 2003;7:183–188. doi: 10.1016/s1367-5931(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 14.Kashlan OB, Scott CP, Lear JD, Cooperman BS. A comprehensive model for the allosteric regulation of mammalian ribonucleotide reductase. Functional consequences of ATP- and dATP-induced oligomerization of the large subunit. Biochemistry. 2002;41:462–474. doi: 10.1021/bi011653a. [DOI] [PubMed] [Google Scholar]
- 15.Roshick C, Iliffe-Lee ER, McClarty G. Cloning and characterization of ribonucleotide reductase from Chlamydia trachomatis. J. Biol. Chem. 2000;275:38111–38119. doi: 10.1074/jbc.M006367200. [DOI] [PubMed] [Google Scholar]
- 16.Högbom M, Stenmark P, Voevodskaya N, McClarty G, Gräslund A, Nordlund P. The radical site in Chlamydial ribonucleotide reductase defines a new R2 subclass. Science. 2004;305:245–248. doi: 10.1126/science.1098419. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Yun D, Saleh L, Barr EW, Xing G, Hoffart LM, Maslak M-A, Krebs C, Bollinger JM., Jr A manganese(IV)/iron(III) cofactor in Chlamydia trachomatis ribonucleotide reductase. Science. 2007;316:1188–1191. doi: 10.1126/science.1141179. [DOI] [PubMed] [Google Scholar]
- 18.Petersson L, Gräslund A, Ehrenberg A, Sjöberg B-M, Reichard P. The iron center in ribonucleotide reductase from Escherichia coli. J. Biol. Chem. 1980;255:6706–6712. [PubMed] [Google Scholar]
- 19.Bollinger JM, Jr, Edmondson DE, Huynh BH, Filley J, Norton JR, Stubbe J. Mechanism of assembly of the tyrosyl radical-dinuclear iron cluster cofactor of ribonucleotide reductase. Science. 1991;253:292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- 20.Hristova D, Wu C-H, Jiang W, Krebs C, Stubbe J. Importance of the maintenance pathway in the regulation of the activity of Escherichia coli ribonucleotide reductase. Biochemistry. 2008;47:3989–3999. doi: 10.1021/bi702408k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C-H, Jiang W, Krebs C, Stubbe J. YfaE, a ferredoxin involved in diferric-tyrosyl radical maintenance in Escherichia coli ribonucleotide reductase. Biochemistry. 2007;46:11577–11588. doi: 10.1021/bi7012454. [DOI] [PubMed] [Google Scholar]
- 22.Roca I, Torrents E, Sahlin M, Gibert I, Sjöberg B-M. NrdI essentiality for class Ib ribonucleotide reduction in Streptococcus pyogenes. J. Bact. 2008;190:4849–4858. doi: 10.1128/JB.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotruvo JA, Jr, Stubbe J. NrdI, an unusual flavodoxin involved in maintenance of the diferric-tyrosyl radical cofactor in Escherichia coli class Ib ribonucleotide reductase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14383–14388. doi: 10.1073/pnas.0807348105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollinger JM, Jr, Tong WH, Ravi N, Huynh BH, Edmondson DE, Stubbe J. Mechanism of assembly of the tyrosyl radical-diiron(III) cofactor of E.coli ribonucleotide reductase. 2. Kinetics of the excess Fe2+ reaction by optical, EPR, and Mössbauer spectroscopies. J. Am. Chem. Soc. 1994;116:8015–8023. [Google Scholar]
- 25.Sturgeon BE, Burdi D, Chen S, Huynh BH, Edmondson DE, Stubbe J, Hoffman BM. Reconsideration of X, the diiron intermediate formed during cofactor assembly in E. coli ribonucleotide reductase. J. Am. Chem. Soc. 1996;118:7551–7557. [Google Scholar]
- 26.Riggs-Gelasco PJ, Shu L, Chen S, Burdi D, Huynh BH, Que L, Jr, Stubbe J. EXAFS characterization of the intermediate X generated during the assembly of the Escherichia coli ribonucleotide reductase R2 diferric tyrosyl radical cofactor. J. Am. Chem. Soc. 1998;120:849–860. [Google Scholar]
- 27.Burdi D, Willems J-P, Riggs-Gelasco P, Antholine WE, Stubbe J, Hoffman BM. The core structure of X generated in the assembly of the diiron cluster of ribonucleotide reductase: 17O2 and H217O ENDOR. J. Am. Chem. Soc. 1998;120:12910–12919. [Google Scholar]
- 28.Mitić N, Clay MD, Saleh L, Bollinger JM, Jr, Solomon EI. Spectroscopic and electronic structure studies of intermediate X in ribonucleotide reductase R2 and two variants: a description of the FeIV-oxo bond in the FeIII-O-FeIV dimer. J. Am. Chem. Soc. 2007;129:9049–9065. doi: 10.1021/ja070909i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han W-G, Liu T, Lovell T, Noodleman L. Density functional theory study of Fe(IV) d-d optical transitions in active-site models of class I ribonucleotide reductase intermediate X with vertical self-consistent reaction field methods. Inorg. Chem. 2006;45:8533–8542. doi: 10.1021/ic060566+. [DOI] [PubMed] [Google Scholar]
- 30.Bollinger JM, Jr, Tong WH, Ravi N, Huynh BH, Edmondson DE, Stubbe J. Mechanism of assembly of the tyrosyl radical-diiron(III) cofactor of E.coli ribonucleotide reductase. 3. Kinetics of the limiting Fe2+ reaction by optical, EPR, and Mösbauer spectroscopies. J. Am. Chem. Soc. 1994;116:8024–8032. [Google Scholar]
- 31.Baldwin J, Krebs C, Ley BA, Edmondson DE, Huynh BH, Bollinger JM., Jr Mechanism of rapid electron transfer during oxygen activation in the R2 subunit of Escherichia coli ribonucleotide reductase. 1. Evidence for a transient tryptophan radical. J. Am. Chem. Soc. 2000;122:12195–12206. [Google Scholar]
- 32.Tong WH, Chen S, Lloyd SG, Edmondson DE, Huynh BH, Stubbe J. Mechanism of assembly of the diferric cluster-tyrosyl radical cofactor of Escherichia coli ribonucleotide reductase from the diferrous form of the R2 subunit. J. Am. Chem. Soc. 1996;118:2107–2108. [Google Scholar]
- 33.Liu KE, Valentine AM, Wang D, Huynh BH, Edmondson DE, Salifoglou A, Lippard SJ. Kinetic and spectroscopic characterization of intermediates and component interactions in reactions of methane monooxygenase from Methylococcus capsulatus (Bath) J. Am. Chem. Soc. 1995;117:10174–10185. [Google Scholar]
- 34.Bollinger JM, Jr, Krebs C, Vicol A, Chen S, Ley BA, Edmondson DE, Huynh BH. Engineering the diiron site of Escherichia coli ribonucleotide reductase protein R2 to accumulate an intermediate similar to Hperoxo, the putative peroxodiiron(III) complex from the methane monooxygenase catalytic cycle. J. Am. Chem. Soc. 1998;120:1094–1095. [Google Scholar]
- 35.Skulan AJ, Brunold TC, Baldwin J, Saleh L, Bollinger JM, Jr, Solomon EI. Nature of the peroxo intermediate of the W48F/D84E ribonucleotide reductase variant: implications for O2 activation by binuclear non-heme iron enzymes. J. Am. Chem. Soc. 2004;126:8842–8855. doi: 10.1021/ja049106a. [DOI] [PubMed] [Google Scholar]
- 36.Yun D, Garcìa-Serres R, Chicalese BM, An YH, Huynh BH, Bollinger JM., Jr (µ-1,2-Peroxo)diiron(III/III) complex as a precursor to the diiron(III/IV) intermediate X in the assembly of the iron-radical cofactor of ribonucleotide reductase from mouse. Biochemistry. 2007;46:1925–1932. doi: 10.1021/bi061717n. [DOI] [PubMed] [Google Scholar]
- 37.Saleh L, Krebs C, Ley BA, Naik S, Huynh BH, Bollinger JM., Jr Use of a chemical trigger for electron transfer to characterize a precursor to cluster X in assembly of the iron-radical cofactor of Escherichia coli ribonucleotide reductase. Biochemistry. 2004;43:5953–5964. doi: 10.1021/bi036099e. [DOI] [PubMed] [Google Scholar]
- 38.Bennati M, Weber A, Antonic JLD, Perlstein, Robblee J, Stubbe J. Pulsed ELDOR spectroscopy measures the distance between the two tyrosyl radicals in the R2 subunit of the E.coli ribonucleotide reductase. J. Am. Chem. Soc. 2003;125:14988–14989. doi: 10.1021/ja0362095. [DOI] [PubMed] [Google Scholar]
- 39.Bennati M, Robblee JH, Mugnaini V, Stubbe J, Freed JH, Borbat P. EPR distance measurements support a model for long-range radical initiation in E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2005;127:15014–15015. doi: 10.1021/ja054991y. [DOI] [PubMed] [Google Scholar]
- 40.Climent I, Sjöberg B-M, Huang CY. Site-directed mutagenesis and deletion of the carboxyl terminus of Escherichia coli ribonucleotide reductase protein R2. Effects on catalytic activity and subunit interaction. Biochemistry. 1992;31:4801–4807. doi: 10.1021/bi00135a009. [DOI] [PubMed] [Google Scholar]
- 41.Ekberg M, Sahlin M, Eriksson M, Sjöberg B-M. Two conserved tyrosine residues in protein R1 participate in an intermolecular electron transfer in ribonucleotide reductase. J. Biol. Chem. 1996;271:20655–20659. doi: 10.1074/jbc.271.34.20655. [DOI] [PubMed] [Google Scholar]
- 42.Rova U, Goodtzova K, Ingemarson R, Behravan G, Gräslund A, Thelander L. Evidence by site-directed mutagenesis supports long-range electron transfer in mouse ribonucleotide reductase. Biochemistry. 1995;34:4267–4275. doi: 10.1021/bi00013a016. [DOI] [PubMed] [Google Scholar]
- 43.Rova U, Adrait A, Pötsch S, Gräslund A, Thelander L. Evidence by mutagenesis that Tyr370 of the mouse ribonucleotide reductase R2 protein is the connecting link in the intersubunit radical transfer pathway. J. Biol. Chem. 1999;274:23746–23751. doi: 10.1074/jbc.274.34.23746. [DOI] [PubMed] [Google Scholar]
- 44.Saleh L, Bollinger JM., Jr Cation mediation of radical transfer between Trp48 and Tyr356 during O2 activation by protein R2 of Escherichia coli ribonucleotide reductase: relevance to R1–R2 radical transfer in nucleotide reduction? Biochemistry. 2006;45:8823–8830. doi: 10.1021/bi060325d. [DOI] [PubMed] [Google Scholar]
- 45.Seyedsayamdost MR, Yee CS, Reece SY, Nocera DG, Stubbe J. pH rate profiles of FnY356-R2s (n = 2, 3, 4) in Escherichia coli ribonucleotide reductase: Evidence that Y356 is a redox-active amino acid along the radical propagation pathway. J. Am. Chem. Soc. 2006;128:1562–1568. doi: 10.1021/ja055927j. [DOI] [PubMed] [Google Scholar]
- 46.Seyedsayamdost MR, Stubbe J. Site-specific replacement of Y356 with 3,4-dihydroxyphenylalanine in the β2 subunit of E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2006;128:2522–2523. doi: 10.1021/ja057776q. [DOI] [PubMed] [Google Scholar]
- 47.Seyedsayamdost MR, Stubbe J. Forward and reverse electron transfer with the Y356DOPA-β2 heterodimer of E. coli ribonucleotide reductase. J. Am. Chem. Soc. 2007;129:2226–2227. doi: 10.1021/ja0685607. [DOI] [PubMed] [Google Scholar]
- 48.Seyedsayamdost MR, Xie J, Chan CTY, Schultz PG, Stubbe J. Site-specific insertion of 3-aminotyrosine into subunit α2 of E. coli ribonucleotide reductase: direct evidence for involvement of Y730 and Y731 in radical propagation. J. Am. Chem. Soc. 2007;129:15060–15071. doi: 10.1021/ja076043y. [DOI] [PubMed] [Google Scholar]
- 49.Voevodskaya N, Narvaez AJ, Domkin V, Torrents E, Thelander L, Gräslund A. Chlamydial ribonucleotide reductase: tyrosyl radical function in catalysis replaced by the FeIII-FeIV cluster. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9850–9854. doi: 10.1073/pnas.0600603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, Bollinger JM, Jr, Krebs C. The active form of Chlamydia trachomatis ribonucleotide reductase R2 protein contains a heterodinuclear Mn(IV)/Fe(III) cluster with S = 1 ground state. J. Am. Chem. Soc. 2007;129:7504–7505. doi: 10.1021/ja072528a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atkin CL, Thelander L, Reichard P, Lang G. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mössbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J. Biol. Chem. 1973;248:7464–7472. [PubMed] [Google Scholar]
- 52.Bossek U, Weyhermüller T, Wieghardt K, Bonvoisin J, Girerd JJ. Synthesis, E.S.R. spectrum and magnetic properties of a heterobinuclear complex containing the {FeIII(µ-O)(µ-MeCO2)2MnIII}2+ core. J. Chem. Soc., Chem. Commun. 1989;10:633–636. [Google Scholar]
- 53.Thelander L, Larsson B, Hobbs J, Eckstein F. Active site of ribonucleoside diphosphate reductase from Escherichia coli. Inactivation of the enzyme by 2'-substituted ribonucleoside diphosphates. J. Biol. Chem. 1976;251:1398–1405. [PubMed] [Google Scholar]
- 54.Sjöberg B-M, Gräslund A, Eckstein F. A substrate radical intermediate in the reaction between ribonucleotide reductase from Escherichia coli and 2'-azido-2'-deoxyribonucleoside diphosphates. J. Biol. Chem. 1983;258:8060–8067. [PubMed] [Google Scholar]
- 55.Fritscher J, Artin E, Wnuk S, Bar G, Robblee JH, Kacprzak S, Kaupp M, Griffin RG, Bennati M, Stubbe J. Structure of the nitrogen-centered radical formed during inactivation of E. coli ribonucleotide reductase by 2'-azido-2'-deoxyuridine-5'-diphosphate: Trapping of the 3'-ketonucleotide. J. Am. Chem. Soc. 2005;127:7729–7738. doi: 10.1021/ja043111x. [DOI] [PubMed] [Google Scholar]
- 56.Voevodskaya N, Lendzian F, Ehrenberg A, Gräslund A. High catalytic activity achieved with a mixed manganese-iron site in protein R2 of Chlamydia ribonucleotide reductase. FEBS Lett. 2007;581:3351–3355. doi: 10.1016/j.febslet.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 57.Bollinger JM, Jr, Jiang W, Green MT, Krebs C. The manganese(IV)/iron(III) cofactor of Chlamydia trachomatis ribonucleotide reductase: Structure, assembly, radical initiation, and evolution. Curr. Opin. Struct. Biol. doi: 10.1016/j.sbi.2008.11.007. accepted. [DOI] [PubMed] [Google Scholar]
- 58.Jiang W, Hoffart LM, Krebs C, Bollinger JM., Jr A manganese(IV)/iron(IV) intermediate in assembly of the manganese(IV)/iron(III) cofactor of Chlamydia trachomatis ribonucleotide reductase. Biochemistry. 2007;46:8709–8716. doi: 10.1021/bi700906g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng M, Khangulov SV, Dismukes GC, Barynin VV. Electronic structure of dimanganese(II,III) and dimanganese(III,IV) complexes and dimanganese catalase enzyme: a general EPR spectral simulation approach. Inorg. Chem. 1994;33:382–387. [Google Scholar]
- 60.Lee S-K, Fox BG, Froland WA, Lipscomb JD, Münck E. A transient intermediate of the methane monooxygenase catalytic cycle containing an FeIVFeIV cluster. J. Am. Chem. Soc. 1993;115:6450–6451. [Google Scholar]
- 61.Lee SK, Nesheim JC, Lipscomb JD. Transient intermediates of the methane monooxygenase catalytic cycle. J. Biol. Chem. 1993;268:21569–21577. [PubMed] [Google Scholar]
- 62.Shu L, Nesheim JC, Kauffmann KE, Münck E, Lipscomb JD, Que L., Jr An Fe2IVO2 diamond core structure for the key intermediate Q of methane monooxygenase. Science. 1997;275:515–518. doi: 10.1126/science.275.5299.515. [DOI] [PubMed] [Google Scholar]
- 63.Nordlund P, Sjöberg B-M, Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature. 1990;345:593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
- 64.Eriksson M, Jordan A, Eklund H. Structure of Salmonella typhimurium nrdF ribonucleotide reductase in its oxidized and reduced forms. Biochemistry. 1998;37:13359–13369. doi: 10.1021/bi981380s. [DOI] [PubMed] [Google Scholar]
- 65.Jiang W, Saleh L, Barr EW, Xie J, Maslak Gardner M, Krebs C, Bollinger JM., Jr Branched activation- and catalysis-specific pathways for electron relay to the manganese/iron cofactor in ribonucleotide reductase from Chlamydia trachomatis. Biochemistry. 2008;47:8477–8484. doi: 10.1021/bi800881m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang W, Xie J, Nørgaard H, Bollinger JM, Jr, Krebs C. Rapid and quantitative activation of Chlamydia trachomatis ribonucleotide reductase by hydrogen peroxide. Biochemistry. 2008;47:4477–4483. doi: 10.1021/bi702085z. [DOI] [PubMed] [Google Scholar]
- 67.Sahlin M, Sjöberg B-M, Backes G, Loehr T, Sanders-Loehr J. Activation of the iron-containing B2 protein of ribonucleotide reductase by hydrogen peroxide. Biochem. Biophys. Res. Comm. 1990;167:813–818. doi: 10.1016/0006-291x(90)92098-k. [DOI] [PubMed] [Google Scholar]
- 68.Willing A, Follmann H, Auling G. Ribonucleotide reductase of Brevibacterium ammoniagenes is a manganese enzyme. Eur. J. Biochem. 1988;170:603–611. doi: 10.1111/j.1432-1033.1988.tb13740.x. [DOI] [PubMed] [Google Scholar]
- 69.Huque Y, Fieschi F, Torrents E, Gibert I, Eliasson R, Reichard P, Sahlin M, Sjöberg B-M. The active form of the R2F protein of class Ib ribonucleotide reductase from Corynebacterium ammoniagenes is a diferric protein. J. Biol. Chem. 2000;275:25365–25371. doi: 10.1074/jbc.M002751200. [DOI] [PubMed] [Google Scholar]