SYNOPSIS
In 2006, eight community tuberculosis (TB) cases and a ninth incarceration-related case were identified during an outbreak investigation, which included genotyping of all Mycobacterium tuberculosis isolates. In 1996, the source patient had pulmonary TB but completed only two weeks of treatment. From February 2005 to May 2006, the source patient lived in four different locations while contagious. The outbreak cases had matching isolate spoligotypes; however, the mycobacterial interspersed repetitive unit (MIRU) patterns from isolates from two secondary cases differed by one tandem repeat at a single MIRU locus. The source patient's isolates showed a mixed mycobacterial population with both MIRU patterns. Traditional and molecular epidemiologic methods linked eight secondary TB cases to a single source patient whose incomplete initial treatment, incarceration, delayed diagnosis, and housing instability resulted in extensive transmission. Adequate treatment of the source patient's initial TB or early diagnosis of recurrent TB could have prevented this outbreak.
Following national trends, the incidence of tuberculosis (TB) in Connecticut has declined from 3.9 cases per 100,000 people in 1998 to 2.5 cases per 100,000 people in 2006, and 60% of TB cases reported in Connecticut are among people born outside the United States.1 However, certain populations, including those who are incarcerated or homeless, are at high risk for TB even in low-incidence states and continue to challenge TB control efforts.2–5
In 2006, eight TB cases from a small community and a ninth incarceration-related case were identified in Connecticut. The source patient, a 29-year-old female, had smear- and culture-positive pulmonary TB diagnosed in 1996 in New Jersey but was lost to further treatment after two weeks of treatment. She later moved to Connecticut, where she was incarcerated from May 2005 through January 2006. While incarcerated, she developed signs and symptoms consistent with pulmonary TB and had abnormal chest radiographs. Her TB went undiagnosed, and she was symptomatic when released. Four months later, smear-positive, cavitary TB was diagnosed at a community hospital. Her Mycobacterium tuberculosis (M. tuberculosis) isolates were susceptible to all first- and second-line anti-TB medications, and she began a standard four-drug regimen by directly observed therapy.
The initial contact investigation for the source case in May 2006 identified four secondary pulmonary TB cases in one adult and three children. In October 2006, three additional adults had pulmonary TB diagnosed, including one person who had a positive tuberculin skin test result during the initial contact investigation but declined isoniazid treatment for latent TB infection at that time. The ninth incarceration-related case was later linked to the source case by traditional and molecular epidemiologic methods.
METHODS
In response to these additional cases, we conducted an outbreak investigation. We reviewed medical records and contact investigation logs, and reinterviewed adult patients. We considered a tuberculin skin test reaction with an induration transverse diameter of ≥5 mm to be positive for any contact.6 Since 2004, Connecticut has used the National TB Genotyping Service. The National TB Genotyping Service is a service funded by the Centers for Disease Control and Prevention that is available to all TB control programs. The objective of the program is universal genotyping of at least one isolate for each new culture-positive TB case in the U.S. at a reference laboratory. We performed genotyping on M. tuberculosis isolates from all patients in this outbreak with culture-positive TB. All isolates were genotyped using two standard methods: spacer oligonucleotide typing (spoligotyping) and mycobacterial interspersed repetitive unit (MIRU)–variable-number tandem repeat analysis.7,8 Select isolates were typed using insertion sequence 6110 (IS6110)-based restriction fragment length polymorphism (RFLP) analysis.9 We considered patients to have a genotype link if their isolate genotype matched that of the source patient.
RESULTS
All patients were U.S.-born and human immunodeficiency virus-seronegative, and had pulmonary TB. All six adults were female with a mean age of 28 years (range: 21–36 years). The three pediatric patients ranged in age from 10–19 months. Eight of nine patients completed treatment; one patient who had clinical, culture-negative disease disappeared shortly after starting treatment.
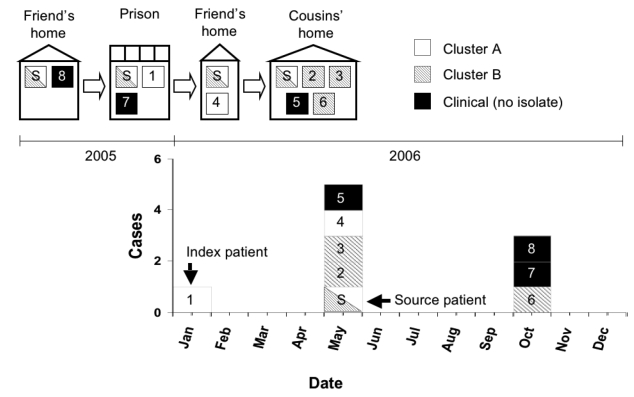
The source patient lived in multiple households before and after incarceration. The Figure shows the epidemiologic curve and source patient's living arrangements in chronological order. Before incarceration, the source patient lived with a friend (Patient 8), who was later diagnosed with smear- and culture-negative TB. During October–November 2005, the source patient lived in the same prison dormitory room as the index patient (Patient 1), who was the first patient to have TB disease diagnosed in January 2006.10 The source patient socialized frequently with a friend (Patient 7) before, during, and after incarceration. Upon release from prison, the source patient lived with another friend and her two children for two weeks; one child (Patient 4) later had TB diagnosed. The source patient then lived with two cousins and their five children for 14 weeks until her diagnosis. Both adult cousins (Patients 3 and 6) and two of the children (Patients 2 and 5) subsequently had TB diagnosed.
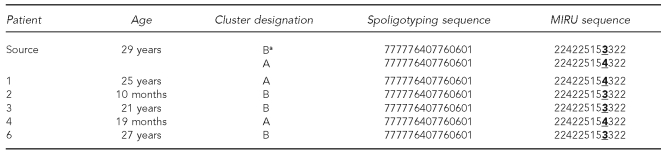
Five (63%) of eight secondary patients had isolates available for genotyping. Three patients did not have isolates: two adults had culture-negative TB, and one child did not have specimens collected. All five isolates had the same spoligotyping pattern as the source patient. Of the three secondary case isolates typed with RFLP, all had the same one-band pattern as the source patient's RFLP pattern from 1996 and 2006. However, the isolates in this outbreak had two distinct MIRU patterns. Based on the difference in the MIRU patterns, we assigned two cluster designations (Cluster A and Cluster B) (Table 1). The difference in these patterns, and therefore clusters, was a single-tandem repeat at locus 27. Because of the strong epidemiologic links between the source patient and all secondary patients, we requested genotyping for all five of the source patient's isolates. On subsequent testing, two of the source patient's isolates showed evidence of a mixed population of M. tuberculosis, with both three and four tandem repeats at MIRU locus 27, consistent with the two distinct clusters.
Table 1.
Genotype patterns of source patient and secondary patients and resulting cluster designations (n=6)
aCluster designation is based on initial genotyping results.
MIRU = mycobacterial interspersed repetitive unit
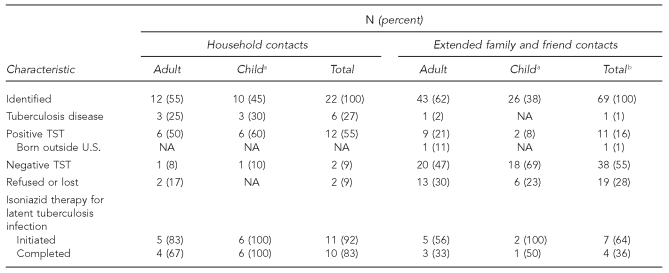
The investigation identified 22 people as household contacts; 12 (55%) were adults and 10 (45%) were children younger than 15 years of age (Table 2). Among household contacts, three (25%) adults and three (30%) children had TB diagnosed, and six (50%) adults and six (60%) children had latent TB infection diagnosed. When we expanded the investigation, we identified 69 additional contacts. Within the wider network, one (2%) adult contact had TB disease and 11 (16%) had latent TB infection. Of the 12 household contacts with latent TB infection, four (67%) adults and all six (100%) children completed nine months of isoniazid treatment. Of the other 11 contacts with latent TB infection, seven (64%) started and four (36%) completed treatment.
Table 2.
Results of a tuberculosis outbreak investigation for the source patient in Connecticut
aChild is defined as age <15 years.
bTotals may not equal 100 due to rounding.
TST = tuberculin skin test
NA = not applicable
DISCUSSION
This outbreak could have been prevented with adequate treatment of the source patient's initial TB in 1996 or early diagnosis of her recurrent TB in 2005. The magnitude of M. tuberculosis transmission was increased by the source patient's housing instability and the number of children living in households she shared. She lived in three separate households and a congregate setting (i.e., prison) while she was contagious. Young children infected with M. tuberculosis are at increased risk for early progression to TB disease.11 Because of limited sleeping accommodations, female family members, friends, and young children regularly slept in the same bedrooms. All the women and young children who shared a bedroom with the source patient subsequently had TB diagnosed.
In addition to the strong epidemiologic links among patients, all isolates from culture-positive cases were linked through one of two distinct genotype patterns to the source patient. The source patient had isolates with three tandem repeats at MIRU locus 27 (Cluster B) and isolates with four tandem repeats at MIRU locus 27 (Cluster A). The two MIRU patterns observed from the source patient might represent clonal evolution. The change at MIRU locus 27 showed a single-tandem repeat difference, which is consistent with a stepwise variable-number tandem repeat mutation mechanism (sequential additions or deletions of repeat units) as reported in the literature for other MIRU loci.12 To our knowledge, this is the first reported TB outbreak in which all the cases were linked by traditional epidemiologic methods, but had distinct MIRU patterns with otherwise matching genotypes. To determine the frequency with which single-locus MIRU changes occurred among patients in the same chain of recent M. tuberculosis transmission would require further field investigations of large outbreaks.
The primary goal of outbreak investigations is to stop the transmission of M. tuberculosis. Since the investigation, no additional cases of TB disease have been identified. However, a secondary patient (Patient 7) disappeared after six weeks of treatment, and efforts by TB control programs, law enforcement, and social service agencies in two states have been unsuccessful in locating her. This untreated patient has the potential to become contagious and the source of a new future TB outbreak.
The secondary goal is to prevent future TB disease by identifying and treating contacts with latent TB infection. The public health district achieved an 83% completion rate for infected household contacts who started treatment using a variety of methods, including direct observation of treatment for latent TB infection, home delivery of medications, and use of incentives (e.g., gift cards) for contacts. An 83% completion rate for latent TB infection treatment approaches the Healthy People 2010 objective of 85%.13
CONCLUSION
This outbreak demonstrated the necessity for early diagnosis and complete treatment of each and every patient with TB disease. The source patient's incarceration, delayed diagnosis, and unstable housing resulted in eight secondary TB cases. In addition, this investigation showed the importance of using both traditional and molecular epidemiologic methods together. The true extent of M. tuberculosis transmission from the source patient would have been underestimated had genotyping results alone been considered.
Figure.
Living arrangements of source patient and secondary patients and tuberculosis cases by month and year of diagnosis, January 2005–December 2006 (n=9)a
aPatients are numbered in order of diagnosis.
Legend: white bar = patients in Cluster A; shaded bar = patients in Cluster B; black bar = patients with clinical tuberculosis (no isolate available)
S = source patient
Acknowledgments
The authors thank Paul Sookram and the staff of the Tuberculosis Control Program, Connecticut Department of Public Health; the American Lung Association of Connecticut; the William W. Backus Hospital; Michigan Department of Community Health Laboratory; and Mycobacteriology Laboratory Branch, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, for their participation and support in this investigation.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) Reported tuberculosis in the United States, 2006. Atlanta: Department of Health and Human Services (US); 2007. [Google Scholar]
- 2.Tuberculosis transmission in multiple correctional facilities—Kansas, 2002–2003. MMWR Morb Mortal Wkly Rep. 2004;53(32):734–8. [PubMed] [Google Scholar]
- 3.MacNeil JR, Lobato MN, Moore M. An unanswered health disparity: tuberculosis among correctional inmates, 1993 through 2003. Am J Public Health. 2005;95:1800–5. doi: 10.2105/AJPH.2004.055442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuberculosis transmission in a homeless shelter population—New York, 2000–2003. MMWR Morb Mortal Wkly Rep. 2005;54(6):149–52. [PubMed] [Google Scholar]
- 5.Lofy KH, McElroy PD, Lake L, Cowan LS, Diem LA, Goldberg SV, et al. Outbreak of tuberculosis in a homeless population involving multiple sites of transmission. Int J Tuberc Lung Dis. 2006;10:683–9. [PubMed] [Google Scholar]
- 6.Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and the CDC. MMWR Recomm Rep. 2005;54(RR-15):1–37. [PubMed] [Google Scholar]
- 7.New CDC program for rapid genotyping of Mycobacterium tuberculosis isolates. MMWR Morb Mortal Wkly Rep. 2005;54(2):47. [Google Scholar]
- 8.Cowan LS, Diem L, Monson T, Wand P, Temporado D, Oemig TV, et al. Evaluation of a two-step approach for large-scale, prospective genotyping of Mycobacterium tuberculosis isolates in the United States. J Clin Microbiol. 2005;43:688–95. doi: 10.1128/JCM.43.2.688-695.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for standardized methodology. J Clin Microbiol. 1993;31:406–9. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosa LE, Lobato MN, Condren T, Williams MN, Hadler JL. Outbreak of tuberculosis in a correctional facility: consequences of missed opportunities. Int J Tuberc Lung Dis. 2008;12:689–91. [PubMed] [Google Scholar]
- 11.Pickering LK, Baker CJ, Long SS, McMillan JA, editors. Red Book®: 2006 report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2006. American Academy of Pediatrics; pp. 678–98. [Google Scholar]
- 12.Savine E, Warren RM, van der Spuy GD, Beyers N, van Helden PD, Locht C, et al. Stability of variable-number tandem repeats of mycobacterial interspersed repetitive units from 12 loci in serial isolates of Mycobacterium tuberculosis. J Clin Microbiol. 2002;40:4561–6. doi: 10.1128/JCM.40.12.4561-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department of Health and Human Services (US) Healthy People 2010: understanding and improving health. 2nd ed. Washington: U.S. Government Printing Office; 2000. [Google Scholar]