Abstract
Integration of multiple signals into the canonical BMP/Smad pathway poses a big challenge during the course of embryogenesis and tissue homeostasis. Here, we show that cyclic guanosine 3′,5′-monophosphate (cGMP)-dependent kinase I (cGKI) modulates BMP receptors and Smads, providing a novel mechanism enhancing BMP signalling. cGKI, a key mediator of vasodilation and hypertension diseases, interacts with and phosphorylates the BMP type II receptor (BMPRII). In response to BMP-2, cGKI then dissociates from the receptors, associates with activated Smads, and undergoes nuclear translocation. In the nucleus, cGKI binds with Smad1 and the general transcription factor TFII-I to promoters of BMP target genes such as Id1 to enhance transcriptional activation. Accordingly, cGKI has a dual function in BMP signalling: (1) it modulates BMP receptor/Smad activity at the plasma membrane and (2) after redistribution to the nucleus, it further regulates transcription as a nuclear co-factor for Smads. Consequently, cellular defects caused by mutations in BMPRII, found in pulmonary arterial hypertension patients, were compensated through cGKI, supporting the positive action of cGKI on BMP-induced Smad signalling downstream of the receptors.
Keywords: bone morphogenetic protein, cGMP-dependent protein kinase, pulmonary arterial hypertension, Smad, TFII-I
Introduction
Bone morphogenetic proteins (BMPs) regulate a plethora of cellular processes in embryonic and mature tissue (Canalis et al, 2003; Lories and Luyten, 2005; Schier and Talbot, 2005; Varga and Wrana, 2005). BMP signalling is strictly regulated at numerous steps, from ligand availability up to the nuclear factors regulating the transcriptional response. The importance of this precise regulation is reflected by the appearance of developmental disorders and dysfunctions in humans such as bone and cartilage diseases or cancer, in which specific components of the BMP pathway are defective (Waite and Eng, 2003; Harradine and Akhurst, 2006; Hartung et al, 2006a, 2006b).
BMPs signal through two specific transmembrane serine/threonine kinase receptors, type I (BMPRI) and type II (BMPRII). Before ligand binding, BMPRs are present at the cell surface as a mixed population comprised of monomers, homodimers and preassembled heteromeric complexes containing both BMPRI and BMPRII (preformed complexes, PFCs) (Gilboa et al, 2000). Ligand binding to PFCs triggers phosphorylation of BMPRI by BMPRII and propagation of the signal by phosphorylation and concomitant activation of receptor-specific Smads the R-Smads1/5/8 (Nohe et al, 2002). The signal is then transduced through complex formation between R-Smad1/5/8 and co-Smad4 and subsequent translocation into the nucleus to regulate BMP-specific target gene expression (Shi and Massague, 2003; Feng and Derynck, 2005). Non-Smad signalling, however, is initiated by binding of BMP-2 to the high affinity receptor BMPRI, which subsequently recruites BMPRII to activate the MAPK pathways (Nohe et al, 2002; Canalis et al, 2003).
BMP signalling is fine tuned at multiple levels, depending on environmental inputs and developmental stage. Ligand accessibility is modulated by antagonists, and receptor activation is controlled by co-receptors, by localization to distinct membrane microdomains, by endocytosis and by receptor-associated proteins (Satow et al, 2006; Hartung et al, 2006a, 2006b). Recently, we showed that BMP R-Smads are phosphorylated, while the activated BMP receptor complex is still at the plasma membrane. The release of Smads from the receptors to translocate into the nucleus requires clathrin-mediated endocytosis of the receptors (Hartung et al, 2006a). Nucleo-cytoplasmic shuttling of Smads is also tightly regulated by the phosphorylation status of R-Smads (Schmierer and Hill, 2007). Thus, phosphorylation of Smad1 in the linker region counteracts Smad1 function, and R-Smad linker phosphorylation by MAPKs (e.g. extracellular signal-regulated kinase) inhibits their nuclear translocation (Kretzschmar et al, 1997; Sapkota et al, 2007). On the other hand, linker phosphorylation by glycogen-synthase kinase 3 targets Smad1 for proteasomal degradation (Fuentealba et al, 2007). Moreover, several studies suggested a dynamic interplay between MAPKs and phosphatases that affects R-Smads. For instance, the phosphatase PP2A as well as small C-terminal phosphatases dephosphorylate both the C-terminal SXS motif and the linker region of Smad1 to modulate BMP signalling (Knockaert et al, 2006; Sapkota et al, 2006; Bengtsson et al, 2009). Finally, nuclear BMP signalling depends on interaction of Smads with proteins of the nuclear envelope such as MAN1 (Osada et al, 2003) and on recruitment of and cooperation with specific transcriptional factors (Feng and Derynck, 2005) to control Smad nucleo-cytoplasmic shuttling, activity status and DNA binding. Together, these mechanisms generate feedback loops and, in crosstalk with other pathways, regulate multiple steps in BMP signalling and prevent its deregulation.
BMPRII not only initiates BMP signalling, but also crosstalks with diverse signalling pathways. For instance, Foletta and co-workers have shown that a key regulator for actin dynamics, LIM kinase I, is inhibited by interaction with the BMPRII-tail, leading to dysregulation of actin depolymerization (Foletta et al, 2003). Mutations in BMPRII were implicated in the development of the vascular disease pulmonary arterial hypertension (PAH) (Lane et al, 2000; Thomson et al, 2000; Machado et al, 2001), and several proteins that bind to the BMPRII-tail, such as Tctex-1 and Tribbles-like protein 3 (Trb-3), were shown to interfere with the pathogenesis of PAH (Machado et al, 2003; Chan et al, 2007). Nevertheless, the role of BMPRII and its crosstalk mechanisms during specific BMP responses are still unclear.
Using a proteomics-based approach (Hassel et al, 2004), we identified cyclic guanosine 3′,5′-monophosphate (cGMP)-dependent kinase I (cGKI) as a binding partner of BMPRII. So far, no function has been assigned to cGKI in BMP signalling. cGKI (PKGI) is a soluble cytoplasmic serine/threonine kinase and one of the major mediators of nitric oxide (NO)/cGMP-triggered signal transduction. It has important functions in many physiological processes such as vascular tone control, platelet activation and synaptic plasticity. It is highly expressed in vascular smooth muscle cells (VSMCs) and regulates gene expression, cell morphology and cell proliferation. Interestingly, alterations in cGKI expression and activity are involved in the pathogenesis of hypertension, atherosclerosis, restenosis and hyperlipemia (Schlossmann et al, 2005; Feil et al, 2005a, 2005b).
cGKI has three functional domains: the N-terminal leucine zipper, the cGMP-binding region and the C-terminal kinase domain. The N terminus exhibits an α and β isoform-specific autoinhibitory/pseudo-substrate site, which blocks the catalytic centre in the inactive state (Orstavik et al, 1997; Francis et al, 2002). In addition, the N terminus mediates homodimerization through a leucine/isoleucine zipper motif and subcellular targeting. It includes autophosphorylation sites involved in the control of the basal activity of cGKI (Smith et al, 1996; Chu et al, 1998; Richie-Jannetta et al, 2003). The regulatory domain comprises two tandem cGMP-binding sites. cGMP binding induces a conformational change, whereby the catalytic centre in the C-terminal kinase domain is released allowing subsequent phosphorylation of substrates (Zhao et al, 1997; Wall et al, 2003; Feil et al, 2005b).
Here, we show that cGKI interacts with and phosphorylates BMPRII. On BMP-2 stimulation, cGKI is released from the receptor to bind R-Smads and the co-Smad4. The cGKI/Smad complexes then translocate into the nucleus, recruit TFII-I, and bind to the promoter of the BMP target gene Id1. These findings show a novel dual role for cGKI in BMP signalling: (1) regulation of BMP receptor and R-Smad activation at the plasma membrane and (2) regulation of the expression of BMP target genes in the nucleus. Our studies provide the first evidence for crosstalk between the cGMP/cGKI and BMP signalling pathways. Importantly, this crosstalk is physiologically relevant, as we show that cGKI can compensate for the aberrant cellular responses to BMP caused by mutations in BMPRII found in PAH patients.
Results
cGKI isoforms interact with BMPRII
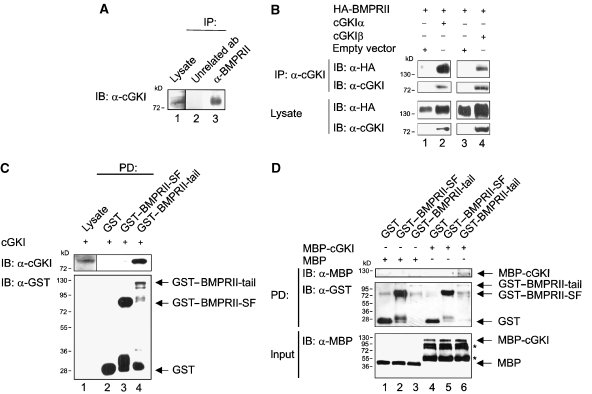
In a proteomics-based screen for BMPRII interactors, we identified several BMPRII-associated proteins (Hassel et al, 2004). Among these interactors not published earlier was cGKI (Supplementary Figure S1). To confirm this interaction in cells, we used co-immunoprecipitation of endogenous proteins in C2C12 cells. As shown in Figure 1A, cGKI co-precipitated with BMPRII as well as BMPRI (data not shown). After knockdown of endogenous BMPRII, BMPRIa did not co-precipitate with cGKI indicating that binding of cGKI to the receptor complex is mediated through BMPRII (Supplementary Figure S2). To investigate whether the cellular localization of cGKI depends on BMPRII, we performed confocal immunofluorescence microscopy in C2C12 cells stably expressing HA-BMPRII (Hassel et al, 2003). After antibody-mediated patching of HA-BMPRII at the cell surface (Gilboa et al, 2000), endogenous cGKI was partially co-localized with HA-BMPRII patches at the cell surface (data not shown). There are two cGKI alternatively spliced cGKI isoforms, α and β, which differ in their N termini (Feil et al, 2005b) (see Supplementary Figure S1). Both isoforms are expressed in C2C12 cells (Casteel et al, 2002); however, the peptides identified in our screen by mass spectrometry (Supplementary Figure S1) did not allow to differentiate between the isoforms (Hassel et al, 2004). cGKIα and β were therefore analysed for co-immunoprecipitation with HA-BMPRII in transiently transfected HEK293T cells. Both cGKI isoforms co-precipitated with HA-BMPRII, with a somewhat higher efficiency for the α isoform (Figure 1B). It is not clear whether this reflects a higher affinity to BMPRII or is merely due to stickiness of the cGKIα leucine zipper. For further experiments, we used either cGKIα or β.
Figure 1.
cGKI isoforms associate with BMPRII. (A) Endogenous complexes of cGKI and the BMP type II receptor in C2C12 cells were analysed by immunoprecipitation using α-BMPRII antibody and subsequent immunoblotting with α-cGKI antibody. Ab, antibody; IB, immunoblotting; IP, immunoprecipitation. (B) cGKI was immunoprecipitated from HEK293T cells transfected with HA-BMPRII and cGKIα or β or empty vector. BMPRII in α-cGKI immunoprecipitates was detected with α-HA antibody. Lysates were controlled for protein expression. (C) GST–BMPRII-SF, GST–BMPRII-tail or GST alone immobilized to glutathione sepharose beads were incubated with C2C12 lysates expressing cGKI. Purified protein complexes and cGKI expression were examined by immunoblotting with α-cGKI antibody, BMPRII fusion proteins with α-GST antibody. PD, pull-down. (D) MBP-cGKI was analysed for in vitro binding to GST–BMPRII-SF or GST–BMPRII-tail, immobilized to glutathione sepharose beads. Precipitates were checked by α-MBP and α-GST immunoblotting. To control input of MBP and MBP-cGKI, immunoblotting with α-MBP antibody was performed on a separate gel. Asterisks mark degradation products of MBP-cGKI.
There are two naturally occurring splice variants of BMPRII. The short form (BMPRII-SF) lacks the long cytoplasmic tail, which is unique to BMPRII among mammalian TGFβ superfamily receptors (Rosenzweig et al, 1995). To map the BMPRII site that interacts with cGKI, we performed pull-down experiments in C2C12 cells overexpressing cGKI, using recombinant GST–BMPRII-tail or GST–BMPRII-SF fusion proteins or GST alone as bait (Figure 1C, Supplementary Figure S3). We observed that cGKI co-precipitates only with BMPRII-tail region (Figure 1C, upper panel, lanes 3 and 4). To investigate whether the interaction between cGKI and BMPRII is direct, we performed in vitro binding assays using recombinant proteins (GST–BMPRII cytoplasmic domains and MBP-cGKI; Figure 1D; Supplementary Figure S2). Recombinant cGKI bound to the BMPRII-tail (Figure 1D, upper panel, lane 6), but not to BMPRII-SF (Figure 1D, upper panel, lane 5). Yet, when we co-expressed BMPRII-SF, BMPRII-LF or BMPRII truncation mutants with cGKI in HEK293T cells, cGKI was immunoprecipitated with BMPRII-SF (Supplementary Figure S4). However, this is caused by ligand independent dimers of BMPRII-SF and BMPRII-LF (Gilboa et al, 2000). This notion is supported by the finding that the shortest truncation mutant BMPRII-TC1, which lacks the kinase domain and tail region and is defective in its ability to dimerize with full-length BMPRII (Gilboa et al, 2000), failed to interact with cGKI (Supplementary Figure S4).
cGKI transphosphorylates BMPRII
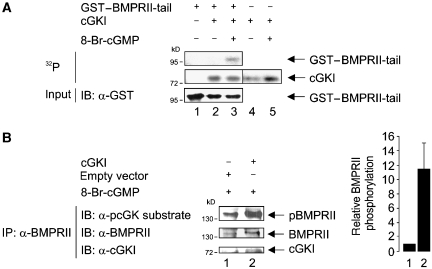
Little is known on the regulation of BMPRII phosphorylation. The BMPRII-associated receptor tyrosine kinase c-Kit shows dual kinase activity and phosphorylates BMPRII at serine 757 in the tail region and thus modulates BMPRII-dependent BMP signalling (Hassel et al, 2006). As cGKI is a kinase, we examined whether it can phosphorylate BMPRII in vitro. The assay used recombinant cGKI in the presence of (γ-32P)-ATP, using GST–BMPRII-tail as substrate (Figure 2A; Supplementary Figure S3). Interestingly, BMPRII-tail, which binds cGKI (Figure 1D), was also phosphorylated by activated cGKI (Figure 2A, upper panel, lane 3). However, cGKI did not phosphorylate BMPRII-SF, as shown with a kinase-deficient BMPRII-SF mutant (BMPRII-SF-K230R (Nohe et al, 2002)) to circumvent concomitant autophosphorylation of the receptor (Supplementary Figure S5). In turn, BMPRII is implicated in regulatory phosphorylation of interaction partners such as Tctex-1 (Machado et al, 2003). In the context of this study, BMPRII kinase did not phosphorylate cGKI (Supplementary Figure S5 and data not shown).
Figure 2.
cGKI phosphorylates BMPRII. (A) GST–BMPRII-tail immobilized to glutathione sepharose beads was subjected to in vitro kinase assay with unactivated or 8-Br-cGMP-stimulated cGKI. Incorporated 32P was detected by autoradiography. Input of the fusion protein was visualized by immunoblotting using α-GST antibody. (B) C2C12 cells transfected with cGKI or empty vector were stimulated with 8-Br-cGMP for 30 min and subjected to immunoprecipitation using α-BMPRII antibody. Phosphorylation of immunoprecipitated endogenous BMPRII through cGKI was analysed using a phospho-cGK/PKA substrate-specific antibody. Pellets and lysates were examined with α-BMPRII or α-cGKI antibodies. Intensities of pBMPRII and BMPRII bands were measured with ImageJ, and the ratio of the intensities (pBMPRII/BMPRII) from two independent experiments (mean±s.d.) is depicted as relative BMPRII phosphorylation.
The phosphorylation of BMPRII by cGKI was next analysed by in vivo phosphorylation assays in C2C12 cells. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation was used in initial studies to analyse cGKI activation (Ruth et al, 1991; Butt et al, 1994; Smolenski et al, 1998; Casteel et al, 2002) (Supplementary Figure S6). Cells transfected with either cGKI or empty vector were stimulated with 8-Br-cGMP before lysis (Figure 2B). Endogenous BMPRII was immunoprecipitated and examined by immunoblot with an antibody specific for substrates phosphorylated by arginine-dependent kinases like cGKs and the cAMP-dependent kinase (PKA). Substrate specificity of this antibody was validated by measuring transphosphorylation of cGKI on recombinant BMPRII-tail, followed by the anti-cGKI substrate antibody (data not shown). Taken together, we conclude that cGKI phosphorylates BMPRII in vitro and in vivo.
cGKI dissociates from BMPRII in response to BMP-2
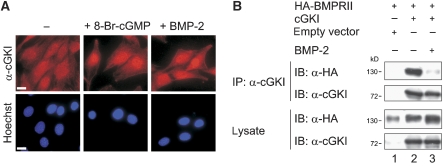
To investigate the cellular localization of cGKI after BMP-2 stimulation, we stained cGKI by immunofluorescence in C2C12 cells (Figure 3A) and HEK293T cells (data not shown). Consistent with published data, endogenous cGKI displayed a pancellular distribution in unstimulated C2C12 cells and other cell lines, whereas 8-Br-cGMP stimulation induced its nuclear translocation (Gudi et al, 1997; Casteel et al, 2002) (Figure 3A). Surprisingly, stimulation with BMP-2 also redistributed cGKI to the nucleus (Figure 3A), indicating its dissociation from the receptor complex.
Figure 3.
cGKI detaches from BMPRII in a BMP-2-dependent manner. (A) Immunofluorescence staining of endogenous cGKI in C2C12 cells after stimulation with 8-Br-cGMP or BMP-2 for 30 min. DNA was stained using Hoechst dye. Bar, 20 μm. (B) HEK293T cells co-transfected with HA-BMPRII and cGKI or empty vector were starved and stimulated with BMP-2 for 10 min or left untreated. cGKI was immunoprecipitated and both precipitates and lysates were analysed by immunoblotting with α-HA and α-cGKI antibodies. A full-colour version of this figure is available at The EMBO Journal Online.
To test this possibility, HEK293T cells were transfected with cGKI and HA-BMPRII, stimulated with BMP-2, and subjected to immunoprecipitation experiments to explore the cGKI-BMPRII association (Figure 3B). In agreement with the immunofluorescence data, stimulation with BMP-2 drastically reduced cGKI-BMPRII interactions.
cGKI translocates into the nucleus together with activated Smads
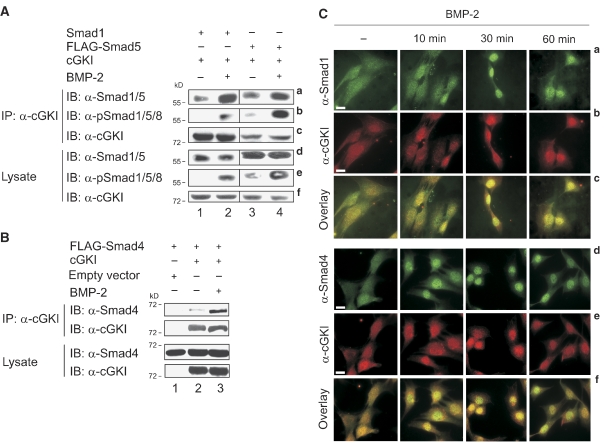
The BMP-dependent dissociation of cGKI from BMPRII and subsequent nuclear translocation of cGKI closely mirrors the route and dynamics of Smads acting as intracellular BMP signal transducers. Therefore, we asked whether cGKI might bind to BMP R-Smads and/or co-Smad4. Transfected HEK293T cells (stimulated with BMP-2 or untreated) were analysed by co-immunoprecipitation to explore cGKI/Smad complexes (Figure 4A). Smad1 (Figure 4A, panel a, lanes 1 and 2) and Smad5 (Figure 4A, panel a, lanes 3 and 4) associated with cGKI already in the absence of ligand. However, complex formation was markedly enhanced on stimulation with BMP-2 (Figure 4A, panel a, lanes 2 and 4). Furthermore, cGKI exhibited BMP-dependent interaction with activated Smad1 and Smad5, which are C-terminally phosphorylated in response to BMP-2 (Figure 4A, panel b, lanes 2 and 4). Moreover, cGKI interacted with Smad4 in a BMP-2-dependent manner (Figure 4B, upper panel, lanes 2 and 3), in accord with their association into mutual complexes before nuclear translocation (Shi and Massague, 2003).
Figure 4.
cGKI binds to activated Smad complexes and undergoes nuclear translocation. (A) HEK293T cells co-transfected with Smad1 or FLAG-Smad5 and cGKI were starved and stimulated with BMP-2 for 30 min or left untreated. cGKI immunoprecipitates were subjected to immunoblotting using α-Smad1/5, α-pSmad1/5/8 and α-cGKI antibodies (panels a–c; in panel a, lanes 3 and 4 were exposed for shorter periods than lanes 1 and 2). Levels of pSmad1/5/8, total Smad and cGKI were detected in lysate controls (panels d–f). (B) As in (A), except that HEK293T cells were co-transfected with FLAG-Smad4 and cGKI or empty vector and Smad4 was assayed for interaction with cGKI. (C) C2C12 cells treated with BMP-2 (10, 30 and 60 min) or without ligand (−) were co-stained for intracellular Smad1 (panels a–c) or Smad4 (panels d–f) and cGKI, respectively, using specific antibodies. Panel sets c and f monitor co-localization by merging the respective upper two panels. Bar, 20 μm.
To further study the subcellular distribution of endogenous cGKI and Smads under physiological conditions, C2C12 cells were stimulated with BMP-2 (10, 30 and 60 min) and examined by immunofluorescence microscopy. In the absence of ligand, the proteins were pancellularly distributed (Figure 4C). On BMP-2 stimulation, cGKI (Figure 4C, panels b and e), Smad1 (Figure 4C, panel a) and Smad4 (Figure 4C, panel d) accumulated in the nucleus at rather similar rates. Moreover, cGKI partly co-localized with both proteins in the cytoplasm as well as in the nucleus of BMP-2-treated cells (Figure 4C, panels c and f). Most of the cGKI and R-Smad/Smad4 complexes were found in the nucleus 30 min after stimulation with BMP-2 (Figure 4C). After 60 min, cGKI, Smad1 and Smad4 started to redistribute into the cytoplasm (Figure 4C). To support these data, we further examined cGKI/Smad complexes by fractionation studies on C2C12 cells. cGKI associated with Smad1 and co-Smad4 in the cytoplasm and more pronounced in the nucleus of BMP-2-treated cells (Supplementary Figure S7).
Taken together, our data suggest that BMP-2 stimulation not only triggers dissociation of cGKI from the BMP receptors but also induces binding of cGKI to activated Smads and subsequent translocation of cGKI/Smad complexes into the nucleus.
Downregulation of endogenous cGKI inhibits BMP-2 signalling
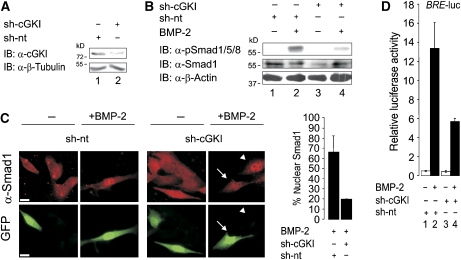
To investigate the impact of endogenous cGKI on BMP signalling, we used short hairpin RNA (sh-RNA) to downregulate both cGKI isoforms in C2C12 cells (Figure 5A). Downregulation of cGKI caused a strong reduction of BMP-2-mediated C-terminal phosphorylation of Smad1/5/8 (Figure 5B, lanes 2 and 4). Consistent with this, overexpression of wild-type cGKI enhanced Smad phosphorylation 2–3-fold, whereas kinase-inactive cGKI-D516A had no effect (Supplementary Figure S8); this suggests that the kinase activity of cGKI is necessary to enhance C-terminal phosphorylation of Smads. Consistent with the results depicted in Figure 5B, knockdown of both cGKI isoforms abolished efficient nuclear translocation of Smad1 (Figure 5C). Accordingly, Smad1/Smad4 complex formation was reduced in cells expressing sh-cGKI relative to cells transfected with a non-targeting sh-RNA (sh-nt) (data not shown).
Figure 5.
Silencing of endogenous cGKI attenuates BMP signalling. (A) cGKI knockdown using a specific sh-RNA (sh-cGKI) for both isoforms compared with a non-targeting sh-RNA (sh-nt) was validated in C2C12 cells by immunoblotting with an α-cGKI antibody. α-β-Tubulin was used as loading control. (B) C2C12 cells transfected with sh-nt or sh-cGKI were incubated with or without BMP-2 for 30 min and subjected to Smad phosphorylation assay using α-pSmad1/5/8, α-Smad1 and α-β-Actin immunoblotting. (C) BMP-2-induced nuclear translocation of endogenous Smad1 was examined by immunofluorescence microscopy in sh-nt/GFP- or sh-cGKI/GFP-transfected C2C12 cells. Quantification was done by determining the respective number of cells with BMP-2-induced nuclear Smad1 from all GFP-positive cells. The results are mean±s.d. of two experiments. Arrow marks transfected and arrowhead non-transfected cells. Bar, 20 μm. (D) C2C12 cells co-transfected with BRE-luc, RL-TK and sh-nt or sh-cGKI were stimulated with BMP-2 for 24 h or left untreated. BRE-driven luciferase activity was measured (Hartung et al, 2006a, 2006b). The results are mean±s.d. of duplicate measurements and represent one out of three independent experiments. A full-colour version of this figure is available at The EMBO Journal Online.
To analyse the functional consequences of cGKI knockdown on the expression of Smad-dependent BMP-2 target genes, we used a luciferase reporter gene under the control of the BMP response element (BRE) from the murine Id1 promoter (Korchynskyi and ten Dijke, 2002). Knockdown of endogenous cGKI using sh-cGKI attenuated BRE reporter gene activity in response to BMP-2 by a factor of 2 relative to control cells (Figure 5D, lanes 2 and 4). In agreement with this result, overexpression of wild-type cGKI increased BRE reporter activity (see Figure 7A), whereas the kinase-inactive mutant cGKI-D516A failed to do so (data not shown). Interestingly, cGKI expression had no effect on BMP-2-induced MAPK p38 activation and on the induction of alkaline phosphatase in C2C12 cells (data not shown).
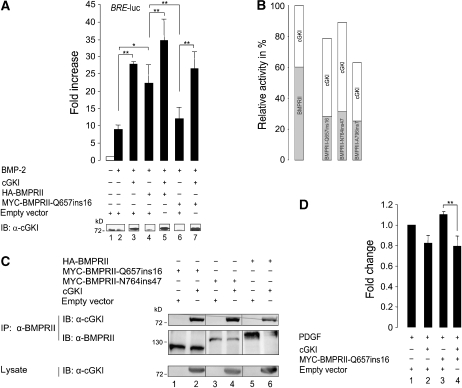
Figure 7.
cGKI counteracts cellular effects caused by BMPRII PAH mutants. (A) C2C12 cells were co-transfected with luciferase reporters and HA-tagged BMPRII or MYC-tagged mutant BMPRII-Q567ins16 (causing idiopathic PAH) and/or cGKI or empty vector. Cells were stimulated with BMP-2 for 24 h or left untreated. Fold changes in BRE reporter activities were normalized to the non-treated empty vector control (mean±s.d.). Data are representative for three independent experiments. cGKI expression was controlled by immunoblotting with α-cGKI antibody. *P<0.05; **P<0.01. (B) Graph shows the reporter gene activities on BMPRII mutant and cGKI coexpression relative to the activity measured for wild-type BMPRII and cGKI. The protein effects were calculated separately to clarify their impact on the overall BRE reporter signal (BMPRII variant effect, light grey fraction; cGKI effect, white fraction). (C) BMPRII mutants were immunoprecipitated using α-BMPRII antibody from transfected HEK293T cells with either cGKI or empty vector and BMPRII, BMPRII-Q567ins16 or BMPRII-N746ins47. Immunoprecipitated complexes and lysates were analysed by α-BMPRII and α-cGKI antibodies. (D) Human aortic smooth muscle cells were transfected with MYC-BMPRII-Q567ins16 and/or cGKI or empty vector and stimulated with PDGF or serum for 24 h. The PDGF- or serum-induced proliferation was measured. Fold changes relative to stimulated, empty vector-transfected cells of two independent experiments are shown (mean±s.d.). **P<0.01.
We conclude that knockdown of endogenous cGKI strongly reduces the BMP-mediated C-terminal phosphorylation of R-Smads, their nuclear translocation and Smad-dependent transcription of distinct target genes, suggesting that cGKI has an activating role in BMP signalling.
Nuclear role of cGKI in BMP signalling
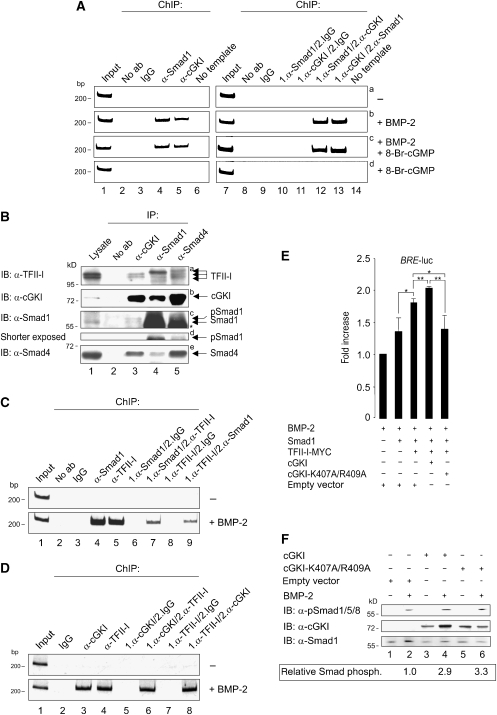
As BMP-2 stimulation induced nuclear translocation of cGKI and cGKI knockdown downregulated BMP-2-induced transcriptional response, we further investigated the nuclear function of cGKI. On nuclear entry, Smads associate with co-factors to assemble transcriptionally active complexes at promoter regions of specific target genes (Ogata et al, 1993; Feng and Derynck, 2005). In this context, we used endogenous chromatin immunoprecipitation (ChIP) assays with either untreated or BMP-2- and/or 8-Br-cGMP-stimulated C2C12 cells to explore whether cGKI is a component of Smad-containing nuclear transcription complexes associated with specific sequences in the Id1 promoter. Binding of Smad1 to the Id1 promoter was undetectable in unstimulated cells (Figure 6A, left, panel a, lane 4), and increasing significantly after BMP-2 stimulation (Lopez-Rovira et al, 2002) (Figure 6A, left, panel b, lane 4). Interestingly, cGKI also bound to this Id1 promoter site on stimulation with BMP-2 (Figure 6A, left, panel b, lane 5), but 8-Br-cGMP alone failed to induce such binding or to enhance the stimulation by BMP-2 (panels c and d, lane 5). This implies that it is the BMP-2 rather than the cGMP signal that directs cGKI binding to the Id1 promoter. Similar results were observed in HEK293T cells (data not shown). To further substantiate that cGKI and Smad1 bind to the Id1 promoter in a common complex, we carried out two-step ChIP experiments (Figure 6A, right). These experiments showed that Smad1 and cGKI indeed complex at the Id1 promoter, both in BMP-2- and BMP-2/8-Br-cGMP-treated C2C12 cells (Figure 6A, right, panels b and c, lanes 12 and 13). This suggests that the BMP/Smad-dependent modulation of gene transcription through cGKI requires the localization of the kinase to the nuclear target gene. This is the first demonstration for signal-dependent and site-specific association of a cGK with DNA.
Figure 6.
cGKI's nuclear function in BMP signalling. (A) C2C12 cells were stimulated with BMP-2 and/or 8-Br-cGMP for 4 h or left unstimulated. Endogenous ChIPs of a specific Id1 promoter fragment was performed using α-Smad1 and α-cGKI antibodies (left). Complex formation of Smad1 and cGKI at the Id1 promoter was analysed by two-step ChIP using α-Smad1 and α-cGKI antibodies in different order (right). As a control, ChIP experiments were performed with IgG or antibody was omitted. (B) Endogenous protein complexes from C2C12 cells containing TFII-I and cGKI, Smad1 or Smad4 were analysed by co-immunoprecipitation with α-cGKI, α-Smad1 or α-Smad4 antibodies. Immunoblotting was performed with the indicated antibodies. (C, D) As in (A), except that the Id1 promoter was assayed for (C) Smad1/TFII-I or (D) cGKI/TFII-I complex formation by one-step and two-step ChIP with the indicated antibodies. (E) C2C12 cells were co-transfected with BRE-luc, RL-TK and the indicated constructs, stimulated with BMP-2 for 24 h of left untreated, and luciferase activities were measured. Fold changes in BRE reporter activities relative to the BMP-2-treated empty vector control from two independent experiments (mean±s.d.) are shown. *P<0.05; **P<0.01. (F) C2C12 cells transfected with cGKI, cGKI-K407A/R409A or empty vector were subjected to Smad phosphorylation assay and analysed by immunoblotting with α-pSmad1/5/8 and α-cGKI antibodies. α-Smad1 was used as a loading control. Intensities of pSmad1/5/8 and Smad1 bands were measured with ImageJ, and the ratio of the intensities (pSmad/Smad) is shown as relative Smad phosphorylation.
To obtain further insight into the molecular mechanisms by which cGKI regulates BMP/Smad target genes, the involvement of the general transcription factor TFII-I, which interacts with and is phosphorylated by cGKIβ (Casteel et al, 2002), was analysed. This interaction was shown to increase the transactivation potential of TFII-I. Furthermore, TFII-I was reported to interact with TGFβ Smads. Therefore, we checked whether TFII-I affects BMP signalling. Co-immunoprecipitation studies in C2C12 cells revealed binding of endogenous TFII-I to cGKI, as well as to Smad1 and Smad4 (Figure 6B, panel a). The TFII-I double band represents two splice variants, β and Δ (Hakre et al, 2006) (Figure 6B, panel a). Interestingly, a slower migrating form of TFII-I co-precipitated with Smad1 and Smad4 (Figure 6B, panel a, lanes 4 and 5). It is not yet clear whether Smad complexes interact preferentially with modified TFII-I, or whether the modification occurs as a consequence of TFII-I interaction with cGKI/Smad complexes and subsequent phosphorylation by cGKI.
Isoform-specific conformation as well as serum starvation, but also growth factor stimulation, regulates the subcellular localization of TFII-I (Hakre et al, 2006). Immunofluorescence microscopy in C2C12 cells using α-pan-TFII-I antibody located TFII-I predominantly in the nucleus independent of BMP-2 treatment (Supplementary Figure S9). In response to BMP-2, Smad1 co-localized with TFII-I in the nucleus (Supplementary Figure S9). As co-localization in a large compartment does not necessarily imply association, we validated by co-immunoprecipitation that TFII-I/Smad1 binding is induced by BMP-2 (Supplementary Figure S10). These observations suggest BMP-2-induced association of Smad1 and TFII-I in the nucleus. To investigate whether TFII-I is associated with cGKI/Smad1 complexes at the Id1 promoter, we extended the ChIP and two-step ChIP experiments using α-TFII-I and α-Smad1 or α-cGKI antibodies. Indeed, TFII-I bound to the Id1 promoter and formed a complex with Smad1 and cGKI at this promoter only in BMP-2- or BMP-2/8-Br-cGMP-treated C2C12 cells (Figure 6C and D and data not shown). Therefore, we propose that cGKI and TFII-I in association with Smad1 has an important function in the regulation of the Id1 promoter.
To test this hypothesis, we measured the effect of TFII-I on BRE reporter gene activity by co-expressing Smad1 and cGKI variants in C2C12 cells (Figure 6E). The BMP-2-induced reporter gene activity was enhanced in cells overexpressing TFII-I and Smad1 as compared with cells transfected with Smad1 alone (Figure 6E, lanes 2 and 3). Coexpression with wild-type cGKI on top of TFII-I/Smad1 added an incremental increase (Figure 6E, lanes 3 and 4). Interestingly, coexpression of a cGKI mutant (cGKI-K407A/R409A (Gudi et al, 1997)), defective in nuclear translocation due to mutations in the nuclear localization sequence (NLS), completely reversed the stimulatory TFII-I effect on BRE activity (Figure 6E, lanes 3 and 5). However, this mutant still interacted with BMPRII (data not shown), and increased Smad1/5/8 C-terminal phosphorylation to the same extent as wild-type cGKI (Figure 6F). We conclude that nuclear translocation of cGKI is crucial for its ability to enhance BMP-2-induced, Smad-dependent transcription. Furthermore, the transcription factor TFII-I seems to be involved, and its effect depends on the presence of cGKI in the nucleus.
cGKI rescues cellular responses arising from BMPRII mutants that cause PAH
PAH is a vascular disease characterized by narrowing of the pulmonary artery caused by vasoconstriction and vascular remodelling through proliferation of VSMCs and endothelial cells (Puri et al, 2007). Genetic studies on PAH (idiopathic and familial) have revealed heterozygous germline mutations in BMPRII (Waite and Eng, 2003). NO, cGMP and cGKs have been implicated in many physiological processes such as vasodilation, cardiac contractility and remodelling of VSMCs. Mice deficient in cGKI show impaired NO/cGMP-dependent dilations of arteries (Hofmann et al, 2006). The fact that specific mutations in BMPRII cause PAH suggested that proteins associated with this receptor might have an important function in PAH and related diseases (Foletta et al, 2003; Machado et al, 2003; Zakrzewicz et al, 2007).
To explore whether the crosstalk between cGKI and BMPRII can modify the role of BMPRII in PAH, we studied the effect of cGKI on the defective BMP signalling mediated by the mutant BMPRII-Q657ins16. This loss-of-function BMPRII-tail mutant was identified in patients with idiopathic PAH (Thomson et al, 2000). BMPRII-Q657ins16 was much less effective in inducing BMP signalling than wild-type BMPRII (Figure 7A, lanes 2, 4 and 6). Coexpression of cGKI with wild-type BMPRII further enhanced signalling (Figure 7A, lanes 2, 4 and 5). Interestingly, despite BMPRII-Q657ins16 failed to induce BMP signalling, cGKI coexpression with this mutant receptor upregulated the reporter gene response, raising its signalling to the same level induced by wild-type BMPRII expression alone (Figure 7A, lanes 2, 4, 6 and 7).
Similar results were obtained with other PAH BMPRII-tail mutants, such as BMPRII-N764ins47 (Machado et al, 2001) and BMPRII-A796ins7 (Thomson et al, 2000). All the PAH mutants showed reduced BMP-2 responsiveness of BRE transcriptional activation, which was rescued by cGKI coexpression (Figure 7B). Notably, the incremental increase in the response to BMP-2 in cells expressing the BMPRII PAH mutants was similar to or even higher than in cells expressing wild-type BMPRII (Figure 7B). Both PAH-BMPRII-tail mutants BMPRII-Q567ins16 and BMPRII-N764ins47 are still competent in binding cGKI (Figure 7C).
In addition to these results, we have investigated the effect of cGKI on BMPRII PAH mutants under more physiological conditions. We transfected human aortic smooth muscle cells with BMPRII-Q657ins16 and/or cGKI or empty vector and stimulated the cells with platelet-derived growth factor (PDGF) or serum. Measurement of the proliferation rates resulted in a reduced proliferation of cGKI-transfected cells (−18%) compared with control cells, whereas transfection of the PAH receptor mutant increased cell proliferation (+11%). Intriguingly, coexpression of cGKI to BMPRII-Q657ins16 lead to a repressed cell growth indicating that the proproliferative effect of the PAH mutant on VSMCs could be compensated for by coexpression of cGKI.
Taken together, our data show that cGKI has an important function in stimulating BMPRII signalling and might be involved in the pathogenesis of PAH in patients with mutations in BMPRII.
Discussion
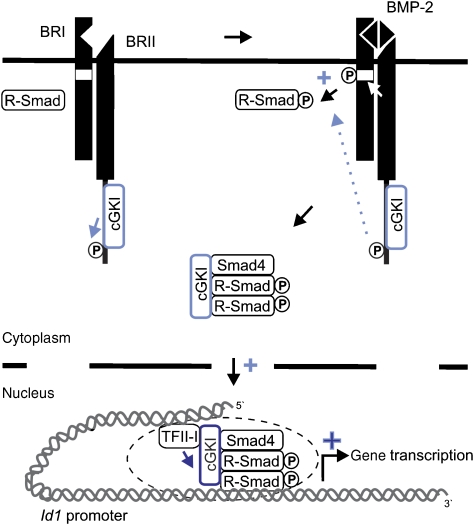
The importance of stringent control of BMP signalling is highlighted by the diseases involving dysfunctions of this pathway, including developmental disorders, fibrosis, cancer and vascular diseases (Waite and Eng, 2003). Indeed, BMP signalling is controlled at multiple levels, including extracellular cues, interactions of BMP receptors at the plasma membrane and intracellular events (Hartung et al, 2006b). Here, we show that the cytoplasmic cGKI enhances Smad signalling at different levels of the BMP pathway. It specifically interacts with BMPRII, phosphorylates the receptor and concomitantly regulates Smad phosphorylation. Furthermore, it translocates with activated R-Smad/co-Smad complexes to the nucleus, and binds together with Smads and TFII-I to the promoter of the target gene Id1 to upregulate its transcription. The model in Figure 8 illustrates this dynamic regulation of BMP signalling by cGKI.
Figure 8.
Model depicting the dual role of cGKI in BMP signalling pathway cGKI regulates BMP signalling through (light blue) modulation of BMP receptors at the cell surface and enhancement of R-Smad phosphorylation and thus Smad activation. After BMP-2-induced association with activated Smad complexes and shuttling into the nucleus, cGKI (dark blue) further controls Smad-mediated transcriptional activation as a nuclear co-factor for Smads, which at least recruits TFII-I. For details, see text.
cGKI, an additional kinase at the BMP receptor complex
cGKI is a serine/threonine kinase, which in the inactive form is blocked by its own pseudo-substrate domain (Francis et al, 2002). Activation of the kinase is achieved by binding of cGMP and subsequent release of the pseudo-substrate. Interestingly, binding of cGKI to BMPRII does not require cGMP, but phosphorylation of BMPRII in the tail domain requires activation of the kinase by cGMP. This suggests that cGKI can bind to the receptor before its activation. As the tyrosine kinase receptor c-Kit (Hassel et al, 2006), cGKI is a BMPRII-associated kinase, which phosphorylates the receptor at the tail region.
The canonical Smad pathway is initiated by BMP binding to BMPRII/BMPRI PFCs (Nohe et al, 2002). Recently, it was shown that the phosphatase Dullard dephosphorylates BMPRIa and inhibits BMP-mediated Smad signalling (Satow et al, 2006). Here, we suggest that cGKI binds BMPRII (and through an indirect mechanism BMPRIa) to support BMP/Smad signalling, most likely by stabilizing the PFCs. Silencing of cGKI using RNAi lead to downregulation of BMPRII-mediated transphosphorylation of BMPRIa at the GS-box (data not shown), supporting a phosphorylation-mediated action of cGKI on the receptor complex.
Taken together, our data suggest that cGKI binds BMPRII in PFCs, phosphorylates BMPRII in the tail region and thereby modulates both BMPRII and BMPRI activities. This leads to enhanced phosphorylation of BMP R-Smads at their C termini, increasing Smad signalling responses.
BMP-mediated piggyback traffic of cGKI and Smads from the receptors to the target genes
Chan and co-workers recently showed that the interaction of BMPRII-tail with Trb-3 enhances BMP/Smad signalling by inducing Smurf1 degradation. On ligand stimulation, Trb3 dissociates from the BMPRII-tail (Chan et al, 2007). This raised the possibility that ligand-induced conformational changes within the receptor complex result in conformations that favour or disfavour specific protein–protein interactions. In accord with this notion, we observed here that cGKI dissociates from the receptor after stimulation with BMP-2. This may occur after a conformational change of the receptor complex induced by BMP-2 binding, which triggers transphosphorylation of BMPRI by BMPRII (Shi and Massague, 2003), or following endocytosis of the receptors, which has been shown to be important for the release of activated R-Smads from the receptor complex (Hartung et al, 2006a).
Subsequent to stimulation with BMP-2, cGKI interacts with activated Smad complexes in the cytoplasm and in the nucleus. As both the association of cGKI with BMPRII and with Smads is regulated by BMP-2, we suggest a sequential binding mechanism where cGKI changes its binding partner. Initially, cGKI binds to BMPRII. After ligand binding to the BMP receptors, BMP R-Smads get phosphorylated. cGKI then associates with the activated Smad proteins, and moves with them to the nucleus.
Smad nucleo-cytoplasmic shuttling dynamics were mainly described for co-Smad4 and the TGFβ R-Smad, Smad2. After being activated, Smads preferentially stay in the nucleus, where they are inactivated by phosphatases such as PPM1A (Lin et al, 2006), which allows their release to the cytoplasm. New signals reactivate cytoplasmic Smad2 to dynamically maintain nuclear accumulation (Schmierer and Hill, 2007; Schmierer et al, 2008). Corresponding studies on Smad1 and Smad5, the BMP R-Smads, are lacking. Here, we show that on BMP-2 stimulation, cGKI leaves the receptor complex piggybacked on activated R-Smads. It will be interesting to investigate the impact of this association on the dynamics of the nucleo-cytoplasmic shuttling of Smad1 or Smad5.
cGMP-induced nuclear translocation of cGKI is mediated by an NLS inside the kinase domain and requires active transport (Gudi et al, 1997). One target gene regulated by activated cGKI is c-fos. The c-fos promoter is induced by cGMP-dependent redistribution of cGKI to the nucleus and by cGKI-mediated phosphorylation of the cAMP-response element binding protein (Gudi et al, 1997, 2000). It is suggested that cell type-specific anchoring proteins regulate the redistribution of cGKI to the nucleus (Casteel et al, 2002). Smads might represent such novel anchoring proteins, which make cGKI responsive to BMP-2-induced nuclear translocation. Thus, BMP-2 is a novel stimulus, uncoupled from cGMP, for the subcellular distribution of cGKI. This opens new avenues for cGKI biological functions. In addition, cGKI represents a novel BMP signalling molecule, which follows a route reminiscent of Smad signalling: from interaction with BMP receptors, through release from the receptors after BMP-2 stimulation, culminating in effects on the transcriptional control of BMP target genes.
Interactions of cGKI in the nucleus
cGKI is a known regulator of transcription factors (Bois et al, 2005; Pilz and Broderick, 2005). Casteel and co-workers showed an interaction of cGKIβ with TFII-I (Casteel et al, 2002), a general transcription factor binding to Inr elements and upstream regulatory sites primarily in TATA-box-less promoters (Roy, 2001). Phosphorylation of Ser371 and 743 in TFII-I is necessary for the induction of c-fos promoter response (Casteel et al, 2002). Earlier studies reported that both serines are phosphorylated after TGFβ-1 stimulation and are important for Smad3/TFII-I complex formation and TGFβ-1-dependent reporter gene response (Stasyk et al, 2005). TFII-I also regulates TGFβ-mediated induction of the goosecoid (gsc) gene in P19 cells by interacting with Smad2 and by recruitment to the gsc promoter after stimulation with TGFβ (Ku et al, 2005). Here, we show that TFII-I and cGKI co-localize with Smad1 at the Id1 promoter after BMP-2 stimulation, suggesting that these proteins form a ternary complex at the DNA. Furthermore, we took advantage of cGKI-K407A/R409A, a cGKI mutant defective in cGMP-mediated nuclear translocation (Gudi et al, 1997). Although this mutant still interacts with BMPRII and enhances Smad phosphorylation, there is no upregulation of BRE reporter gene activity (data not shown), concomitant with its defective nuclear translocation. Interestingly, expression of TFII-I stimulates BMP signalling similar to wild-type cGKI, whereas the cGKI NLS mutant significantly represses the activating effect of TFII-I. This suggests that the presence of TFII-I in the nucleus is not sufficient to induce Smad signalling; cGKI redistribution to the nucleus on BMP-2 stimulation is also required to enable the TFII-I effect on Smad target gene activation. Only then the transcriptional complex at the promoter site of Smad target genes is complete and most efficient.
cGMP/cGKI and BMP signalling in hypertension disease
PAH is characterized by thickening of pulmonary arteries due to abnormal proliferation and apoptosis of cells and remodelling of the small arteries. Accompanied with vasoconstriction, PAH patients suffer from elevated pressure in the pulmonary artery and heart failure (Puri et al, 2007). PAH (idiopathic and familial) has been shown to be associated with heterozygous germline mutations in BMPRII (Waite and Eng, 2003). Smooth muscle-specific expression of mutant BMPRII in transgenic mice results in increased thickness of pulmonary arteries and increased muscularization of small pulmonary arteries. This suggests that loss of BMPRII function in smooth muscle cells is sufficient to cause a PAH phenotype (West et al, 2004). Crosstalk to other signalling pathways increases the complexity of BMP signalling. Thus, crosstalk mechanisms involving BMPRII are assumed to influence pulmonary hypertension diseases (Foletta et al, 2003; Machado et al, 2003; Chan et al, 2007). For instance, the BMPRII-associated receptor for activated C-kinase 1 (Rack-1) appears to be important for the pathology of hypertension diseases. Rack-1 binding to PAH BMPRII mutants is weaker, enhances the antiproliferative BMP signalling and is thus a negative regulator of cell proliferation (Zakrzewicz et al, 2007). cGKI, characterized here mainly as a modulator of BMP signalling in the myoblastic C2C12 cells, is itself a key regulator of vasodilation (Hofmann et al, 2006). Its potential relevance to PAH is underlined by the involvement of increased cGMP levels in muscle relaxation; the PDE5 inhibitor Sildenafil, used to treat PAH patients, supports pulmonary vasodilation (Ghofrani et al, 2006; Hemnes and Champion, 2006). Importantly, the overexpression of cGKI restores normal BMP responsiveness in cells expressing signalling deficient PAH mutant receptors such as the mutant BMPRII-Q657ins16 (Thomson et al, 2000), suggesting that cGKI is able to overcome deficient BMP signalling in cells expressing PAH mutants of BMPRII.
A multiplicity of studies describe an antiproliferative role of the cGMP/cGKI pathway in VSMC differentiation in cell culture, although the overall mechanisms involved in growth and proliferation of VSMCs are still controversial (Lincoln et al, 2006). We have found an antiproliferative action of overexpressed cGKI in aortic VSMCs, which moreover overwrote the proproliferative effect of PAH mutant receptors such as BMPRII-Q657ins16. Further studies should be directed to determine the detailed mechanism underlying the cooperation between cGMP/cGKI signalling and BMPRII in PAH and related diseases. The modulation of BMP receptor and Smad activity might represent a cGKI-dependent regulatory mechanism in addition to earlierdescribed mechanisms, such as transcriptional control of specific genes through cGMP/cGKI in the vascular system (Pilz and Broderick, 2005).
In summary, we propose that the ability of cGKI to compensate for deficient BMP signalling by PAH BMPRII mutants is due to cGKI-induced enhancement of Smad phosphorylation, acting downstream of the receptors. The cGKI and BMP pathways have been ascribed high importance in hypertension diseases, but they were each considered separately. The present studies provide novel evidence that these pathways are integrated. Thus, the crosstalk between cGKI and BMP signalling not only expands the functional flexibility of the cGMP/cGKI pathway, but also opens new prospects for the investigation of BMP/cGKI/Smad signalling pathways and for the development of new treatments to vascular diseases.
Materials and methods
Cell culture and transfection
HEK293T and C2C12 cells (both from ATCC) were cultivated in Dulbecco's modified eagle medium (DMEM) supplemented with 10% (v/v) FBS and 100 U/ml penicillin and 100 mg/ml streptomycin. Human aortic VSMCs were obtained from U Rauch (Charite, Berlin, Germany) and cultivated in medium 231 with 5% (v/v) of the smooth muscle growth supplement SMGS (both Cascade Biologics). HEK293T cells were transfected with polyethylenimine (PEI) (Roth). For transfection of C2C12 cells, PEI or Lipofectamine 2000 (Invitrogen) were used according to manufacturer's instructions. VSMCs were transfected with Fugene HD (Roche Diagnostics). Cells were assayed 24–48 h after transfection.
Immunoprecipitation
C2C12 cells or transfected HEK293T cells were either lysed directly or starved for 3 h in DMEM/0.5% (v/v) FBS and stimulated with 10 nM BMP-2 (W. Sebald, University of Wuerzburg, Wuerzburg, Germany) for 10–30 min. Cell lysis was carried out using lysis buffer (1% (v/v) Triton X-100, 150 mM NaCl, 20 mM Tris/HCl pH 7.5, COMPLETE® EDTA-free protease inhibitors (Roche Diagnostics), 1 mM phenylmethylsulfonylfluoride) and immunoprecipitation was performed with 0.5–1 μg of antibody overnight under rotation at 4°C. After intense washing, precipitated proteins were separated by SDS–PAGE, transferred to 0.2 μm nitrocellulose membrane and analysed by immunoblotting.
Pull-down for in vivo and in vitro binding
For in vivo binding, C2C12 cells expressing cGKI were lysed in lysis buffer. A measure of 1 μg of GST, GST–BMPRII-SF or GST–BMPRII-tail bound to glutathione sepharose were incubated with cell lysate overnight at 4°C. For in vitro binding, 1 μg of GST, GST–BMPRII-SF or GST–BMPRII-tail bound to glutathione sepharose were incubated for 1 h at 4°C with 1 μg of MBP or MBP-cGKI in 50 μl of binding buffer (0.1% (v/v) Nonidet P-40, 150 mM NaCl, 20 mM Tris–HCl, pH 7.5, 1 mM EDTA, 0.5 mM dithiothreithol, 0.1% bovine serum albumine, 10% (v/v) glycerol, COMPLETE® EDTA-free protease inhibitors, 1 mM phenylmethylsulfonylfluoride). After intense washing, BMPRII-bound protein was isolated on glutathione sepharose beads, eluted in SDS sample buffer and examined by SDS–PAGE and immunoblot.
In vitro kinase assay
Recombinant BMPRII cytoplasmic domains and cGKI (Promega) were supplemented with 25 μl kinase buffer (150 mM NaCl, 20 mM Hepes pH 7.4, 75 mM MgCl2, 500 μM ATP, 1 mM dithiothreithol), either with or without 25 μM 8-Br-cGMP (Biolog). Phosphorylation was initiated by addition of 1 μCi of (γ-32P)ATP (Hartmann Analytics) and samples were incubated for 30 min at 30°C. Phosphorylated proteins, separated on SDS–PAGE and transferred to nitrocellulose membrane, were detected using X-ray films. Protein loading was determined by subsequent immunoblotting.
In vivo kinase assay
Transfected C2C12 cells, starved for 3 h and stimulated with 1 μM 8-Br-cGMP for 30 min, were lysed in lysis buffer containing phosphatase inhibitors. Cleared lysates were subjected to immunoprecipitation with α-BMPRII antibody (N-terminal) for BMPRII protein enrichment. After SDS–PAGE and immunoblotting, samples were probed with α-pcGK substrate antibody. BMPRII phosphorylation was quantified relative to BMPRII protein amount using ImageJ (Wayne Rasband (National Institutes of Health, NIH); http://rsb.info.nih.gov/ij).
Immunofluorescence microscopy
C2C12 cells were starved for 3 h and incubated with or without 10 nM BMP-2 and/or 1 mM 8-Br-cGMP for 30 min. Immunofluorescence staining and microscopy were performed as described (Bengtsson and Wilson, 2006) using specific primary antibodies (α-cGKI (C-terminal) antibody (Stressgen), α-Smad1 and α-Smad4 antibodies (Santa Cruz Biotechnology) and α-TFII-I antibody (BD Biosciences)), and fluorescent dye-coupled secondary antibodies (goat α-mouse IgG (H+L), conjugated to Alexa Fluor 594 or 488 or goat α-rabbit IgG (H+L), conjugated to Alexa Fluor 594 (Invitrogen)). After mounting the slides (Fluoromount G; Southern Biotech), cells were viewed using a fluorescence microscope (Axiovert 200; Zeiss) equipped with a camera (Axiocam HRM; Zeiss) and a PlanApochromat 63/1.4 oil objective (Zeiss). Images were analysed using Axiovision (Zeiss) and Photoshop software (Adobe).
Chromatin immunoprecipitation
ChIP was performed as described earlier (Weiske and Huber, 2006) with minor modifications. Briefly, C2C12 cells were grown to a confluence of 80–90% (10 cm dish). After stimulation with 10 nM BMP and/or 1 μM 8-Br-cGMP for 4 h, cells were washed with PBS, fixed with 2 mM disuccinimidyl-glutarate and cross-linked with 1% (v/v) formaldehyde and samples were subjected to immunoprecipitation with 2.5–5 μg of a specific antibody (α-Smad1 (Santa Cruz Biotechnology), α-cGKI (C-terminal) antibody (Stressgen) or α-TFII-I antibody (BD Biosciences)). For two-step ChIP, immunocomplexes of the first ChIP were eluted by adding 100 μl 10 mM dithiothreithol (30 min, 37°C) and diluted in ChIP dilution buffer followed by incubation with second-step antibody. ChIP and two-step ChIP were performed in the same way. For subsequent PCR analysis, extracted DNA was used as a template to amplify an Id1 promoter fragment using specific oligodeoxynucleotides (5′-GGAGCGGAGAATGCTCCAG-3′ (forward), 5′-GAAGGCCTCCGAGCAAGC-3′ (reverse)). PCR products were separated on 8% polyacrylamide gels and analysed under UV light.
Other assays
For analysing Smad phosphorylation, transfected C2C12 cells were treated (10 nM BMP-2) and examined as described (Hartung et al, 2006a). Quantification was done using ImageJ (Wayne Rasband, NIH). Analysis of Smad-dependent target gene transcription in C2C12 cells was performed as described (Hartung et al, 2006a). For investigation of VASP phosphorylation, C2C12 cells were starved for 24 h, stimulated with 1 or 100 μM 8-Br-cGMP (as indicated) for 30 min and analysed as described for Smad phosphorylation assay. For studying the effect of cGKI knockdown, C2C12 cells were transfected with sh-cGKI or sh-nt, and 48 h after transfection, cells were subjected to immunoblotting, Smad phosphorylation assay, immunofluorescence (on coexpression of GFP), immunoprecipitation or BRE luciferase reporter gene assay (Hartung et al, 2006a). To analyse cell proliferation, human aortic smooth muscle cells were used. Transfected cells were starved for 24 h and stimulated either with 20 nM PDGF-BB or with 10% of the smooth muscle growth supplement SMGS for 24 h. Proliferation was measured with the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) according to manufacturer's instructions.
Data presentation and statistical analysis
Immunoblots, autoradiographs and PCRs are shown from representative experiments that were reproduced at least three times with similar results. Pictures were processed with Photoshop software (Adobe). The means of indicated groups were compared using two-tailed Student's t test. P-values of <0.05 and <0.01 were considered to indicate statistical significance.
Supplementary Material
Supplementary Information
Acknowledgments
We thank S Hassel, S Souchelnytskyi and U Hellmann for their contribution to the initial proteomics studies. We thank R Pilz for diverse constructs, H Volkmer for providing us with sh-RNA constructs, and U Rauch for VSMCs. We are grateful to C Hiepen, J Börgermann, C Sieber and F Bormann for excellent technical assistance and R Pilz and L Bengtsson for helpful discussions and for critical reading of the manuscript. This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG, grant number Kn 332/8-1, to PK) and the German-Israeli Foundation for Scientific Research and Development (GIF, grant number 932-244.13/2006, to YIH and PK). YIH is an incumbent of the Zalman Weinberg Chair in Cell Biology.
References
- Bengtsson L, Schwappacher R, Roth M, Boergermann JH, Hassel S, Knaus P (2009) PP2A regulates BMP signalling by interacting with BMP receptor complexes and by dephosphorylating both the C-terminus and the linker region of Smad1. J Cell Sci 122(Pt 8): 1248–1257 [DOI] [PubMed] [Google Scholar]
- Bengtsson L, Wilson KL (2006) Barrier-to-autointegration factor phosphorylation on Ser-4 regulates emerin binding to lamin A in vitro and emerin localization in vivo. Mol Biol Cell 17: 1154–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois PR, Brochard VF, Salin-Cantegrel AV, Cleveland JL, Grosveld GC (2005) FoxO1a-cyclic GMP-dependent kinase I interactions orchestrate myoblast fusion. Mol Cell Biol 25: 7645–7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U (1994) cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269: 14509–14517 [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E (2003) Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev 24: 218–235 [DOI] [PubMed] [Google Scholar]
- Casteel DE, Zhuang S, Gudi T, Tang J, Vuica M, Desiderio S, Pilz RB (2002) cGMP-dependent protein kinase I beta physically and functionally interacts with the transcriptional regulator TFII-I. J Biol Chem 277: 32003–32014 [DOI] [PubMed] [Google Scholar]
- Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du K, Lagna G, Hata A (2007) A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol 27: 5776–5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Francis SH, Thomas JW, Maksymovitch EA, Fosler M, Corbin JD (1998) Activation by autophosphorylation or cGMP binding produces a similar apparent conformational change in cGMP-dependent protein kinase. J Biol Chem 273: 14649–14656 [DOI] [PubMed] [Google Scholar]
- Feil R, Feil S, Hofmann F (2005a) A heretical view on the role of NO and cGMP in vascular proliferative diseases. Trends Mol Med 11: 71–75 [DOI] [PubMed] [Google Scholar]
- Feil R, Hofmann F, Kleppisch T (2005b) Function of cGMP-dependent protein kinases in the nervous system. Rev Neurosci 16: 23–41 [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R (2005) Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693 [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O (2003) Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Poteet-Smith C, Busch JL, Richie-Jannetta R, Corbin JD (2002) Mechanisms of autoinhibition in cyclic nucleotide-dependent protein kinases. Front Biosci 7: d580–d592 [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131: 980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani HA, Osterloh IH, Grimminger F (2006) Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 5: 689–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P (2000) Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol Biol Cell 11: 1023–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi T, Casteel DE, Vinson C, Boss GR, Pilz RB (2000) NO activation of fos promoter elements requires nuclear translocation of G-kinase I and CREB phosphorylation but is independent of MAP kinase activation. Oncogene 19: 6324–6333 [DOI] [PubMed] [Google Scholar]
- Gudi T, Lohmann SM, Pilz RB (1997) Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase: identification of a nuclear localization signal. Mol Cell Biol 17: 5244–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakre S, Tussie-Luna MI, Ashworth T, Novina CD, Settleman J, Sharp PA, Roy AL (2006) Opposing functions of TFII-I spliced isoforms in growth factor-induced gene expression. Mol Cell 24: 301–308 [DOI] [PubMed] [Google Scholar]
- Harradine KA, Akhurst RJ (2006) Mutations of TGFbeta signaling molecules in human disease. Ann Med 38: 403–414 [DOI] [PubMed] [Google Scholar]
- Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P (2006a) Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol 26: 7791–7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung A, Sieber C, Knaus P (2006b) Yin and Yang in BMP signaling: impact on the pathology of diseases and potential for tissue regeneration. Signal Transduction 6: 314–328 [Google Scholar]
- Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S (2004) Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 4: 1346–1358 [DOI] [PubMed] [Google Scholar]
- Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, Ehrlich M, Henis YI, Sebald W, Knaus P (2003) Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am 85-A (Suppl 3): 44–51 [DOI] [PubMed] [Google Scholar]
- Hassel S, Yakymovych M, Hellman U, Ronnstrand L, Knaus P, Souchelnytskyi S (2006) Interaction and functional cooperation between the serine/threonine kinase bone morphogenetic protein type II receptor with the tyrosine kinase stem cell factor receptor. J Cell Physiol 206: 457–467 [DOI] [PubMed] [Google Scholar]
- Hemnes AR, Champion HC (2006) Sildenafil, a PDE5 inhibitor, in the treatment of pulmonary hypertension. Expert Rev Cardiovasc Ther 4: 293–300 [DOI] [PubMed] [Google Scholar]
- Hofmann F, Feil R, Kleppisch T, Schlossmann J (2006) Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23 [DOI] [PubMed] [Google Scholar]
- Knockaert M, Sapkota G, Alarcon C, Massague J, Brivanlou AH (2006) Unique players in the BMP pathway: small C-terminal domain phosphatases dephosphorylate Smad1 to attenuate BMP signaling. Proc Natl Acad Sci USA 103: 11940–11945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchynskyi O, ten Dijke P (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem 277: 4883–4891 [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J (1997) Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature 389: 618–622 [DOI] [PubMed] [Google Scholar]
- Ku M, Sokol SY, Wu J, Tussie-Luna MI, Roy AL, Hata A (2005) Positive and negative regulation of the transforming growth factor beta/activin target gene goosecoid by the TFII-I family of transcription factors. Mol Cell Biol 25: 7144–7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA III, Loyd JE, Nichols WC, Trembath RC (2000) Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 26: 81–84 [DOI] [PubMed] [Google Scholar]
- Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, Shi Y, Chen YG, Meng A, Feng XH (2006) PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 125: 915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Wu X, Sellak H, Dey N, Choi CS (2006) Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367 [DOI] [PubMed] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F (2002) Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem 277: 3176–3185 [DOI] [PubMed] [Google Scholar]
- Lories RJ, Luyten FP (2005) Bone morphogenetic protein signaling in joint homeostasis and disease. Cytokine Growth Factor Rev 16: 287–298 [DOI] [PubMed] [Google Scholar]
- Machado RD, Pauciulo MW, Thomson JR, Lane KB, Morgan NV, Wheeler L, Phillips JA III, Newman J, Williams D, Galie N, Manes A, McNeil K, Yacoub M, Mikhail G, Rogers P, Corris P, Humbert M, Donnai D, Martensson G, Tranebjaerg L et al. (2001) BMPR2 haploinsufficiency as the inherited molecular mechanism for primary pulmonary hypertension. Am J Hum Genet 68: 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC (2003) Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet 12: 3277–3286 [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P (2002) The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277: 5330–5338 [DOI] [PubMed] [Google Scholar]
- Ogata T, Wozney JM, Benezra R, Noda M (1993) Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc Natl Acad Sci USA 90: 9219–9222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik S, Natarajan V, Tasken K, Jahnsen T, Sandberg M (1997) Characterization of the human gene encoding the type I alpha and type I beta cGMP-dependent protein kinase (PRKG1). Genomics 42: 311–318 [DOI] [PubMed] [Google Scholar]
- Osada S, Ohmori SY, Taira M (2003) XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development 130: 1783–1794 [DOI] [PubMed] [Google Scholar]
- Pilz RB, Broderick KE (2005) Role of cyclic GMP in gene regulation. Front Biosci 10: 1239–1268 [DOI] [PubMed] [Google Scholar]
- Puri A, McGoon MD, Kushwaha SS (2007) Pulmonary arterial hypertension: current therapeutic strategies. Nat Clin Pract Cardiovasc Med 4: 319–329 [DOI] [PubMed] [Google Scholar]
- Richie-Jannetta R, Francis SH, Corbin JD (2003) Dimerization of cGMP-dependent protein kinase Ibeta is mediated by an extensive amino-terminal leucine zipper motif, and dimerization modulates enzyme function. J Biol Chem 278: 50070–50079 [DOI] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K (1995) Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 92: 7632–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AL (2001) Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene 274: 1–13 [DOI] [PubMed] [Google Scholar]
- Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F (1991) The activation of expressed cGMP-dependent protein kinase isozymes I alpha and I beta is determined by the different amino-termini. Eur J Biochem 202: 1339–1344 [DOI] [PubMed] [Google Scholar]
- Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J (2007) Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25: 441–454 [DOI] [PubMed] [Google Scholar]
- Sapkota G, Knockaert M, Alarcon C, Montalvo E, Brivanlou AH, Massague J (2006) Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-beta pathways. J Biol Chem 281: 40412–40419 [DOI] [PubMed] [Google Scholar]
- Satow R, Kurisaki A, Chan TC, Hamazaki TS, Asashima M (2006) Dullard promotes degradation and dephosphorylation of BMP receptors and is required for neural induction. Dev Cell 11: 763–774 [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS (2005) Molecular genetics of axis formation in zebrafish. Annu Rev Genet 39: 561–613 [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Feil R, Hofmann F (2005) Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci 10: 1279–1289 [DOI] [PubMed] [Google Scholar]
- Schmierer B, Hill CS (2007) TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982 [DOI] [PubMed] [Google Scholar]
- Schmierer B, Tournier AL, Bates PA, Hill CS (2008) Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system. Proc Natl Acad Sci USA 105: 6608–6613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Smith JA, Francis SH, Walsh KA, Kumar S, Corbin JD (1996) Autophosphorylation of type Ibeta cGMP-dependent protein kinase increases basal catalytic activity and enhances allosteric activation by cGMP or cAMP. J Biol Chem 271: 20756–20762 [DOI] [PubMed] [Google Scholar]
- Smolenski A, Bachmann C, Reinhard K, Honig-Liedl P, Jarchau T, Hoschuetzky H, Walter U (1998) Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem 273: 20029–20035 [DOI] [PubMed] [Google Scholar]
- Stasyk T, Dubrovska A, Lomnytska M, Yakymovych I, Wernstedt C, Heldin CH, Hellman U, Souchelnytskyi S (2005) Phosphoproteome profiling of transforming growth factor (TGF)-beta signaling: abrogation of TGFbeta1-dependent phosphorylation of transcription factor-II-I (TFII-I) enhances cooperation of TFII-I and Smad3 in transcription. Mol Biol Cell 16: 4765–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE et al. (2000) Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 37: 741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AC, Wrana JL (2005) The disparate role of BMP in stem cell biology. Oncogene 24: 5713–5721 [DOI] [PubMed] [Google Scholar]
- Waite KA, Eng C (2003) From developmental disorder to heritable cancer: it's all in the BMP/TGF-beta family. Nat Rev Genet 4: 763–773 [DOI] [PubMed] [Google Scholar]
- Wall ME, Francis SH, Corbin JD, Grimes K, Richie-Jannetta R, Kotera J, Macdonald BA, Gibson RR, Trewhella J (2003) Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase. Proc Natl Acad Sci USA 100: 2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiske J, Huber O (2006) The histidine triad protein Hint1 triggers apoptosis independent of its enzymatic activity. J Biol Chem 281: 27356–27366 [DOI] [PubMed] [Google Scholar]
- West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM (2004) Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114 [DOI] [PubMed] [Google Scholar]
- Zakrzewicz A, Hecker M, Marsh LM, Kwapiszewska G, Nejman B, Long L, Seeger W, Schermuly RT, Morrell NW, Morty RE, Eickelberg O (2007) Receptor for activated C-kinase 1, a novel interaction partner of type II bone morphogenetic protein receptor, regulates smooth muscle cell proliferation in pulmonary arterial hypertension. Circulation 115: 2957–2968 [DOI] [PubMed] [Google Scholar]
- Zhao J, Trewhella J, Corbin J, Francis S, Mitchell R, Brushia R, Walsh D (1997) Progressive cyclic nucleotide-induced conformational changes in the cGMP-dependent protein kinase studied by small angle X-ray scattering in solution. J Biol Chem 272: 31929–31936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information