Abstract
Proneurotrophins mediate neuronal apoptosis using a dual receptor complex of sortilin and p75NTR. Although p75NTR is highly expressed on the plasma membrane and accessible to proneurotrophin ligands, sortilin is primarily localized to intracellular membranes, limiting the formation of a cell surface co-receptor complex. Here, we show that the mammalian p75NTR homologue NRH2 critically regulates the expression of sortilin on the neuronal cell surface and promotes p75NTR and sortilin receptor complex formation, rendering cells responsive to proneurotrophins. This is accomplished by interactions between the cytoplasmic domains of NRH2 and sortilin that impair lysosomal degradation of sortilin. In proneurotrophin-responsive neurons, acute silencing of endogenous NRH2 significantly reduces cell surface-expressed sortilin and abolishes proneurotrophin-induced neuronal death. Thus, these data suggest that NRH2 acts as a trafficking switch to impair lysosomal-dependant sortilin degradation and to redistribute sortilin to the cell surface, rendering p75NTR-expressing cells susceptible to proneurotrophin-induced death.
Keywords: apoptosis, NRH2, p75NTR, proneurotrophin, sortilin
Introduction
Neurotrophins are growth factors that play crucial roles in the development and maintenance of the vertebrate nervous system through two structurally unrelated receptors, the Trk receptor tyrosine kinases and the neurotrophin receptor p75NTR. Trk receptors regulate neuronal survival, differentiation, and synaptic plasticity through well defined signalling pathways (Patapoutian and Reichardt, 2001; Chao, 2003), whereas p75NTR mediates numerous actions, including neuronal apoptosis or axonal repulsion that are dependant on its co-receptor partners and preferred ligands (Hempstead, 2002; Roux and Barker, 2002; Barker, 2004). Our earlier studies indicate that proforms of neurotrophins (proneurotrophins; i.e. proNGF or proBDNF) selectively bind to p75NTR to induce cell death (Lee et al, 2001; Teng et al, 2005). ProNGF is secreted and can induce cell death under pathologic conditions, including spinal cord injury, CNS lesion, and seizure induction (Beattie et al, 2002; Harrington et al, 2004; Volosin et al, 2006), and is more abundant in Alzheimer's brains, as compared with age-matched controls (Peng et al, 2004), suggesting a pathophysiological role of proNGF in vivo.
ProNGF interacts with a dual receptor complex of p75NTR and sortilin, a Vps10p domain-containing transmembrane protein that is highly expressed in the vertebrate CNS (Sarret et al, 2003; Nykjaer et al, 2004; Jansen et al, 2007). Biochemical studies suggest that the prodomains of neurotrophins bind sortilin, whereas the mature domains bind p75NTR on the cell surface to mediate cell death (Nykjaer et al, 2004). Earlier studies, however, indicate that sortilin is predominantly intracellular in location, in the trans-Golgi network (TGN) where it participates in intracellular trafficking, and <10% of the total sortilin pool is expressed on the plasma membrane (Petersen et al, 1997; Nielsen et al, 1999, 2001; Mazella, 2001; Mari et al, 2008). As p75NTR is primarily localized to the plasma membrane, these observations suggest that mechanisms which promote sortilin expression on the cell surface could determine cellular responsiveness to proneurotrophins. Indeed, a recent report indicates that sortilin is co-expressed with p75NTR on the plasma membrane of mouse retinal ganglion cells (RGCs) when p75NTR-dependant developmentally regulated RGC death is robust (embryonic day 13–15.5). In contrast, at later developmental stages of the retina (postnatal day 0–6), sortilin is preferentially expressed in the Golgi complex and p75NTR-dependant RGC death is not observed (Frade and Barde, 1999; Harada et al, 2006; Nakamura et al, 2007). However, the molecular mechanisms that regulate sortilin localization during neuronal development are unknown.
A mammalian homologue of p75NTR, NRH2 (also termed as PLAIDD or NRADD) shares some sequence similarity to p75NTR, but contains a unique truncated ectodomain that does not bind to neurotrophins (Frankowski et al, 2002; Kanning et al, 2003; Wang et al, 2003; Murray et al, 2004). Although NRH2 has been suggested to function like p75NTR, the cytoplasmic domains of NRH2 and p75NTR indeed share <40% amino acid identity (Murray et al, 2004), suggesting that NRH2 might not merely mimic p75NTR function, but might perform additional biological actions. NRH2 is developmentally regulated, and is expressed at high levels during embryonic development, but downregulated in adult tissues (Frankowski et al, 2002; Wang et al, 2003). NRH2 is co-expressed with p75NTR in subpopulations of cells in the spinal cord, neonatal retina, dorsal root ganglion, or cultured sympathetic neurons (Kanning et al, 2003; Murray et al, 2004), regions where sortilin is expressed, and proneurotrophin-induced cell death has been reported (Sarret et al, 2003; Nykjaer et al, 2004; Domeniconi et al, 2007; Nakamura et al, 2007), suggesting that NRH2 might play a role in proneurotrophin-mediated cell death.
In this study, we investigate the potential actions of NRH2 in regulating proneurotrophin-induced neuronal apoptosis. We find that NRH2 expression is dynamically regulated during development, with increased expression during periods when proneurotrophin-induced apoptosis occurs. NRH2 interacts with both p75NTR and sortilin, facilitating the formation of a p75NTR and sortilin complex. This is achieved by a new mechanism, by which NRH2 acts as a trafficking switch to impair lysosome-dependant sortilin degradation and to redistribute sortilin to the cell surface. These results identify a new process that regulates neuronal responsiveness to pro-apoptotic ligands, in which NRH2 regulates sortilin expression and directs cell surface localization to promote p75NTR–sortilin receptor complex formation and proneurotrophin-induced cell death.
Results
NRH2 interacts with sortilin as well as p75NTR
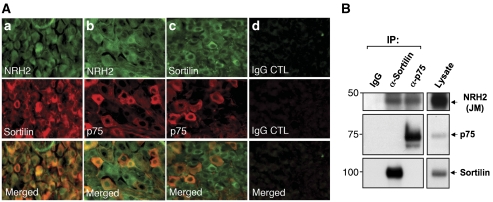
To assess a potential role for NRH2 in regulating proneurotrophin signalling, we first examined whether NRH2 co-localizes with the proneurotrophin receptors, p75NTR and sortilin in vivo. To this end, immunofluorescence microscopy was carried out using tissues where NRH2 and/or p75NTR are known to be expressed (Kanning et al, 2003; Murray et al, 2004). We detected prominent NRH2 immunoreactivity throughout the dorsal root ganglion (DRG) of P1 mice (Figure 1A, a and b), and co-immunostaining with sortilin and NRH2 antibodies showed that most DRG neurons express both NRH2 and sortilin (Figure 1A, a). In contrast, p75NTR is expressed by a subpopulation of neurons that also express both NRH2 and sortilin (Figure 1A, b and c). Co-expression of NRH2 with sortilin or p75NTR is also observed in a subset of spinal motor neurons of P1 mice (Supplementary Figure S1) and in cultured rat superior cervical ganglion (SCG) or DRG neurons (data not shown). Strong co-immunoreactivity of sortilin and NRH2 is also detected in the ganglion cell layer (GCL) of E15.5 mouse retina (Supplementary Figure S2B) where proNGF is expressed and p75NTR-dependant developmentally regulated apoptosis is robust (Frade and Barde, 1999; Harada et al, 2006; Nakamura et al, 2007). However, NRH2 and sortilin immunoreactivity is markedly reduced in the GCL at a later developmental time when apoptosis is not apparent (Supplementary Figure S2C). To determine if NRH2 interacts with p75NTR and sortilin in vivo, we immunoprecipitated p75NTR or sortilin from rat brain, DRG or spinal cord lysates and observed that NRH2 interacts with p75NTR (Figure 1B and Supplementary Figure S3), consistent with earlier studies of overexpression in HEK-293 cells (Frankowski et al, 2002). Interestingly, we could also detect a new interaction of NRH2 with sortilin in brain lysates (Figure 1B and Supplementary Figure S3). Collectively, these results suggest that NRH2 is expressed by p75NTR- and sortilin-expressing, and proneurotrophin-responsive neurons, and that NRH2 interacts with both p75NTR and sortilin in vivo.
Figure 1.
NRH2 is co-expressed and interacts with the proneurotrophin receptors, sortilin and p75NTR. (A) Co-expression of NRH2, sortilin and p75NTR in a subpopulation of dorsal root ganglion (DRG) neurons. DRG sections from C57/BL6 mice (P1) were double-immunostained using anti-NRH2 (1074), anti-sortilin (BAF2934) or anti-p75NTR antibodies (BAF1157 or 9992). Scale bars, 20 μm. (B) Endogenous interaction of NRH2 with sortilin or p75NTR was assessed by immunoprecipitation of rat brain lysates from E17 animals with the indicated antibody, followed by probing with anti-NRH2 (juxtamembrane (JM)), anti-sortilin or anti-p75NTR antisera as noted. Lysate, 40 μg.
NRH2 enhances p75NTR and sortilin association
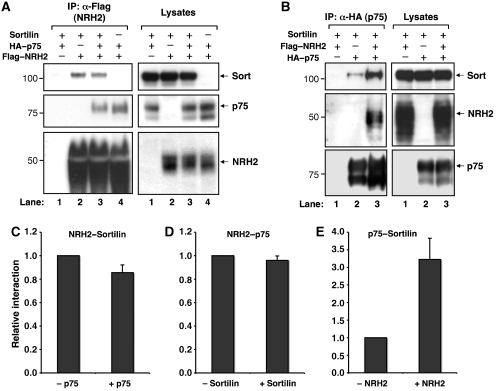
To identify a potential role for NRH2 in regulating the formation of p75NTR and sortilin receptor complexes, we expressed NRH2, p75NTR, and sortilin in heterologous cells and examined the molecular interactions among them. Immunoprecipitation of NRH2 followed by western blot analysis confirmed interactions of NRH2 with both sortilin and p75NTR (Figure 2A). Surprisingly, we found that the interaction between p75NTR and sortilin is significantly increased when NRH2 is co-expressed (Figure 2B). Quantification of western blots indicated that NRH2 expression induces a 3.2-fold increase in the association of p75NTR with sortilin (Figure 2E, n=3), suggesting that NRH2 positively regulates p75NTR–sortilin association by interacting with sortilin and/or p75NTR.
Figure 2.
NRH2 promotes the interaction of p75NTR and sortilin. (A) HEK 293 cells were transfected with plasmids encoding sortilin (2 μg), HA–p75NTR (2 μg) or FLAG–NRH2 (2 μg) as indicated, and the association of NRH2 with p75NTR or sortilin was determined by immunoprecipitation with anti-FLAG antibody followed by immunoblot analysis. Lysates, 10 μg per lane. (B) HEK 293 cells were transfected with 2 μg of sortilin, HA–p75NTR or FLAG–NRH2, and cell lysates were immunoprecipitated with anti-HA antibody followed by immunoblot analysis. Lysates, 10 μg per lane. (C) Quantitation of western blots for the NRH2 and sortilin association represented in (A), lanes 2 and 3. To determine the relative NRH2–sortilin interaction, sortilin bands co-immunoprecipitated with NRH2 were normalized by the band intensities both in lysates and NRH2 immunoprecipitates, and values in the absence and presence of p75NTR expression were compared (mean±s.e.m., n=3). (D) Quantitation of western blots for the p75NTR and NRH2 association represented in (A), lanes 3 and 4. Values were normalized as described above (n=3). (E) Quantitation of western blots for the sortilin and p75NTR association represented in (B), lanes 2 and 3. Values were normalized as described above (n=3).
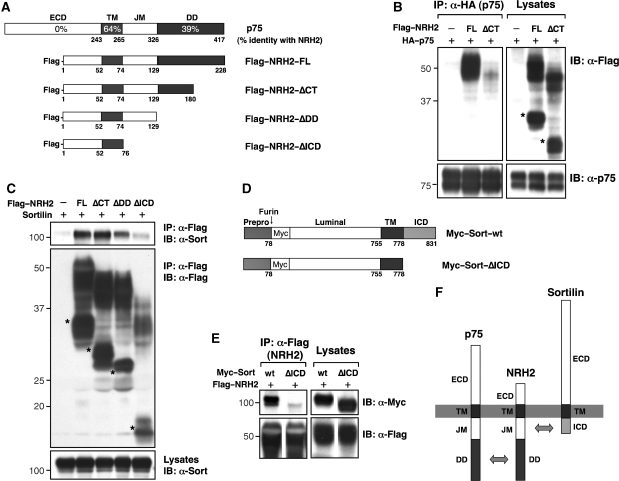
To determine the domains of NRH2 that selectively promote interaction with p75NTR or sortilin, we generated C-terminal serially deleted NRH2 constructs lacking the entire or part of the intracellular domain (FLAG–NRH2–ΔCT, FLAG–NRH2–ΔDD and FLAG–NRH2–ΔICD; Figure 3A). Deletion of a part or the entire NRH2 death domain markedly reduces its association with p75NTR (Figure 3B and data not shown). Deletion of part of the NRH2 death domain (NRH2–ΔCT) does not impair association with sortilin. However, further deletions in the cytoplasmic domain of NRH2 (NRH2–ΔDD and NRH2–ΔICD) progressively decrease its interaction with sortilin (Figure 3C and Supplementary Figure S4). To test whether the intracellular domain of sortilin is required for this interaction, a deletion mutant of sortilin lacking the cytoplasmic tail (Myc–Sort–ΔICD, Figure 3D) was coexpressed with NRH2. Deletion of the sortilin ICD prevented association with NRH2 (Figure 3E), suggesting that the juxtamembrane region of NRH2 and the sortilin cytoplasmic domain promote their association, whereas the death domain of NRH2 is required for interaction with p75NTR (Figure 3F).
Figure 3.
Association of NRH2 with p75NTR and sortilin. (A) Diagrammatic representation of full-length or truncated NRH2 constructs. Sequence similarity between murine p75NTR and murine NRH2 is shown as a % identity for each domain. (B) Death domain of NRH2 is necessary for the interaction with p75NTR. HEK 293 cells were co-transfected with HA–p75NTR and FLAG–NRH2–FL or FLAG–NRH2–ΔCT, and cell lysates were subjected to immunoprecipitation of p75NTR. (C) The juxtamembrane domain of NRH2 promotes sortilin binding. Full-length or truncated NRH2 s were co-expressed with sortilin in HEK 293 cells and their associations were assessed by co-immunoprecipitation with NRH2. Asterisks indicate immaturely N-glycosylated NRH2 intermediates verified by N-glycanase reaction (data not shown). (D) Diagrammatic representation of Myc-tagged full-length and truncated sortilin constructs. (E) ICD deletion of sortilin shows impaired NRH2 binding, as assessed by co-immunoprecipitation of NRH2 in HEK 293 cells. (F) Summary of molecular interactions among p75NTR, NRH2 and sortilin. NRH2-DD is required to interact with p75NTR, whereas NRH2-JM and sortilin-ICD promote their association.
NRH2 selectively increases cell surface expression of sortilin
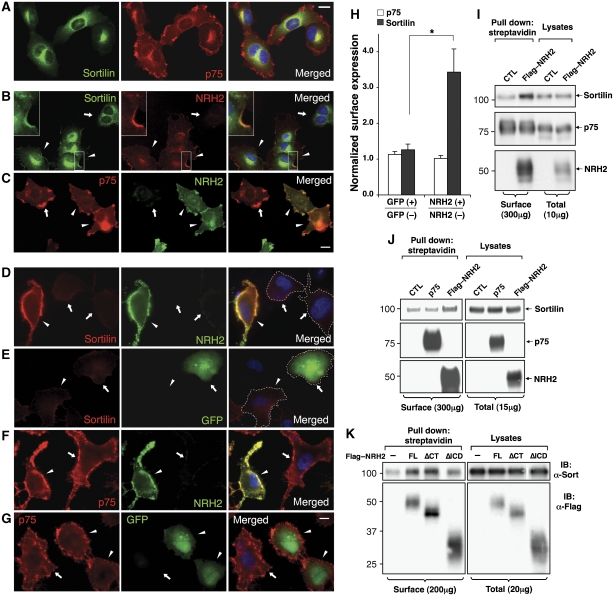
Recent studies suggest that cell surface expression of sortilin is dynamically regulated during development and correlates with cell apoptosis (Nakamura et al, 2007). Therefore, we speculated that NRH2 might regulate the cell surface expression of sortilin to enhance p75NTR and sortilin co-localization. Using HT-1080 cells that stably express both p75NTR and sortilin (HT-1080P/S), we assessed cellular localization of sortilin and p75NTR in the presence or absence of NRH2 using three complementary techniques. First, using double immunofluorescence microscopy of membrane-permeabilized cells lacking NRH2, sortilin was detected predominantly in the perinuclear region of the cells, reflecting its known localization to the Golgi and ER membranes (verified by co-immunostaining with anti-Golgi-Zone antibody and ER stain, data not shown) and endosomes, whereas p75NTR was detected at the leading edge or plasma membrane of cells as well as in the perinuclear region (Figure 4A). Merged images indicate no obvious co-localization of p75NTR and sortilin at the plasma membrane. However, distinctive and prominent sortilin immunoreactivitiy at the edge of cells was detected in NRH2-coexpressing cells (Figure 4B, arrowheads). To directly test whether NRH2 increases cell surface expression of sortilin, immunofluorescence microscopy was carried out in a membrane non-permeabilized condition (Figure 4D–H and Supplementary Figure S5). We detected a significant increase of sortilin immunoreactivity on the cell surface in NRH2-coexpressing cells (Figure 4D, arrowhead), which was not observed neither in NRH2-nonexpressing (Figure 4D, arrows), nor in GFP-expressing control cells (Figure 4E). Quantification of images indicates a 2.7-fold increase of sortilin immunoreactivity on the cell surface upon NRH2 co-expression as compared with the GFP-expressing cells (Figure 4H, *P<0.05, n=4). By contrast, NRH2 does not alter p75NTR cell surface expression, as comparable levels of p75NTR immunoreactivity are detected in cells with and without NRH2 expression (Figure 4C, F and G). Lastly, cell surface biotinylation of HT-1080P/S cells, transfected with NRH2 or control vector (CTL), corroborate these results that NRH2 expression increases cell surface expression of sortilin (2.1±0.25-fold increase, n=4) but not p75NTR (Figure 4I). Given the modest extent of sequence similarity between NRH2 and p75NTR, we asked whether targeting of sortilin to the cell surface is selectively promoted by NRH2. Unlike NRH2, p75NTR expression in sortilin-expressing cells (HT-1080S) fails to increase the level of sortilin detected on the cell surface (Figure 4J). To further determine whether the association of sortilin with NRH2 is required for sortilin relocalization, we carried out cell surface biotinylation of HT-1080S cells expressing full-length or deletion mutants of NRH2 (Figure 4K). Expression of NRH2–ΔICD, which is impaired in sortilin binding (Figure 3C and Supplementary Figure S4), does not increase cell-surface expression levels of sortilin, whereas sortilin-interacting NRH2–FL and NRH2–ΔCT do (Figure 4K), suggesting that sortilin is selectively redistributed to the plasma membrane on its association with NRH2.
Figure 4.
NRH2 selectively relocalizes sortilin to the plasma membrane. (A) Immunofluorescence detection of p75NTR and sortilin in membrane permeabilized HT-1080P/S cells. Scale bar, 20 μm. (B, C) NRH2 redistributes sortilin but not p75NTR. HT-1080P/S cells were transfected with FLAG–NRH2, and the effect of NRH2 expression on sortilin (B) or p75NTR localization (C) was monitored by immunofluorescence microscopy in membrane-permeabilized cells. Arrowheads and arrows indicate NRH2-expressing and non-expressing cells, respectively. Insets, higher magnification of co-localization of NRH2 and sortilin at the leading edge of the cell. Scale bars, 20 μm. (D, E) Cell surface expression of sortilin is markedly increased by NRH2 co-expression. HT-1080P/S cells were transfected with FLAG–NRH2 (D) or GFP (E), and sortilin expression on the cell surface was monitored by immunofluorescence microscopy in a membrane non-permeabilized condition. (F, G) NRH2 does not alter p75NTR expression on the cell surface. Immunoreactivity of p75NTR in FLAG–NRH2 (F) or GFP-transfected HT-1080P/S cells (G) was visualized by immunoflurescence microscopy in a membrane non-permeabilized condition. Arrowheads indicate NRH2 or GFP-expressing cells, and arrows indicate non-transfected reference cells. (H) Quantification of p75NTR or sortilin immunoreactivity represented in (D)–(G). Locations containing both transfected and non-transfected cells were chosen and staining intensity of sortilin or p75NTR in NRH2 (+) or GFP (+) cells was normalized by corresponding intensity in NRH2 (−) or GFP (−) cells in the same picture (mean±s.e.m., n=4, *P<0.05). (I) HT-1080P/S cells were transfected with control vector or NRH2 encoding vector, followed by cell surface biotinylation and streptavidin pull-down of cell lysates, showing that NRH2 increases cell surface expression of sortilin but not p75NTR. (J) NRH2 but not p75NTR increases sortilin expression on the cell surface assessed by cell surface biotinylation in transfected HT-1080S. (K) NRH2–ΔICD mutant, which does not interact with sortilin, fails to increase cell surface expression of sortilin in HT-1080S cells.
Cell surface expression of sortilin is crucially regulated by endogenous NRH2 in neurons
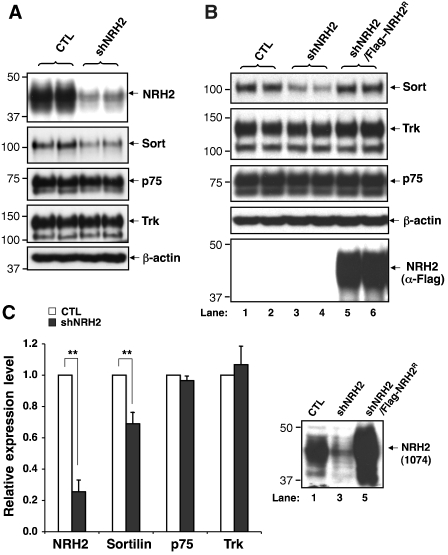
To determine whether endogenous NRH2 in neurons regulates sortilin localization, we delivered short hairpin RNA (shRNA) targeting a sequence in the transmembrane domain of rat NRH2. As DRG neurons express high levels of NRH2, sortilin, and p75NTR (Supplementary Figure S6), we infected DRG neurons with shNRH2-expressing lentivirus (shNRH2). This reduced NRH2 expression by approximately 75%, as compared with the control lentivirus (CTL)-infected cells (Figure 5A and B). Unexpectedly, overall sortilin levels are significantly reduced in NRH2-silenced DRG neurons (69.0±7.2% of control, n=6, P<0.01) (Figure 5A and B). To verify that the reduction in sortilin expression in DRG neurons is mediated by NRH2, we co-expressed an shNRH2-resistant, FLAG-tagged murine NRH2, using the shNRH2-expressing lentiviral vector (shNRH2/FLAG–NRH2R). The reduction in sortilin expression induced by NRH2 knockdown is effectively rescued by co-expression of FLAG–NRH2R (Figure 5C), indicating that the level of sortilin expression is selectively regulated by NRH2, rather than an off target effect of shRNA. This conclusion is also supported by comparable expression levels of other proteins including p75NTR, Trk, and β-actin after silencing or restoring of NRH2 expression in DRG neurons (Figure 5A–C).
Figure 5.
Sortilin expression levels are selectively reduced in NRH2-depleted DRG neurons. (A) DRG neurons infected with CTL or shNRH2-expressing lentivirus were harvested at DIV7, and expression of NRH2, p75NTR, sortilin or Trk was examined by immunoblot analysis. (B) Quantitation of western blots represented in (A). Mean proportions±s.e.m. were determined from six independently carried out experiments at DIV7-9, **P<0.01. (C) Sortilin levels are rescued upon restoring NRH2 expression. Dissociated DRG cultures were infected with lentiviruses (CTL, shNRH2, or shNRH2/FLAG–NRH2R), and western blot analysis was carried out at DIV7. Bottom, NRH2 Western blot using anti-NRH2 (1074) antibody for the lysates represented in lanes 1, 3 and 5 of upper panels.
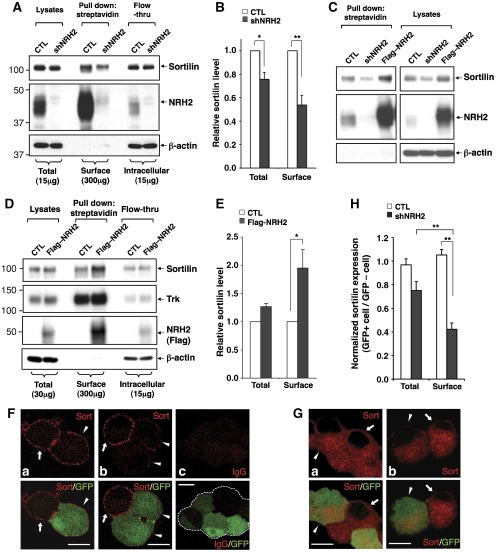
To assess whether NRH2 regulates sortilin expression on the neuronal cell surface, CTL or shNRH2 expressing DRG neurons were biotinylated, and surface proteins were detected by streptavidin-pull down and western blot analysis (Figure 6A). On acute silencing of NRH2, we observed a reduction of sortilin expression on the cell surface (46.3% reduction compared with control, n=3, **P<0.01) that was more pronounced than the total decrease in sortilin expression (24.4% decrease compared with control, n=3, *P<0.05) (Figure 6B). In addition, augmentation of NRH2 expression in DRG neurons (Figure 6C) or cortical neurons (Figure 6D), where NRH2 is expressed at low levels (Supplementary Figure S5), significantly increases the level of surface-expressed sortilin (2.0±0.32-fold, n=3) with a moderate increase in total sortilin (1.3±0.05-fold, n=3) in cortical neurons (Figure 6E). To determine whether endogenous NRH2 in DRG neurons preferentially regulates cell surface localization of sortilin, DRG neurons were infected with CTL or shNRH2-expressing lentiviruses and immunofluorescence microscopy was carried out in both membrane non-permeabilized (Figure 6F) and permeabilized conditions (Figure 6G), and staining intensity present on the cell surface and in total was compared. We detected a significant decrease of sortilin immunoreactivity in membrane non-permeabilized, NRH2-silenced cells (60% reduction compared with control, **P<0.01, n=5; Figure 6F arrowheads and Figure 6H). In contrast, only a modest decrease was observed in membrane permeabilized, NRH2-silenced cells (22% reduction compared with control, n=4; Figure 6G arrowheads and Figure 6H). Collectively, these studies indicate that NRH2 critically regulates the cell surface localization of endogenous sortilin in two classes of neurons.
Figure 6.
NRH2 preferentially regulates cell surface expression of sortilin in primary neurons. (A) Reduced cell surface expression of sortilin in NRH2-silenced DRG neurons. Control or NRH2-silenced DRG neurons were biotinylated at DIV7-8, and cellular lysates (300 μg) were subjected to streptavidin-precipitation (surface). Lysates (15 μg) harvested before and after precipitation represented as ‘total' and ‘intracellular' fractions, respectively. (B) Quantitation of sortilin immunoreactive bands represented in (A). n=3, mean±s.e.m., *P<0.05 and **P<0.01. (C) Control, NRH2-silenced (shNRH2) or NRH2-overexpressed (FLAG–NRH2) DRG neurons were subjected to cell surface biotinylation at DIV7 followed by western blot analysis. (D) Augmentation of NRH2 expression in rat cortical neurons increases sortilin levels on the cell surface, assessed by cell surface biotinylation (DIV7-8). (E) Quantitation of sortilin levels represented in (D). n=3, *P<0.05. (F) DRG cultures infected with CTL (a, c) or shNRH2-expressing lentiviruses (b) were subjected to immunocytochemical staining with anti-sortilin antibody (a, b) or control IgG (c) in a membrane non-permeabilized condition, and surface expression of sortilin in the neuronal cell body was visualized by confocal microscopy. Arrowheads indicate CTL or shNRH2-expressing cells identified by GFP expression, and arrows indicate GFP-negative non-infected cells. Representative images are shown. Scale bars, 10 μm. (G) Total sortilin expression in CTL (a) or NRH2-depleted (b) DRG neuronal cell body was visualized by immunofluorescence confocal microscopy carried out after membrane permeabilization. Scale bars, 10 μm. (H) Quantification of sortilin immunoreactivity represented in (F) and (G). Staining intensity of GFP-positive cells was normalized by the intensity of GFP-negative cells in the same field. Mean±s.e.m. was obtained from four (total) or five (surface) independently conducted experiments, **P<0.01.
NRH2 regulates sortilin expression in neurons by altering sortilin degradation
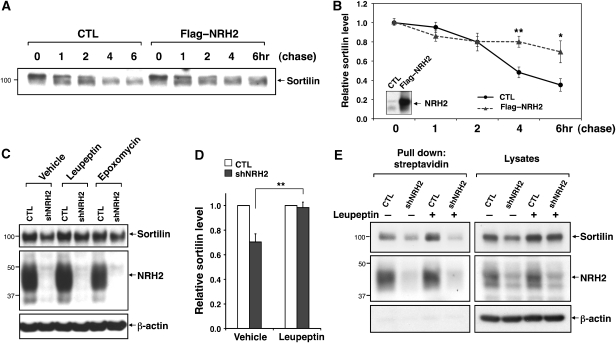
As acute silencing of endogenous NRH2 leads to a moderate reduction in total sortilin levels, we carried out pulse-chase experiments to determine whether sortilin degradation was affected. In control neurons, sortilin levels fall significantly at 4 and 6 h of chase conditions (52 and 65% reduction compared with 0 hr of chase). In contrast, in neurons overexpressing NRH2, sortilin levels at these time points are largely maintained (20 and 30% reduction, *P<0.05 and **P<0.01, n=4) (Figure 7A and B), suggesting that NRH2 expression stabilizes sortilin by attenuating its degradation. Recent studies suggest that sorting nexin-1 (SNX-1), a component of retromer, interacts with the sortilin cytoplasmic tail and facilitates endosome–TGN transport of sortilin. Depletion of SNX-1 decreases the sortilin pool in the TGN and increases its lysosomal degradation (Canuel et al, 2008; Mari et al, 2008). Thus, we postulated that the interaction of NRH2 with sortilin might impair the trafficking of sortilin to the lysosome for degradation. To test this hypothesis, DRG neurons infected with CTL or shNRH2-expressing lentiviruses were incubated with lysosomal or proteosomal inhibitors, and sortilin levels in cell lysates were analysed by western blot analysis. Treatment with the lysosomal inhibitor leupeptin restored sortilin levels in NRH2-depleted cells to levels close to those observed in control conditions (98.3±2.6%, **P<0.01, n=3) (Figure 7C and D). However, proteosomal inhibitors, including epoxomycin and MG-132 did not significantly augment sortilin protein levels in NRH2-silenced neurons (Figure 7C and data not shown), demonstrating that a primary effect of NRH2 is to interfere with sortilin degradation in the lysosomal dependant pathway. To determine whether lysosomal inhibition also restores sortilin surface expression in NRH2-silenced cells, control or NRH2-silenced DRG neurons were treated with leupeptin or not, followed by cell surface biotinylation (Figure 7E). Leupeptin treatment rescues total levels of sortilin but not the surface-expressed sortilin in NRH2-depleted neurons (Figure 7E). These studies suggest that enhanced cell surface localization of sortilin is not a direct consequence of increased sortilin stability, and that association of sortilin with NRH2 may be necessary for this process.
Figure 7.
NRH2 regulates sortilin expression by altering lysosomal degradation of sortilin. (A) Cortical neurons were electroporated (Amaxa) with control or FLAG–NRH2 encoding plasmids and subjected to pulse-chase [35S]Cys/Met labelling at DIV5. Biosynthetically labelled sortilin was immunoprecipitated from cell lysates (300 μg), harvested at the indicated times, followed by autoradiography. (B) Quantitation of autoradiographs in (A). The levels of endogenous biosynthetically labelled sortilin are significantly higher after 4 and 6 h of chase upon NRH2 overexpression (*P<0.05 and **P<0.01, n=4). Inset, NRH2 expression levels in control or FLAG–NRH2-transfected cortical neurons, verified by immunoblotting with NRH2 antibody (1074). (C) Control or NRH2-silenced DRG neurons were treated with vehicle (0.01% DMSO), leupeptin (50 μM) or epoxomycin (1 μM) for 20 h at 37°C (DIV7–8), and subjected to western blot analysis. (D) Quantification of western blots in (A). Each bar graph represents a mean±s.e.m. from three experiments, **P<0.01. (E) Leupeptin treatment (50 μM, 20 h) effectively rescues total expression of sortilin in NRH2-depleted DRG neurons but not surface expression of sortilin, determined by cell surface biotinylation followed by immunoblot analysis.
NRH2 promotes proNGF binding/internalization
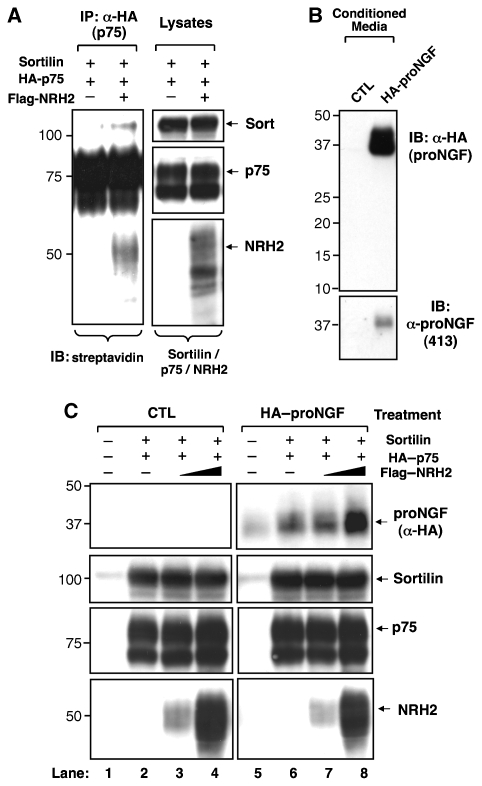
As NRH2 facilitates the redistribution of sortilin to the cell surface and increases the association between sortilin and p75NTR, we next assessed whether NRH2 promotes the formation of a p75NTR and sortilin complex on the cell surface. Cells expressing p75NTR and sortilin, with or without NRH2, were subjected to surface biotinylation and cell lysates were immunoprecipitated with p75NTR antibodies. Immunoblotting with streptavidin–HRP detects three prominent bands (∼110, ∼75, and ∼50 kDa), corresponding to sortilin, p75NTR and NRH2, respectively, as verified by western blot analysis (data not shown), and shows that NRH2 expression markedly increases the p75NTR and sortilin association on the cell surface (3.3-fold increase compared with control, n=2) (Figure 8A). To determine whether this increased formation of a p75NTR–sortilin complex on the cell surface enhances proNGF binding or internalization, we treated p75NTR, sortilin, or NRH2 expressing-heterologous cells with HA–proNGF (Lee et al, 2001). Immunodetection with anti-HA is approximately 10-fold more sensitive than anti-proNGF (0.1 ng versus 1.0 ng per lane, respectively), thus improving detection of proNGF binding (Figure 8B). Cell-associated proNGF is increased in p75NTR and sortilin-expressing cells (Figure 8C, lane 6) as compared with control (Figure 8C, lane 5), and further augmented on co-expression of NRH2 with p75NTR and sortilin (Figure 8C, lane 8). These data strongly suggest that NRH2 expression facilitates p75NTR–sortilin receptor complex formation on the cell surface to promote proneurotrophin binding.
Figure 8.
NRH2 increases the p75NTR–sortilin complex on the cell surface and promotes proNGF binding/internalization. (A) NRH2 facilitates the formation of p75NTR–sortilin complex on the cell surface. Transfected HEK 293 cells, with or without FLAG–NRH2, were surface biotinylated, and cell lysates (1 mg) were immunoprecipitated with p75NTR. Cell surface association with p75NTR were determined by western blotting with streptavidin–HRP (left panel). Lysates, 20 μg per lane. (B) Control or HA–proNGF-containing conditioned media were collected from HEK 293 cells, and analysed by western blotting with anti-HA (HA.11) and anti-proNGF (413) antibodies (40 μl per lane). (C) Enhanced proNGF binding to p75NTR and sortilin expressing cells upon NRH2 co-expression. HEK 293 cells were co-transfected with p75NTR, sortilin and increasing amounts of FLAG–NRH2 (0, 0.2 and 2 μg for lanes 2–4, and 6–8, respectively), treated with CTL– or HA–proNGF-conditioned media (B) for 60 min at 37°C, and cell lysates (30 μg) were subjected to immunoblot analysis.
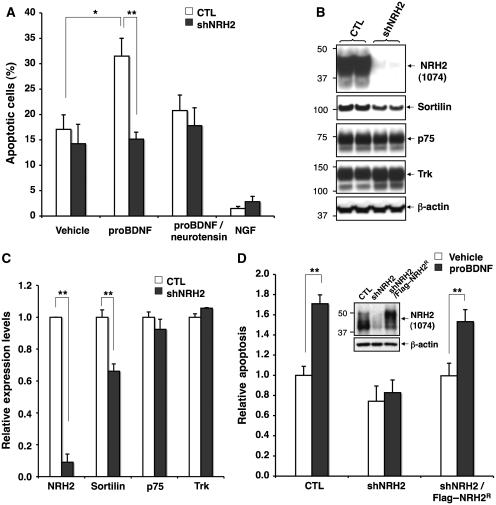
Acute depletion of NRH2 abolishes proBDNF-induced sympathetic neuronal death
To determine whether altered targeting of sortilin induced by NRH2 regulates proneurotrophin-induced cell death, rat sympathetic neurons were treated with proBDNF after acute silencing of NRH2 (Figure 9A). Consistent with our earlier results (Teng et al, 2005), proBDNF treatment induced apoptosis in neurons infected with control lentivirus (1.84-fold increase compared with the control treatment, n=4, *P<0.05) (Figure 9A). This effect is blocked by neurotensin, which binds sortilin and impairs proneurotrophin binding (Nykjaer et al, 2004; Teng et al, 2005) (Figure 9A). In contrast, NRH2-silenced neurons were resistant to proBDNF-induced killing as compared with the control cells (n=4, **P<0.01, Figure 9A). Immunoblot analysis verified efficient knockdown of NRH2 in sympathetic SCG neurons to <10% of that observed in control cells (Figure 9B and C). Consistent with the observations in DRG neurons (Figure 5), NRH2 silencing moderately reduced sortilin levels (34% reduction compared with control, n=3, **P<0.01) but not p75NTR, Trk or β-actin expression in SCG neurons (Figure 9B and C). Importantly, restoration of NRH2 expression (Figure 8D; insets) re-sensitized SCG cells to proBDNF (1.57-fold increase as compared with the control treatment, n=10, **P<0.01) (Figure 9D). Collectively, these results indicate that NRH2 plays a critical role in regulating proneurotrophin-induced neuronal apoptosis.
Figure 9.
NRH2 is indispensable for proBDNF-induced SCG neuronal death. (A) At DIV7–8, control or NRH2-silenced SCG neurons were treated with PBS (Vehicle), proBDNF (10 ng/ml), with or without neurotensin (20 μM), or mature NGF (50 ng/ml) for 36 h, and the percentage of apoptotic cells were scored. Each bar indicates mean±s.e.m. from four independently conducted experiments (*P<0.05 and **P<0.01). (B) Reduced sortilin expression by NRH2 knockdown in SCG neurons (DIV7). (C) Quantitation of western blots in (B). Values are means±s.e.m., n=3 and **P<0.01. (D) Restoring NRH2 expression resensitizes proBDNF-induced cell death. SCG neurons were infected with lentivirus-expressing CTL (n=10), shNRH2 (n=6), or shNRH2/FLAG–NRH2R (n=10). At DIV 7–8, cells were treated with PBS (Vehicle) or 5 ng/ml proBDNF for 30-40 h, then apoptotic cells were counted. Each bar graph represents mean±s.e.m., **P<0.01. Insets, silenced or restored NRH2 expression in infected SCG neurons verified by immunoblotting with NRH2 antibody (1074).
Discussion
The goal of this study is to identify a mechanism that regulates sortilin expression on the cell surface to promote the formation of p75NTR–sortilin complexes and to render neurons responsive to proneurotrophins. Unlike the other neurotrophin receptors, p75NTR and Trks, which are expressed at high levels on the plasma membrane, sortilin transports proteins including lysosomal hydrolases from TGN to endosomes and lysosomes (Nielsen et al, 1999; Lefrancois et al, 2003; Chen et al, 2005; Shi and Kandror, 2005; Ni and Morales, 2006). The interaction of the sortilin cytoplasmic domain with the Golgi-localized gamma-ear-containing Arf-binding proteins (GGAs) to promote TGN-to-endosome/lysosome trafficking, as well as with the retromer to direct endosomal to TGN targeting (Nielsen et al, 2001; Canuel et al, 2008; Mari et al, 2008), might provide important mechanisms to limit cell surface expression of sortilin, and thus responsiveness to proneurotrophins. However, during neural development and after nerve injury, proneurotrophin-induced cell death is robust (Beattie et al, 2002; Harrington et al, 2004; Jansen et al, 2007), implicating an endogenous mechanism to acutely relocalize sortilin to the plasma membrane to enhance cellular responsiveness to proneurotrophins.
Here, we provide evidence that NRH2, a mammalian homologue of p75NTR, plays this crucial role in regulating sortilin localization on the cell surface. Our results indicate that NRH2 interacts with sortilin in vivo, promotes sortilin expression on the plasma membrane, and increases the formation of a p75NTR–sortilin–proneurotrophin complex on the cell surface. Most importantly, the reduction of apoptosis in NRH2-silenced sympathetic neurons suggests that NRH2 expression is indispensable for proneurotrophin-induced cell death in these neurons. Thus, our findings suggest that NRH2-mediated targeting of sortilin to the plasma membrane, and the resulting increase in p75NTR–sortilin receptor complex formation on the cell surface are critical to initiate proneurotrophin signalling in neurons.
How does NRH2 enhance sortilin expression on the cell surface? The sortilin cytoplasmic domain contains sorting motifs that promote endocytosis and TGN-to-endosome/lysosome trafficking of sortilin through interactions with GGAs, AP-1, and AP-2 to target cargo proteins to their intended destinations (Bonifacino and Traub, 2003). In addition, recent studies co-localize sortilin with SNX-1 to direct trafficking from the early endosome to TGN. As a result, more than 90% of sortilin is targeted to the TGN, endosomes, or lysosomes (Mazella, 2001; Nielsen et al, 2001; Mari et al, 2008). To increase sortilin expression on the cell surface, NRH2 might act to override trafficking signals that direct sortilin to those sorting or recycling routes, and retarget it to the plasma membrane. Indeed, in the absence of NRH2 expression in DRG neurons, lysosome-dependant sortilin degradation is elevated, resulting in preferential reduction of sortilin expression on the cell surface. Similarly, acute silencing of SNX-1 reduces the cellular levels of sortilin by 50%, by mistargeting sortilin to the lysosome for degradation (Mari et al, 2008). Thus, it is plausible that NRH2 interacts with sortilin primarily to interfere with lysosomal trafficking and degradation of sortilin to reroute it to the plasma membrane.
Although NRH2 interacts with both p75NTR and sortilin, NRH2 expression does not relocalize p75NTR, nor alter p75NTR expression levels. This might reflect the dominant role of the p75NTR extracellular-stalk domain in directing p75NTR to the plasma membrane (Yeaman et al, 1997). More importantly, even though both p75NTR and NRH2 interact with sortilin, p75NTR expression does not alter sortilin localization. How might NRH2 selectively regulate sortilin trafficking? Firstly, NRH2 and p75NTR seem to interact with each other by homotypic association between their death domains, as most death domain-containing proteins do. By contrast, the sortilin cytoplasmic tail and the juxtamembrane region of NRH2, which is very different from the p75NTR juxtamembrane region (Figure 3A) (Kanning et al, 2003; Murray et al, 2004), seem necessary for the interaction of sortilin and NRH2, suggesting that discrete molecular interactions mediated by the NRH2–JM and the sortilin–ICD might provide selectivity of NRH2 to regulate sortilin localization. It is not clear whether the molecular association between NRH2 and p75NTR independent of sortilin might regulate proneurotrophin signaling. This study suggests that NRH2 enhances p75NTR–sortilin interaction on the cell surface to facilitate proNGF binding. However, NRH2 does not directly bind to mature neurotrophins (Murray et al, 2004), and thus a direct association with ligand is not anticipated. Future structural studies will be necessary to identify the stoichiometry and molecular interactions between proneurotrophins, p75NTR, sortilin, and NRH2.
The dynamic expression of NRH2 during the development of the mammalian nervous system (Frankowski et al, 2002; Wang et al, 2003) suggests that downregulation in postnatal life might provide a mechanism to limit proneurotrophin responsiveness. Our studies suggest that Trk activation does not regulate NRH2 expression (Supplementary Figure S7B), suggesting that other regulatory mechanisms are involved. In addition, the limited tissue distribution of NRH2 might act to facilitate proneurotrophin actions in the nervous system, but not in other organs. For example, RGCs undergo developmental-stage specific, p75NTR- and sortilin-dependant apoptosis (Frade and Barde, 1999; Harada et al, 2006; Jansen et al, 2007). This cell death correlates with the level of cell surface expression of sortilin, and determines the proNGF susceptibility in these cells (Nakamura et al, 2007). Our studies indicate that NRH2 expression coincides with cell surface expression of sortilin in RCGs at E15.5, but not P1 (Supplementary Figure S2), to render these cells transiently competent to respond to locally synthesized proNGF.
The ability of NRH2 to act as a transmembrane chaperone to specifically redirect sortilin trafficking provides a new mechanism to regulate receptor localization and biological responsiveness. The best characterized chaperone proteins for receptors include the heat shock family of proteins, with well-defined interactions of hsp90/hsp70 with glucocorticoid receptors (Pratt et al, 2006), and interaction of heat shock protein gp96 with the Toll-like receptors (Harding, 2007). Other receptors, including the G protein-coupled receptors (GPCRs), utilize type I and type II transmembrane proteins to direct cell surface expression in neurons, and other highly polarized cells (Tan et al, 2004). For example, the cell surface localization of odorant receptors in Caenorhabditis elegans is dependant on interaction with a neuronally expressed syntaxin-like molecule, ODR-4 (Dwyer et al, 1998), and the surface localization of the calcitonin receptor-like receptor requires interaction with the type I transmembrane protein RAMPs (receptor activity modifying proteins) (McLatchie et al, 1998). One example of receptor heterodimerization that seems to override ER retension signals is the GABAB receptor. The GB1 subunit encodes a C-terminal ER retention motif, which is masked through interactions of GB1 and GB2 through C-terminal coiled coil α helices (Margeta-Mitrovic et al, 2000). However, these strategies seem to be utilized primarily to confer cell-type specific surface expression, whereas our studies suggest a more dynamic model, in which developmental regulation of NRH2 might act to limit proneurotrophin responsiveness.
In summary, our findings identify a new mechanism for regulating apoptotic signalling in neurons: the requirement for a chaperone protein, NRH2, to impair lysosomal degradation of sortilin, and to promote enhanced expression on the cell surface. This provides a mechanism for tightly regulating the susceptibility of p75NTR and sortilin-expressing neurons to proneurotrophin-dependant death, and might allow p75NTR to subserve different cellular functions, such as axonal repulsion, migration, or myelination (Barker, 2004).
Materials and methods
Reagents
Murine NGF was obtained from Harlan Bioproducts (Indianapolis, IN) and the furin-resistant His-tagged proBDNF was generated as described earlier (Teng et al, 2005). The rabbit anti-p75NTR (9992) (Esposito et al, 2001), anti-proNGF (413) (Beattie et al, 2002), and anti-sortilin (727, for detecting the extracellular domain) antibodies were generated in the laboratory, and the anti-NRH2 antibody (1074) was generously provided by Moses Chao (Murray et al, 2004). Anti-human p75NTR monoclonal antibody (MAB367), biotinylated goat anti-mouse sortilin (BAF2934) and anti-mouse p75NTR (BAF1157) antibodies, and biotinylated normal goat IgG were obtained from R&D Systems (Minneapolis, MN). The rabbit polyclonal antibody for Trk (C-14) was from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-FLAG-tag (M2 and rabbit polyclonal) and anti-β-actin monoclonal antibodies were from Sigma (St Louis, MO). Monoclonal antibody for sortilin was obtained from BD Bioscience (San Jose, CA), and anti-Myc polyclonal antibody was from Bethyl Laboratories Inc. (Montgomery, TX).
DNA constructs
N-terminal FLAG-tagged murine NRH2 (Murray et al, 2004) was a generous gift from Moses Chao(Murray et al, 2004). N-terminally FLAG-tagged NRH2 constructs encoding C-terminus, death domain, and cytoplasmic domain truncations (FLAG–NRH2–ΔCT1−180, FLAG–NRH2–ΔDD1−129 and FLAG–NRH2–ΔICD1−76 respectively) were generated by PCR amplification.
Cell cultures, immunoprecipitation and immunoblotting
Cell cultures, primary neuronal cultures, immunoprecipitation, and immunoblotting were carried out as described in Supplementary data. To detect endogenous interactions among NRH2, sortilin, and p75NTR, whole brains were dissected from embryonic day 17 (E17) rats, homogenized, and lysed in TNE buffer with protease and phosphatase inhibitors (Sigma). After centrifugation and preclearing with immobilized streptavidin (Pierce Biotechnology, Rockford, IL), lysates (3 mg per IP) were incubated with biotinylated anti-p75NTR (BAF1157), anti-sortilin (BAF2934), or normal goat IgG (BAF108). The immunocomplexes were precipitated using immobilized streptavidin and analysed by western blotting with anti-NRH2 (1074), anti-sortilin (monoclonal), and anti-p75NTR (9992) antibodies.
Immunofluorescence microscopy
Immunocytochemical or immunohistochemical staining and fluorescence microscopy were carried out as described earlier (Murray et al, 2004; Chen et al, 2005). To selectively label sortilin, NRH2, or p75NTR present on the plasma membrane, cells were incubated with pre-cooled blocking buffer and primary antibodies detecting ECDs of target proteins followed by incubation with secondary antibodies at 4°C. HT-1080P/S cells were examined by epifluorescence microscopy and staining intensity of each fluor in individual cells was integrated using Image J software (NIH, Bethesda, MD). Ten to 20 cells/constructs/conditions were analysed at random locations in each experiment, and normalized with NRH2 or GFP non-expressing adjacent cells. Confocal fluorescence microscopy was carried out on DRG neuron specimens by using an LSM510 microscope (Carl Zeiss, Oberkochen, Germany). Twenty to 40 lentivirus-infected cells (GFP-positive) were examined randomly in each experiment and staining intensity was analysed using Image J software. Further details are described in Supplementary data.
Cell surface biotinylation
Cells were carefully rinsed twice with ice-cold PBS containing 1 mM CaCl2 and 0.5 mM MgCl2 (PBS+) and incubated with 0.5 mg/ml sulfo-NHS-LC-biotin (Pierce Biotechnology) dissolved in biotinylation buffer (10 mM TEA pH 7.4, 2 mM CaCl2, and 150 mM NaCl) for 20 min at 4°C. Cells were then quenched with PBS+ containing 50 mM Tris pH 7.4 for 10 min at 4°C, washed with PBS+, and lysed in RIPA buffer (1% NP-40, 0.1% SDS, 0.1% deoxycholate, 150 mM NaCl, 1 mM EDTA, and 10 mM Tris pH 7.4) with protease inhibitors. Clarified lysates were then immunoprecipitated with immobilized streptavidin, or anti-HA matrix.
Lentiviral application
Lentiviral stocks were prepared according to manufacturer's instruction (Invitrogen). See Supplementary data for details. To silence, rescue, or augment NRH2 expression in primary neurons, cells at DIV2 were infected with control, shNRH2, or shNRH2/FLAG–NRH2R-expressing lentivirus for 2–3 days in the presence of 5-FUDR and/or NGF (for DRG and SCG). Cultures were then treated or not, and harvested at DIV7-9. In each experiment, expression of GFP or FLAG–NRH2 was verified by western blotting or fluorescence microscopy.
Pulse-chase experiment
Cortical neurons were dissociated and electroporated (Amaxa) with control or FLAG–NRH2 plasmids, and on DIV5, cells were incubated in methionine-free, cysteine-free DMEM supplemented with 5% dialysed FBS for 30 min. Cells were pulse-labelled by the addition of 250 μCi/ml [35S] cysteine/methionine mix (PerkinElmer, Waltham, MA) in methionine and cysteine free media for 1 h, and cell media was replaced with media containing 5 mM methionine and cysteine for 0, 1, 2, 4, and 6 h, respectively. Cells were then washed twice with ice-cold PBS, lysed in RIPA buffer, and cell lysates (300 μg per IP) were subjected to immunoprecipitation with biotinylated anti-sortilin antibody (BAF2934) followed by streptavidin pull-down. The samples were subjected to 8% SDS–PAGE and analysed by autoradiography.
Assessment of apoptosis
Dissociated SCG neurons were prepared from wild-type P1 rats, infected with lentiviruses, treated with NGF, proBDNF, neurotensin, or vehicle for 36 h, and apoptotic cells were scored as described earlier (Teng et al, 2005). At least 200 cells were counted for each culture condition, and the level of NRH2, sortilin, Trk, β-actin, or p75NTR was monitored by western blotting from the equivalent cultures.
Statistics
Statistical significance was typically determined by two-tailed Student's t-test after testing for normal distribution.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Moses Chao and Simon Murray for generously providing an antibody and DNA construct for NRH2, and Philip Barker and Kuo-Fen Lee for providing additional antibodies against NRH2. HA-tagged proNGF and myc-tagged sortilin constructs were generously provided by Francis Lee and Zhe-Yu Chen. We also thank Kenneth Teng, Francis Lee, Timothy McGraw, and Sarah Felice for helpful discussions and critical comments on the manuscript, Matthew Light for providing recombinant proBDNF, Chia-Jen Siao for DRG, spinal cord, and retinal sections, and other Hempstead laboratory members for general support. This work was supported by a grant from NIH, NS30687.
References
- Barker PA (2004) p75NTR is positively promiscuous: novel partners and new insights. Neuron 42: 529–533 [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO (2002) ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 36: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447 [DOI] [PubMed] [Google Scholar]
- Canuel M, Lefrancois S, Zeng J, Morales CR (2008) AP-1 and retromer play opposite roles in the trafficking of sortilin between the Golgi apparatus and the lysosomes. Biochem Biophys Res Commun 366: 724–730 [DOI] [PubMed] [Google Scholar]
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309 [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25: 6156–6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV (2007) Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci 34: 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Troemel ER, Sengupta P, Bargmann CI (1998) Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell 93: 455–466 [DOI] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL (2001) The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem 276: 32687–32695 [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA (1999) Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development 126: 683–690 [DOI] [PubMed] [Google Scholar]
- Frankowski H, Castro-Obregon S, del Rio G, Rao RV, Bredesen DE (2002) PLAIDD, a type II death domain protein that interacts with p75 neurotrophin receptor. Neuromolecular Med 1: 153–170 [DOI] [PubMed] [Google Scholar]
- Harada C, Harada T, Nakamura K, Sakai Y, Tanaka K, Parada LF (2006) Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye. Dev Biol 290: 57–65 [DOI] [PubMed] [Google Scholar]
- Harding CV (2007) gp96 leads the way for toll-like receptors. Immunity 26: 141–143 [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM (2004) Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA 101: 6226–6230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL (2002) The many faces of p75NTR. Curr Opin Neurobiol 12: 260–267 [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A (2007) Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci 10: 1449–1457 [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC (2003) Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci 23: 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945–1948 [DOI] [PubMed] [Google Scholar]
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR (2003) The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J 22: 6430–6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27: 97–106 [DOI] [PubMed] [Google Scholar]
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, Cullen PJ, Klumperman J, Geuze HJ (2008) SNX1 defines an early endosomal recycling exit for sortilin and mannose 6-phosphate receptors. Traffic 9: 380–393 [DOI] [PubMed] [Google Scholar]
- Mazella J (2001) Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal 13: 1–6 [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339 [DOI] [PubMed] [Google Scholar]
- Murray SS, Perez P, Lee R, Hempstead BL, Chao MV (2004) A novel p75 neurotrophin receptor-related protein, NRH2, regulates nerve growth factor binding to the TrkA receptor. J Neurosci 24: 2742–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Namekata K, Harada C, Harada T (2007) Intracellular sortilin expression pattern regulates proNGF-induced naturally occurring cell death during development. Cell Death Differ 14: 1552–1554 [DOI] [PubMed] [Google Scholar]
- Ni X, Morales CR (2006) The lysosomal trafficking of acid sphingomyelinase is mediated by sortilin and mannose 6-phosphate receptor. Traffic 7: 889–902 [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM (1999) Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem 274: 8832–8836 [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, Pohlmann R, Petersen CM (2001) The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J 20: 2180–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM (2004) Sortilin is essential for proNGF-induced neuronal cell death. Nature 427: 843–848 [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF (2001) Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280 [DOI] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M (2004) Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol 63: 641–649 [DOI] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK (1997) Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 272: 3599–3605 [DOI] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Murphy M, Harrell M (2006) Chaperoning of glucocorticoid receptors. Handb Exp Pharmacol 172: 111–138 [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67: 203–233 [DOI] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A (2003) Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol 461: 483–505 [DOI] [PubMed] [Google Scholar]
- Shi J, Kandror KV (2005) Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell 9: 99–108 [DOI] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE (2004) Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609 [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25: 5455–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ (2006) Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci 26: 7756–7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shao Z, Zetoune FS, Zeidler MG, Gowrishankar K, Vincenz C (2003) NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ 10: 580–591 [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E (1997) The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol 139: 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information