Abstract
Naïve CD8 T cells respond to signals provided by Ag, costimulation and cytokines by proliferating and differentiating to develop effector functions. Following initial clonal expansion, however, the cells develop activation-induced non-responsiveness (AINR), a form of anergy characterized by an inability to produce IL-2. Cells in the AINR state can carry out effector functions (cytolysis, IFN-γ production) but cannot continue to proliferate and expand in the face of persisting Ag. AINR limits the ability of activated CTL to control tumor growth but can be reversed by IL-2, provided either therapeutically or by activated CD4 T helper cells, to allow continued expansion.
Keywords: CD8 T lymphocyte, anergy, tumor, tolerance
1. Introduction
Most approaches to tumor immunotherapy target the activation of tumor-specific CD8 T cell responses. Numerous MHC class I-restricted peptide epitopes have been identified that are over-expressed, or uniquely expressed, on various types of tumors, and many are being tested in clinical trials for their ability to activate the generation of effective CTL responses. The importance of adjuvants in eliciting responses to peptides and other forms of antigen (Ag) is widely appreciated, and numerous forms of these are also being tested.
In addition to Ag and costimulatory ligands on Ag-presenting cells, cytokines play an important role in signaling for the generation and maintenance of a productive CD8 T cell response. Over the past several years, considerable evidence has accumulated for there being at least two critical cytokine-dependent checkpoints in the response. The first occurs early, when the CD8 T cell is undergoing its initial proliferation in response to Ag and costimulation. A ‘third signal’ from an inflammatory cytokine (IL-12 or Type I IFN) is required at this point to support differentiation so that the cells acquire effector functions and avoid being tolerized. The second occurs at the peak of the initial clonal expansion. At this point the cells display a form of split anergy termed ‘activation-induced non-responsiveness’ (AINR); they can carry out effector functions but cannot produce IL-2 and continue to expand in response to Ag. If IL-2 is available the cells proliferate and anergy is reversed so that proliferation can continue to occur in response to Ag.
These two checkpoints in the CD8 T cell response, their biochemical basis, and their implications for approaches to tumor immunotherapy, are reviewed here. The discussion focuses primarily on the AINR checkpoint, as this may present the greatest challenge to successful CD8-targeted therapies.
2. Initiating the CD8 T cell response
Naïve CD8 T cells normally encounter Ag when it is being presented by dendritic cells (DC) in the lymph nodes that drain the site of infection or tumor growth. This can lead to the induction of tolerance if the DC have not received activating signals. Alternatively, if the DC are activated by CD4 T helper cells or by engagement of their Toll-like receptors (TLR), the CD8 T cells respond productively by proliferating, clonally expanding, and developing effector functions [1–3]. Activated DC express higher levels of Ag and costimulatory molecules, including B7-1 (CD80) and B7-2 (CD86), and this contributes to their ability to activate CD8 T cells. There is now considerable evidence, however, that CD8 T cells also need a ‘third signal’ to be productively activated and avoid tolerance induction, and that this can be provided by either IL-12 or Type I IFNs (IFN-α/β).
A requirement for a third signal was first suggested by in vitro studies demonstrating that naïve CD8 T cells failed to clonally expand or become effector cells when stimulated with artificial APC having Ag and B7-1 on the surface, while memory CD8 cells expressing the same TCR made a strong response. Addition of either IL-12 [4] or IFN-α/β [5] to the cultures resulted in vigorous clonal expansion of naïve cells and development of cytolytic function and the capacity to produce IFN-γ. In vivo experiments examining immunization with peptide Ag provided further evidence that these cytokines supply a critical third signal to CD8 T cells. Immunization with peptide Ag in the absence of an adjuvant normally results in tolerance [6,7], and it was shown that co-administration of either IL-12 [8,9] or IFN-α [10–12] could replace the requirement for adjuvant to support a productive effector response and development of memory. That the cytokines were acting directly on the CD8 T cells was shown by the fact that they were ineffective if the CD8 T cell was deficient for the cytokine receptor, and were effective if only the CD8 T cells expressed the receptor.
There is accumulating evidence for the importance of IL-12 and/or IFN-α/β in signaling directly to CD8 T cells to support responses to pathogens. Kolumam et.al. [11] showed that the response to lymphocytic choriomeningitis virus (LCMV) infection by virus-specific CD8 T cells was reduced by more than ninety-nine percent when the CD8 T cells lacked the Type I IFN receptor. Responses to Listeria monocytogenes (LM) and vaccinia virus (VV) were much less affected [10,13]. We have found that responses to LM and VV depend upon both IFN-α/β and IL-12 that are produced in response to the infections, and CD8 T cells that lack receptors for both IFN-α/β and IL-12 make almost no response (Xiao and Mescher, manuscript in preparation). DC produce IL-12 and IFN-α/β in response to engagement of TLR by ligands produced by pathogens, but can also produce IL-12 in response to engagement of CD40 on their surface [14]. This suggested that CD40-dependent ‘conditioning’ of DC by CD4 helper T cells to make them more effective in activating CD8 T cells [15–17] might involve up-regulation of IL-12 production by the DC. Filatenkov et.al. [18] directly demonstrated this in an ectopic heart transplant model in which rapid rejection depended on both CD4 and CD8 T cells. DC produced IL-12 in response to CD4 T cell help, but only if the CD4 T cells expressed CD40 ligand (CD40L; CD154), and the CD8 T cells required IL-12 to mediate rapid graft rejection. Whether other cytokines, or surface ligands, can provide this third signal in some cases remains to be determined.
Both IL-12 and IFN-α/β have been studied extensively as tumor immunotherapeutic agents, and significant effects have been demonstrated in numerous models and in clinical trials. While both cytokines have effects on many cell types, including DC and NK cells, it is likely that their direct effects on CD8 T cells also contribute in many cases. For example, we have recently shown that administration of IL-12 to mice bearing an E.G7 tumor (EL-4 thymoma transfected with ovalbumin (OVA)) greatly increases the response of adoptively transferred OT-I CD8 T cells specific for OVA, with a concomitant reduction in tumor growth, and this requires that the OT-I cells express the IL-12 receptor (Curtsinger et.al., submitted for publication). In other experiments employing adoptive transfer of OT-I CD8 T cells, we have found that administering OVA peptide along with IL-12 to mice having established B16-OVA melanoma tumors results in induction of a strong OT-I response and decreased tumor growth, and requires that the OT-I cells express the IL-12 receptor (Popescu and Mescher, manuscript in preparation). Both IL-12 [19,20] and IFN-α [21] have been shown to have adjuvant-like effects on CD8 T cell responses to peptides in human melanoma patients, and it is likely that these also involve direct effects of the cytokines on the T cells.
Thus, there is now considerable evidence that productive activation of naïve CD8 T cells requires the classical two signals, Ag and costimulation, but also requires a third signal that can be provided by IL-12 and/or IFN-α/β (reviewed in [22,23]). Most adjuvants activate the production of these cytokines by Ag-presenting DC. A wide array of adjuvants can be effective in eliciting primary CTL responses to tumor Ags in murine models, and many are being developed for clinical testing. Thus, it is likely that multiple approaches involving various adjuvants, or DC vaccines, will be successful in initiating tumor-specific responses. However, even when all three signals are available and a productive response is initiated, the responding cells encounter another checkpoint that limits their ability to continue to expand if Ag persists.
3. Expanding and sustaining the effector phase of the CD8 T cell response
When a CD8 T cell receives all three signals that it needs to productively respond, it undergoes multiple rounds of division over the next three days resulting in a large expansion in the number of cells, and the cells differentiate to develop effector functions, including cytolytic activity and the ability to produce IFN-γ upon re-encountering Ag. During this time the cells also develop a form of anergy, characterized by an inability to produce IL-2, that limits the extent to which clonal expansion can continue to occur.
3.1. Activation-induced non-responsiveness (AINR) in CD8 T cells
Naïve CD8 T cells rapidly upregulate expression of IL-2 mRNA and protein in response to Ag and costimulation, and this makes a major contribution to clonal expansion in vitro. IL-2 appears to be somewhat less important in vivo; cells still undergo clonal expansion in the absence of IL-2 but the magnitude of the response is decreased [24]. In contrast to naïve cells, the effector cells that develop within three days of encountering Ag have lost the ability to produce IL-2. The cells can clearly still signal through the TCR, as demonstrated by their ability to kill Ag-bearing targets and produce IFN-γ in response to Ag, and continue to express CD28 on their surface, but cannot upregulate IL-2 mRNA expression [25]. This resembles the classical anergy that develops in CD4 T cells when they receive a TCR stimulus in the absence of costimulation [26,27]. It differs, however, in that the anergy develops even though the CD8 T cells have initially received a full set of signals and become effector cells. Thus, to distinguish this non-responsive state from anergy that develops in the absence of costimulation, we have termed it ‘activation-induced non-responsiveness’ (AINR).
AINR was initially demonstrated in vitro [25], but has also been shown to occur when CD8 T cells are responding to tumor [25,28] or virus [29,30] in vivo. Furthermore, AINR develops whether Ag persists at the peak of an effector response to syngeneic tumor [28] or persistent virus [30], or is cleared at the peak of a response to allogeneic tumor [25] or LCMV [29]. Thus, it appears very likely that development of AINR is a normal part of the CD8 T cell differentiation process, and that it limits the extent to which the cells can continue to expand even when Ag and co-stimulation are present.
Although they cannot make IL-2, effector CD8 T cells in the AINR state can proliferate in response to IL-2 if it is available. Furthermore, when effector cells are provided with IL-2 and proliferate for one to two days, the AINR state is reversed so that the cells regain the ability to upregulate IL-2 mRNA and protein in response to Ag stimulation [31]. Reversal of AINR with IL-2 has implications for novel approaches to CD8-targeted tumor immunotherapy, and is discussed in section 3.5.
3.2 Biochemical signaling in AINR CD8 T cells
CD4 T cells that have become anergic as a result of TCR stimulation in the absence of costimulatory signals are defective in their ability to upregulate the ras-MAP-kinase pathway that is essential for IL-2 production. Activation of p21 ras leads to phosphorylation and activation of ERK and JNK, and these MAPKs transactivate AP-1, a transcription factor essential for IL-2 mRNA transcription. Engagement of TCR and CD28 on anergic CD4 T clones fails to upregulate p21 ras [32] or ERK and JNK [33,34] in response to TCR and CD28 engagement.
A similar inability to upregulate the MAP-kinase pathway is seen in effector CD8 T cells that have developed AINR [35]. When naïve CD8 T cells are stimulated with artificial aAPC (aAPC) having Ag alone or Ag along with B7-1, ERK is upregulated by TCR engagement and costimulation does not increase the activation. JNK upregulation, however, requires costimulation, as it does in CD4 T cells [34,36]. Another member of the MAPK family that may be important for IL-2 production, p38, was also upregulated in naïve CD8 T cells via the TCR, but activation further increased when B7-1 was present [35]. Specific inhibitors of ERK (PD98059) and p38 (SB202190) inhibited IL-2 production and proliferation of naïve cells responding to Ag and B7-1, suggesting that both are required for an optimal response. Activation of all three MAPKs was reduced in AINR CD8 T cells examined three days after initial stimulation. In comparison to naïve cells, ERK activation was much more transient, JNK activation was reduced by more than half, and there was no detectable activation of p38. Thus, inability of the AINR cells to upregulate these MAPKs accounts, at least in part, for the inability of these cells to continue to proliferate in response to Ag. Activation of p21 ras, which is upstream of ERK, JNK and p38 in the MAPK pathway, is defective in anergic CD4 T cells [32], and it is likely that this is also the case in AINR CD8 T cells. This has not been directly examined, but would be consistent with the finding that AINR CD8 T cells respond to calcium ionophore and phorbol myristate acetate (PMA), which activates p21 ras, by activating ERK, JNK and p38, upregulating IL-2 production and proliferating [35].
The LFA-1 integrin receptor on T cells can also provide a potent costimulatory signal to CD8 T cells to stimulate IL-2 production when it binds to its ligand, ICAM-1 [37]. LFA-1 and CD28 activate some of the same signaling pathways, including the MAPK pathway and the phosphatidylinositol 3-kinase pathway [38,39]. There are differences, however, in that inhibiting the PI 3-kinase pathway blocks proliferation in response to LFA-1-dependent but not CD28-dependent costimulation. CD8 T cells develop AINR when costimulation is provided through either LFA-1 or CD28, and cells in the AINR state cannot respond to costimulation through either receptor [25]. In contrast to LFA-1 and CD28, the TNFR family of costimulatory receptors, including OX40, 4-1BB and CD27, signal through distinct proximal signaling pathways to lead to MAPK pathway activation [40,41]. These receptors are upregulated on T cells following stimulation with Ag, and have the effect of prolonging cell division and/or survival beyond the initial CD28-dependent period of clonal expansion. Although not directly examined in a model where CD8 T cells were shown to be in the AINR state, the effects of costimulation through these receptors in several experimental systems would appear to be consistent with the possibility that AINR cells can respond to this form of costimulation.
When AINR cells are provided with IL-2 and proliferate in response to it, the AINR state is reversed; the cells regain the ability to upregulate the MAPKs and produce IL-2 mRNA and protein [31]. Furthermore, some ‘re-wiring’ occurs during the reversal, so that costimulatory signals are no longer required. Engagement of just the TCR is sufficient to upregulate ERK, JNK and p38 activity to result in IL-2 production. Following reversal of AINR by IL-2, the cells can continue to proliferate in response to Ag for a prolonged period without any additional exogenous IL-2. Whether AINR cells can respond to the TNFR family member costimulatory receptors and, if so, whether this reverses the AINR state remains to be determined. IL-2 provided by CD4 T helper cells may provide a means of driving effector CTL through the AINR checkpoint to allow them to continue to respond and expand in the face of persisting Ag, and evidence for this is discussed below (section 3.5).
3.3 Basis for signaling alteration in AINR cells
The changes that occur upon differentiation of naïve CD8 T cells to result in altered signaling in the AINR effector cells, and that occur upon reversal of AINR, might result from changes intrinsic to the developmental program of the cells that alter expression of TCR, costimulatory receptors, or components of the signaling pathways. Alternatively, AINR might involve inhibitory signaling by a receptor(s) whose expression is upregulated upon differentiation and downregulated upon reversal. AINR does not appear to result from downregulation of TCR or costimulatory molecules, as expression of TCR, CD8, LFA-1 and CD28 are the same or higher than on naïve cells [25,28].
Alterations in components of the signaling pathways appears likely to play an important role in CD4 T cell anergy. Considerable evidence has accumulated for an important role for E3 ubiquitin ligases in establishing and maintaining anergy in these cells. Ubiquitination can alter the functioning of a protein by altering its abundance (through targeted degradation), or its subcellular localization or activity. Several E3 ubiquitin ligases have been implicated as acting as repressors that can limit IL-2 production and proliferation of CD4 T cells by altering components of the TCR/CD28 signaling pathways (reviewed in [42]). Given the similarities in altered MAPK pathway activation in anergic CD4 T cells and CD8 T cells in the AINR state, it would seem likely that similar mechanisms may be involved in establishment and maintenance of the AINR state.
Recent evidence has demonstrated a role for degradation of signaling pathway components in CD8 T cell anergy. In studying anergic human CD8 T cell lines, Uhlin et.al. [43] found that lck, a src-family protein kinase involved in proximal TCR signaling, was rapidly degraded upon TCR engagement. A role for this degradation in maintaining the anergic state was demonstrated in experiments showing that inhibiting the degradation by pharmacological blockade, or transfecting the cells with an lck expression vector, restored responsiveness. The effects of pharmacological agents indicated that lck degradation was independent of proteosome or lysosome pathways, and the proteases and targeting mechanisms involved remain to be determined. Whether the anergy examined in these human CD8 T cell lines and the AINR state that develops at the peak of a primary murine effector response have the same molecular basis is unclear, but it appears that they may differ. In contrast to primary murine effectors, development and maintenance of anergy in the human CD8 T cells was not affected by exogenously added IL-2, but lck expression and responsiveness could be restored by 1L-15 or IFN-α [43].
There is also the possibility that establishment and maintenance of the AINR state in effector CD8 T cells might be an actively signaled process, with signals from a surface receptor interfering with the TCR/CD28 signaling pathway. Although CTLA-4 can deliver inhibitory signals upon binding to its B7 ligands, it does not appear to be involved in establishment or maintenance of AINR. Development of AINR in vitro [25] or in vivo [44] is not prevented or reversed when CTLA-4 binding to its ligands is blocked. Another inhibitory member of the CD28 family, programmed death (PD)-1, can also negatively regulate T cell responses and contribute to peripheral T cell tolerance [45,46]. PD-1 expression is upregulated on activated CD8 T cells, and the properties of PD-1 mediated inhibition resemble the AINR state in that proliferation and IL-2 production are decreased, and the inhibition can be overcome by addition of exogenous IL-2 [47]. In contrast to AINR cells, however, CD8 T cells subject to PD-1 inhibition are also defective in cytolytic activity and secretion of cytokines other than IL-2 [48]. AINR develops when naïve CD8 T cells are stimulated in vitro with artificial APC that do not have a PD-1 ligand on the surface [25,31,35], suggesting that AINR may represent a distinct mechanism of non-responsiveness. However, activated CD8 T cells also express PD-L1 [49], one of the PD-1 ligands, raising the possibility that PD-1 might recognize ligand on adjacent cells in the culture and cause inhibition. Clearly, further work will be needed to determine the relationship between AINR and PD-1-mediated inhibition.
3.4. The post-stimulation program of CD4 T cells: help for AINR CD8 T cells
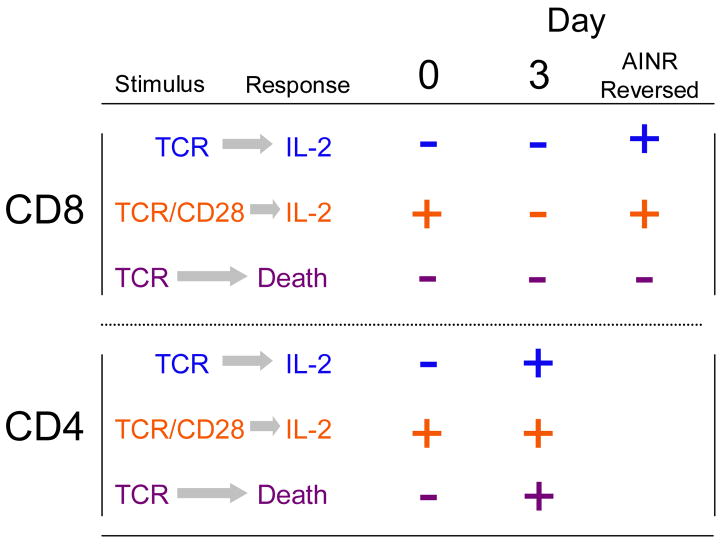
CD4 T cells become anergic when they respond to TCR engagement in the absence of costimulation, but do not develop a non-responsive state comparable to AINR if costimulation is present along with the TCR ligand [50]. When purified CD4 T cells are stimulated in vitro with artificial APC having anti-TCR mAb and B7-1 on the surface they produce IL-2, proliferate and expand in number over several days. Unlike CD8 T cells, they retain the ability to rapidly up-regulate IL-2 message and protein over this entire time. However, within two to three days, the CD4 T cells become sensitized to undergo Fas-mediated activation-induced cell death (AICD) upon re-stimulation through the TCR. Again, this is in contrast to CD8 T cells where, under these experimental conditions, the effector cells are not subject to Fas-mediated death [50]. The distinct properties of the post-stimulation programs of CD4 and CD8 T cells are summarized in Fig. 1.
Figure 1. Comparison of responses in post-stimulation CD4 and CD8 T cells.
T cells were either naïve (Day 0) or stimulated with anti-TCR mAb and B7-1 in vitro for the indicated times. Cells were then washed, stimulated with the indicated stimulus, and their IL-2 production and survival assessed. In addition, CD8 T cells were harvested on day 3, cultured for 2 additional days in IL-2 to reverse AINR, and restimulated (‘AINR Reversed’). A + indicates that response occurred, a – indicates that it did not. nd, not determined. (Adapted from ref. 50).
Blocking Fas-mediated death with anti-FasL mAb or a caspase inhibitor made it possible to study upregulation of the MAPKs upon re-stimulation of responding CD4 T cells [50]. Consistent with their ability to up-regulate IL-2 and proliferate, CD4 T cells retained the ability to upregulate ERK and JNK throughout their response over five days. However, a ‘re-wiring’ occurred similar to that seen following reversal of AINR in CD8 T cells. Naïve CD4 T cells require both TCR and CD28 engagement to upregulate JNK, but JNK upregulation became costimulation independent within two days after the initial stimulation. Thus, CD4 T cells retain the ability to produce IL-2 as they undergo the transition to effector cells, but lose the requirement for costimulation, so that upon re-encountering Ag they rapidly produce IL-2 but are also signaled to undergo AICD.
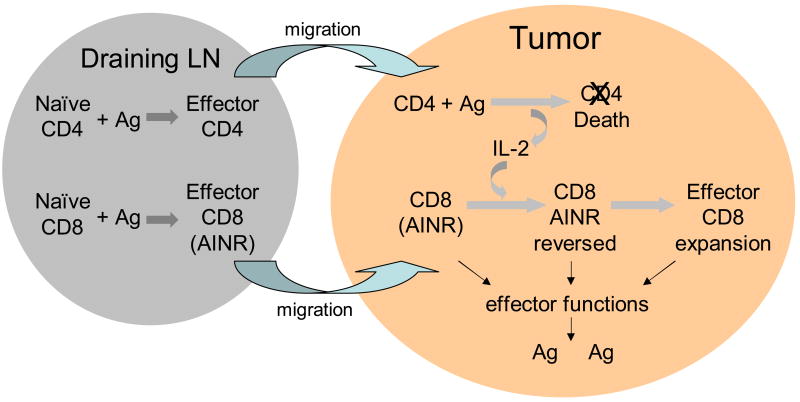
These results suggest a model by which CD4 T cells may provide IL-2 dependent help to sustain and expand a CD8 T cell response at a peripheral site of infection or tumor (Fig. 2). Both naïve CD4 and CD8 T cells would initially encounter Ag in draining lymph nodes and undergo clonal expansion and differentiation over the next two days. At this stage, CD4 T cells may provide some help by ‘conditioning’ DC to more effectively activate the naïve CD8 T cells [15–17], possibly by up-regulating production of signal 3 cytokines, IL-12 and IFN-α, by the DC [18]. By day 3, the responding cells will migrate out of the lymph nodes and traffic to the peripheral site of Ag. Upon arrival, the effector CTL will be able to carry out their lytic effector function and produce cytokines, including IFN-γ, but will not undergo further expansion because they are in the AINR state. However, further expansion of the CTL population will occur if effector CD4 T cells arriving at the site re-encounter their Ag and produce IL-2 to reverse the AINR of the CD8 T cells. Because re-wiring has occurred, IL-2 production by the CD4 T cells will not require costimulation, and may therefore occur effectively in response to Ag being presented by tissue macrophages, rather than requiring professional Ag-presenting cells. The CD4 T cells may also undergo AICD in a relatively short time, and this might be important in limiting IL-2 production to avoid IL-2-dependent induction of apoptosis in the re-activated CD8 T cells.
Figure 2. Model for IL-2 dependent CD4 T cell help at a peripheral site to reverse AINR in effector CD8 T cells.
See text for discussion.
3.5. AINR and CD8 T cell responses to tumors
The use of adoptive transfer of T cells from TCR transgenic mice [51,52] has made it possible to directly visualize and quantitate CD8 T cell responses to tumors, since the numbers, locations, phenotypes and functions of the tumor-specific T cells can be characterized during the course of the response. Use of this approach has allowed us to examine the in vivo development of AINR in CD8 T cells responding to model murine tumors in considerable detail, and the results have implications for immunotherapeutic vaccine approaches that target tumor-specific CD8 T cells. Much of this work has examined responses to E.G7 tumor [53], the EL-4 lymphoma transfected with ovalbumin (OVA), by adoptively transferred CD8 T cells from OT-I mice having a transgenic TCR specific for H-2Kb and OVA257–264 peptide [54]. Thus, OVA serves as a pseudo-tumor Ag. While this very artificial model does not have direct therapeutic relevance, it has provided substantial new insights into how CD8 T cells behave when responding to a developing tumor, and suggested novel approaches for testing in more relevant models of therapy.
When E.G7 tumor is injected i.p. the tumor grows in the peritoneal cavity, predominantly as single cells in the ascites fluid (although solid tumors develop with the peritoneal cavity late in the course of tumor growth). If mice first receive OT-I CD8 T cells by adoptive transfer, and are challenged with E.G7 one to two days later, the numbers of OT-I cells in the lymph nodes and spleen begin to decline on days 4 and 5 post-challenge, concomitant with the appearance of blasting OT-I cells in the peritoneal cavity [28]. The number of OT-I cells in the peritoneal cavity increases over the next two to three days (Fig. 3), the cells develop lytic effector function (as determined by direct ex vivo chromium release assay), and tumor growth is controlled in comparison to growth in control mice that did not receive OT-I cells. However, the number of OT-I cells in the peritoneal cavity then begins to decline as the cells migrate out into the spleen and lymph nodes. These changes in OT-I distribution were demonstrated to be the result of migration, not due to cells dying in the peritoneal cavity and expanding in the lymphoid organs. By about day twelve, few OT-I cells remain in the peritoneal cavity and uncontrolled tumor growth has resumed, while OT-I cells are present in large numbers in the spleen and lymph nodes.
Figure 3. Time course for the response of CD8 T cells to tumor growing in the peritoneal cavity, and the effects of IL-2 administration at varying times.
Mice received OT-I CD8 T cells by adoptive transfer, and were then challenged by i.p. injection of E.G7 tumor. Left panel: The numbers of OT-I T cells and E.G7 tumor cells in the peritoneal cavity, and the OT-I T cells in the spleen during the course of tumor growth. Lettered black bars indicate the times of treatment with low dose IL-2. Right panel: The tumor load in groups of mice treated with IL-2 at the times indicated in the left panel, expressed as a percent of the tumor burden in untreated control mice. (Adapted from ref. 64).
The OT-I cells present in the spleen by day ten were lytic effector cells, able to kill E.G7 target cells directly ex vivo [28]. However, they were unable to respond when placed in culture and re-stimulated with Ag. This was not due to suppressive effects of the tumor environment, since OT-I cells recovered from mice challenged with EL-4 tumor made a vigorous in vitro response to OVA peptide Ag. While OT-I cells that had responded to E.G7 and then migrated to the spleen could not respond to Ag, they proliferated and clonally expanded when IL-2 was added to the cultures. Thus, following a vigorous response to tumor Ag in the peritoneal cavity and transient control of tumor load, the effector cells migrated away from the site of tumor to the lymphoid organs where they retained effector functions but were in the AINR state and unable to continue to expand in response to Ag.
The ability of IL-2 to reverse AINR in vitro suggested that it might also have this effect if administered in vivo to tumor-bearing mice at the appropriate time, and this was confirmed. When mice challenged with E.G7 were given low dose IL-2 (2,000 IU/day) on days 1 and 2 after tumor inoculation, there was no effect on either the OT-I T cell response or on tumor growth (Fig. 3, group A). In contrast, IL-2 given on days 4 and 6 had the effect of extending the OT-I response in the peritoneal cavity and prolonging control of tumor growth. By day 10, the number of OT-I cells in the treated mice was about five-fold higher than the number in untreated mice, and tumor load was significantly lower in the treated mice (Fig. 3, group B). Thus, IL-2 given early when the OT-I response is being initiated has no effect, suggesting that the cells either make sufficient IL-2 at this point, or that IL-2 does not make a major contribution to the initial proliferation [24]. In contrast, when IL-2 is given at the time that AINR is developing in the responding CD8 T cells it supports a prolonged response by the cells. Consistent with IL-2 reversing AINR, the OT-I cells present at the site of the tumor are still blasting, indicating that they are continuing to respond to tumor Ag long after the exogenous IL-2 is gone.
IL-2 administration was also effective at later times, when the majority of AINR OT-I cells had migrated out of the peritoneal cavity. When IL-2 was given on days 16 and 18, and the mice evaluated on day 26 in comparison to untreated mice, there was a substantial reduction in tumor load (Fig. 3, group D) and a large increase in the number of OT-I T cells. The small number of OT-I cells present in the peritoneal cavity of untreated mice were not blasting, while over half of the OT-I cells in the treated mice were, indicating that AINR had been reversed and the cells were continuing to proliferate in response to Ag. In similarly treated groups of mice, IL-2 treatment on days 16 and 18 resulted in a median survival time of 60 days, in comparison to 40 days for the untreated controls.
While administering low dose IL-2 for just two days significantly increased OT-I cell numbers and reduced tumor, these effects were lost when IL-2 was given for a longer time. Thus, while IL-2 on days 16 and 18 was effective, as described above (Fig. 3, group D), mice that received IL-2 every other day from days 16 to 24 (5 times) had low numbers of OT-I cells, and tumor load (Fig. 3, group E) and survival was comparable to untreated controls (Fig. 3, group E). IL-2 is known to stimulate apoptotic death of activated T cells [55–57], and this probably accounts for the decreased efficacy of more prolonged administration. Mice that received prolonged IL-2 administration had fewer OT-I cells than untreated mice, and a substantial fraction of the OT-I cells that were still present appeared to be dying by apoptosis, as indicated by staining with Annexin V.
Further study of this model demonstrated that CD4 T cell help could also prolong the OT-I response to E.G7 tumor [44], but did not do so during the normal course of the response. CD4 T cell depletion did not change the OT-I response to E.G7 in the peritoneal cavity; the expansion, tumor control and development of AINR were the same as in normal hosts. However, when mice were primed with a CD4 T cell-specific OVA peptide epitope prior to tumor challenge, the OT-I response and tumor growth control were prolonged. By day 35, the number of OT-I cells was about ten-fold higher than in unprimed controls, and tumor load was reduced about four-fold.
The failure of CD4 T cells to provide help in the absence of priming suggested that tolerance might be induced in response to Ag from the growing tumor, and suggested a possible role for CTLA-4. CTLA-4 can mediate induction of tolerance in CD4 T cells [58–60], and blocking CTLA-4 function can enhance T cell-dependent responses to tumors [61–63]. When anti-CTLA-4 mAb was administered to mice challenged with E.G7, the OT-I response and tumor growth control were again prolonged [44]. This effect required CD4 T cells, since CTLA-4 blockade had no effect in CD4 T cell-depleted mice. The prolonged OT-I response upon CTLA-4 blockade depended on IL-2, as demonstrated by blocking with anti-IL-2 antibody. Together, these results indicated CD4 T cells can produce IL-2 to reverse AINR that develops in the responding OT-I T cells to allow a prolonged response to tumor, but only if induction of CD4 T cell tolerance is avoided by either priming or CTLA-4 blockade.
The OT-I response to E.G7 tumor in the peritoneal cavity thus provides a clear example of development of AINR limiting the CD8 T cell response to tumor, and the potential for prolonging the response if AINR is reversed by IL-2 provided either exogenously or by CD4 helper T cells [28,44,64]. More recently, we have found that a short pulse of low-dose IL-2 can also prolong CD8 T cell activation and tumor control when delivered a few days after peptide/adjuvant immunization of mice bearing established E.G7 or B16 melanoma subcutaneous tumors (Popescu and Mescher, manuscript in preparation). Results similar to those described here for the tumor system have been obtained in examining responses to virus. Virus-specific CD8 T cells are in an AINR state at the peak of the response to LCMV, as evidenced by an inability to upregulate ERK or produce IL-2 in response to re-stimulation [29], and providing IL-2 at this time results in higher numbers of CD8 T cells for a more prolonged period of time [65].
The use of Mart-1/HLA-A2 tetramers made it possible to identify tumor-specific cells in cancer patients, and revealed that the activation state of the cells differs for different individuals [66–69]. For some, the cells have a naïve phenotype, indicating that they are ignorant. For other patients, the cells have a memory phenotype, indicating that they have responded to Ag but have failed to control the tumor and are no longer responding. In some cases, this may represent cells that are in the AINR state and thus no longer able to continue to expand in response to Ag. This is suggested by the fact that cells from some patients will proliferate when placed in culture with IL-2 [70]. For other patients the cells fail to respond to IL-2, suggesting a more profound tolerance [68], perhaps as a result of responding to Ag in the absence of a signal 3 cytokine to support differentiation and development of effector functions. For those patients having IL-2 responsive cells, there is the potential that a short course of low-dose IL-2 might reactivate the cells and provide some therapeutic benefit. Similarly, vaccine immunotherapy approaches that target CD8 T cells might benefit from provision of IL-2 at the time that the cells are becoming AINR. IL-2 clearly has opposing effects, however, in that it can reverse AINR and re-activate the cells but can also induce apoptosis once the cells have been re-activated ([64]). While optimum dose and timing for AINR reversal without death can be readily evaluated in murine models, this is not the case for human therapy. Thus, alternative means of reversing AINR that do not also induce death of the re-activated cells might be more efficacious. This might include agonists for members of the TNFR family of costimulatory receptors that, as discussed above, may be able to costimulate CD8 T cells that are in the AINR state. Agonists for these receptors have demonstrated some efficacy in murine tumor immunotherapy models [40,41]. It might also include IL-7 and/or IL-15, as discussed below.
4. Development of memory CD8 T cells: reversal of AINR
When Ag is successfully cleared by the initial CTL response and the effector cells are in the AINR state, reversal must also occur to result in development of memory cells that are again able to respond to Ag. Studies of the CD8 T cell response to LCMV suggest that this occurs over about two weeks [29]. At the peak of the response the effector cells are unable to upregulate IL-2 or proliferate in response to Ag, and are defective in their ability to activate ERK. Within about two weeks, however, the cells that remain have regained these functions. We have obtained similar results in examining CD8 T cells responding to immunization with peptide Ag and adjuvant. Here too, the AINR cells present at the peak of the response regained the ability to upregulate IL-2 and proliferate in response to Ag within about two weeks (Hammerbeck and Mescher, unpublished results).
IL-7 contributes to survival of resting naïve and memory T cells, and to the establishment of memory [71–76], and appears to be good candidate for driving the reversal of AINR following clearance of Ag. Expression of the high affinity IL-7Rα chain on CD8 T cells is regulated by TCR signaling; naïve CD8 T cells express high IL-7Rα levels, rapidly down-regulate the receptor upon encounter with Ag, and rapidly re-express IL-7Rα when they are no longer receiving Ag-dependent signals (Hammerbeck and Mescher, manuscript submitted). Kaech et.al. [77] showed that the small population of CD8 effector cells that were IL-7Rα high at the peak of a response to LCMV were the cells that could survive long term to become memory cells, while subsequent work showed that not all IL-7Rα high cells present at the peak of a response survived long term ([78–80] and Hammerbeck and Mescher, manuscript submitted). Thus, the evidence suggests that re-expression of IL-7Rα by effector CD8 T cells may be necessary, but not sufficient, for their survival and conversion to a memory population.
We have found that addition of IL-7 to AINR CD8 T cells in vitro stimulates proliferation of the cells, and that the AINR state is reversed within two days as the cells regain the ability to produce IL-2 and proliferate in response to Ag (Hammerbeck and Mescher, manuscript in preparation). Although not yet examined at the level of activation of MAPKs, it appears likely that re-wiring similar to that seen with IL-2 occurs, since costimulation is not required for IL-2 production following reversal with IL-7. Thus, IL-7 can reverse AINR in vitro; whether it also mediates reversal in vivo during the effector to memory conversion remains to be determined.
Irrespective of whether IL-7 is normally responsible for driving the effector to memory conversion when Ag is cleared in vivo, it has the potential to be more efficacious than IL-2 for reversing AINR in CD8 T cells responding to tumors since it does not induce apoptosis in activated T cells. Ag remains present when tumor is not cleared and the responding CD8 T cells become AINR and this could result in low IL-7Rα expression on the cells, making them unresponsive to the cytokine. However, in the E.G7 (Fig. 3) and B16 (Popescu and Mescher, manuscript in preparation) tumor models, the CD8 T cells do not remain at the tumor site after AINR develops. Thus, they may re-express IL-7Rα in the lymphoid organs where Ag levels are low, and preliminary results suggest that this may be the case for OT-I cells that have responded to the E.G7 tumor (unpublished results). IL-15 also has a role in maintaining memory cells, and the receptor for this cytokine is not down-regulated when CD8 T cells respond to Ag. Whether IL-15 is also able to reverse AINR remains to be determined. If so, it will also be interesting to evaluate its ability to prolong responses to tumors.
5. Conclusion
There are two cytokine-dependent checkpoints in the CD8 T cell response; a requirement for a signal 3 cytokine (IL-12 or IFN-α/β) to support differentiation during the period of initial proliferation to Ag, and a requirement for IL-2 to reverse the anergy (AINR) that develops in the effector cells at the peak of the response. Thus, achieving effective CD8 T cell responses to tumors, or tumor Ags, requires that the appropriate cytokines are available to pass these checkpoints in the response. CD4 T helper cells can potentially provide for this, by stimulating DC to produce IL-12 and/or IFN-α/β at the initiation of the response, and by providing IL-2 to reverse AINR. However, relatively few tumor-specific CD4 T cell epitopes have been identified, and the requirements for activating these cells to provide optimum help are not well defined.
There are alternatives, however, for passing these checkpoints. Many of the adjuvants currently being studied are likely to stimulate Ag-presenting DC to produce IL-12 and/or IFN-α/β to support CD8 T cell differentiation. The more difficult challenge is finding ways to drive the CD8 T cells through the AINR checkpoint so that the response can be maintained and expanded. Brief administration of IL-2 can do this, but defining appropriate doses and timing to allow reversal of AINR while avoiding the induction of apoptosis in the re-activated cells may be difficult. There may be other ways of reversing or bypassing AINR, however. IL-7 (or IL-15) may reverse AINR without inducing apoptosis. Indeed, IL-15 and IL-7 contribute to the anti-tumor activity of adoptively transferred CD8 T cells [81,82], and this might potentially involve reversal of AINR. Alternatively, costimulation through the TNFR family of co-receptors may bypass the inability of the cells to respond to CD28 and LFA-1-dependent costimulation. Finally, if the AINR state is due to PD-1-mediated inhibition, then blockade of this inhibitory pathway may bypass or reverse AINR. Manipulations of these cytokines and receptors have been shown to have effects in various tumor models, but it is not clear whether they are acting to reverse or bypass AINR, or by some other mechanism. If a different mechanism(s) is involved, then combining these strategies with one that reverses AINR would have the potential to yield synergistic therapeutic effects.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 CA088956 and PO1 AI35296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Ann Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 4.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide third signals for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 5.Curtsinger JM, Valenzuela JO, Agarwal P, Lins DC, Mescher MF. Cutting edge: Type I interferons provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol CE. 2005 doi: 10.4049/jimmunol.174.8.4465. in press. [DOI] [PubMed] [Google Scholar]
- 6.Aichele P, Brduscha-Reim K, Zinkernagel R, Hengartner H, Pircher H. T cell primingversus T cell tolerance induced by synthetic peptides. J Exp Med. 1995;182:261–66. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyburz D, Aichele P, Speiser D, Hengartner H, Zinkernagel R, Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–62. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 8.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- 10.Aichele P, Unsoeld H, Koshella M, Schweier O, Kalinke U, Vucikuja S. Cutting Edge: CD8 T cells specific for lymphocytic choriomeningitis virus require Type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 11.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by Type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic T-cell responses is mediated by CD40 signaling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 16.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 17.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 18.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection. J Immunol. 2005 doi: 10.4049/jimmunol.174.11.6909. in press. [DOI] [PubMed] [Google Scholar]
- 19.Hamid O, Solomon JC, Scotland R, Garcia M, Sian S, Ye W, Groshen SL, Weber JS. Alum with interleukin-12 augments immunity to a melanoma peptide vaccine: correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res. 2007;13:215–222. doi: 10.1158/1078-0432.CCR-06-1450. [DOI] [PubMed] [Google Scholar]
- 20.Lee P, Wang F, Kuniyoshi J, Rubio V, Stuges T, Groshen S, Gee C, Lau R, Jeffery G, Margolin K, et al. Effects of interleukin-12 on the immune response to a multipeptide vaccine for resected metastatic melanoma. J Clin Oncol. 2001;19(18):3836–47. doi: 10.1200/JCO.2001.19.18.3836. [DOI] [PubMed] [Google Scholar]
- 21.Di Pucchio T, Pilla L, Capone I, Ferrantini M, Montefiore E, Urbani F, Patuzzo R, Pennacchioli E, Santinami M, Cova A, et al. Immunization of stage IV melanoma patients with Melan-A/MART-1 and gp100 peptides plus IFN-alpha results in the activation of specific CD8(+) T cells and monocyte/dendritic cell precursors. Cancer Res. 2006;66:4943–4951. doi: 10.1158/0008-5472.CAN-05-3396. [DOI] [PubMed] [Google Scholar]
- 22.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunological Reviews. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 24.D’Souza WN, Lefrancois L. IL-2 Is Not Required for the Initiation of CD8 T Cell Cycling but Sustains Expansion. J Immunol. 2003;171(11):5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 25.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163(1):102–10. [PubMed] [Google Scholar]
- 26.Mueller D, Jenkins M. Molecular mechanisms underlying function T-cell unresponsiveness. Current Opinion in Immunol. 1995;7:375–81. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RH. T cell anergy. Ann Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 28.Shrikant P, Mescher MF. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol. 1999;162:2858–2866. [PubMed] [Google Scholar]
- 29.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and Functional Profiling of Memory CD8 T Cell Differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. PNAS. 2004;101(45):16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tham EL, Shrikant P, Mescher MF. Activation-induced nonresponsiveness: A Th-dependent regulatory checkpoint in the CTL response. J Immunol. 2002;168:1190–1197. doi: 10.4049/jimmunol.168.3.1190. [DOI] [PubMed] [Google Scholar]
- 32.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 33.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. Anergic T cells are defective in both jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J Exp Med. 1996;183(5):2017–23. doi: 10.1084/jem.183.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 35.Tham EL, Mescher MF. Signaling alterations in activation-induced non-responsive (AINR) CD8 T cells. J Immunol. 2001;167:2040–2048. doi: 10.4049/jimmunol.167.4.2040. [DOI] [PubMed] [Google Scholar]
- 36.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 37.Deeths MJ, Mescher MF. ICAM-1 and B7-1 provide similar but distinct costimulation for CD8+ T cells, while CD4+ T cells are poorly costimulated by ICAM-1. Eur J Immunol. 1999;29:45–53. doi: 10.1002/(SICI)1521-4141(199901)29:01<45::AID-IMMU45>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Ni H-T, Deeths MJ, Li W, Mueller DL, Mescher MF. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–5189. [PubMed] [Google Scholar]
- 39.Ni H-T, Deeths MJ, Mescher MF. LFA-1-mediated costimulation of CD8+ T cell proliferation requires phosphatidylinositol 3-kinase activity. J Immunol. 2001;166:6523–6529. doi: 10.4049/jimmunol.166.11.6523. [DOI] [PubMed] [Google Scholar]
- 40.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 41.Watts TH. TNF/TNFR Family Members in Costimulation of T Cell Responses. Annual Review of Immunology. 2004;23(0):23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 42.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 43.Uhlin M, Masucci MG, Levitsky V. Regulation of lck degradation and refractory state in CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:9264–9269. doi: 10.1073/pnas.0406333102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Ann Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 46.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Carter LL, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 49.Lactchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tham EL, Mescher MF. The poststimulation program of CD4 versus CD8 T cells (death versus activation-induced nonresponsiveness) J Immunol. 2002;169(4):1822–8. doi: 10.4049/jimmunol.169.4.1822. [DOI] [PubMed] [Google Scholar]
- 51.Kearney E, Walunas T, Karr R, Morton P, Loh D, Bluestone J, Jenkins M. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–36. [PubMed] [Google Scholar]
- 52.Pape K, Kearney E, Khoruts A, Mondino A, Merica R, Chen Z, Ingulli E, White J, Johnson J, Jenkins M. Use of adoptive transfer of T-cell antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 53.Moore M, Carbone F, Bevan M. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 54.Hogquist K, Jameson S, Heath W, Howard J, Bevan M, Carbone F. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 55.Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG. The role of the common cytokine receptor gamma-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol. 1999;163(6):3131–7. [PubMed] [Google Scholar]
- 56.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 57.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–61. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 58.Chambers CA, Krummel MF, Boitel B, Hurwitz A, Sullivan TJ, Fournier S, Cassell D, Brunner M, Allison JP. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 59.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6(4):411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 60.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 61.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Imm. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 62.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade [see comments] Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 63.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57(18):4036–41. [PubMed] [Google Scholar]
- 64.Shrikant P, Mescher MF. Opposing effects of interleukin-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- 65.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 66.D’Souza S, Rimoldi D, Lienard D, Lejeune F, Cerottini JC, Romero P. Circulating Melan-A/Mart-1 specific cytolytic T lymphocyte precursors in HLA-A2+ melanoma patients have a memory phenotype. Int J Cancer. 1998;78(6):699–706. doi: 10.1002/(sici)1097-0215(19981209)78:6<699::aid-ijc6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 67.Jantzer P, Schendel DJ. Human renal cell carcinoma antigen-specific CTLs: antigen-driven selection and long-term persistence in vivo. Cancer Res. 1998;58(14):3078–86. [PubMed] [Google Scholar]
- 68.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature Medicine. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 69.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Lienard D, Cerottini JC, Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998;188(9):1641–50. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yannelli JR, Hyatt C, McConnell S, Hines K, Jacknin L, Parker L, Sanders M, Rosenberg SA. Growth of tumor-infiltrating lymphocytes from human solid cancers: summary of a 5-year experience. Int J Cancer. 1996;65(4):413–21. doi: 10.1002/(SICI)1097-0215(19960208)65:4<413::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 71.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 73.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 74.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 76.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a non-redundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 78.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 79.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8 memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 80.Xiao Z, Curtsinger JM, Prlic M, Jameson SC, Mescher MF. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of funtional responses. Int Immunol. 2007 doi: 10.1093/intimm/dxm039. in press. [DOI] [PubMed] [Google Scholar]
- 81.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T Cells. PNAS. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang LX, Li R, Yang G, Lim M, O’ara A, Chu Y, Fox BA, Restifo NP, Urba WJ, Hu HM. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]