SYNOPSIS
Objective.
Intrauterine environmental factors, including maternal diet, may play an etiologic role in acute lymphoblastic leukemia (ALL), a common childhood cancer. Expanding on previous findings from phase 1 of the Northern California Childhood Leukemia Study (NCCLS), a population-based case-control study, we sought to further elucidate and replicate the relationships between maternal diet and ALL risk.
Methods.
We matched 282 case-control sets of children (205 pairs and 77 triplets) from phases 1 and 2 of the NCCLS on sex, date of birth, mother's race, Hispanic racial/ethnic status, and county of residence at birth. We used an interviewer-administered food frequency questionnaire to obtain information on maternal dietary intake in the 12 months prior to pregnancy.
Results.
Risk of ALL was inversely associated with maternal consumption of vegetable (adjusted odds ratio [AOR] = 0.65, 95% confidence interval [CI] 0.50, 0.84); protein sources (AOR=0.55, 95% CI 0.32, 0.96); fruit (AOR=0.81, 95% CI 0.65, 1.00); and legume food groups (AOR=0.75, 95% CI 0.59, 0.95). The risk reduction was strongest for consumption of the protein sources and vegetable food groups, independent of the child's diet up to age 2 years, and consistent across phases 1 and 2 of data collection for vegetable consumption.
Conclusions.
These data suggest that it may be prudent for women to consume a diet rich in vegetables and adequate in protein prior to and during pregnancy as a possible means of reducing childhood ALL risk in their offspring.
Childhood leukemia is the primary cause of cancer-related mortality of children in the United States.1 Of children with leukemia, 78% are diagnosed with acute lymphoblastic leukemia (ALL), the etiology of which is largely unknown.2,3 However, as summarized by Maia et al.,4 observations on clonal markers in leukemic cells from monozygotic twins concordant for leukemia, and evaluation of neonatal blood spots for clonotypic fusion gene sequences have provided compelling evidence that a substantial portion of childhood ALLs originate in utero, and that chromosome translocations are very early or initiating events.4 These findings underscore the need to examine potential risk factors in the fetal environment, such as maternal diet.
In a previous report of 138 case-control pairs from phase 1 of the Northern California Childhood Leukemia Study (NCCLS), maternal consumption of the vegetable and protein sources food groups was inversely associated with offspring ALL.5 Expanding on this research, we undertook a comprehensive analysis of 282 case-control sets from phases 1 and 2 of the NCCLS to further elucidate and replicate the relationships between maternal diet and ALL risk. Our analyses also took into account the potential confounding effect of the child's early diet, which has recently been reported to be associated with childhood acute leukemia risk.6 We hypothesized that maternal diet is associated with offspring risk of ALL, independent of the child's early diet, and that observed relationships would be consistent across phase 1 and 2 data.
METHODS
Study population
The NCCLS is a matched case-control study, and the analysis presented in this article consisted of data collected from August 19, 1995, to November 30, 1999 (phase 1), and from December 1, 1999, to November 30, 2002 (phase 2). We identified incident leukemia cases on the basis of International Classification of Diseases for Oncology, Third Edition criteria,7 using a rapid case-ascertainment procedure from pediatric hospitals in the northern and central California study region. During phase 1, the study area encompassed seven pediatric hospitals in 17 counties in the greater San Francisco-Oakland Bay area. During phase 2, the study area expanded to encompass nine pediatric hospitals and 35 counties including the Central Valley of California. Comparison with the statewide California Cancer Registry for 2000 showed that the NCCLS protocol identified 95% of eligible cases among residents in the five-county San Francisco-Oakland metropolitan statistical area and 76% of eligible cases in the other 30 counties of the study area. We considered case subjects to be eligible if they were younger than 15 years of age, had no prior cancer diagnosis, lived within the study region, and had parents who spoke either English or Spanish. The University of California Committee for the Protection of Human Subjects, the California Health and Human Services Agency Committee for the Protection of Human Subjects, and the Institutional Review Boards of the participating hospitals approved the study. We obtained written informed consent from parents of all participating children.
After identifying each case, we selected control subjects from birth certificates through the California Office of Vital Records, and matched the control subjects 1:1 (phase 1) or 1:2 (phase 2) to the case subjects on date of birth, sex, Hispanic status (if either parent was Hispanic, then we considered the child Hispanic), maternal race/ethnicity, and maternal county of residence at birth (phase 1 only; matching on maternal county of residence at birth was not continued in phase 2 because of concerns regarding overmatching on potential environmental exposures related to leukemia risk). For the 7% of case children not born in California, we selected controls from the case child's county of residence at diagnosis. Out-of-state case children were comparable to case children born in California on age, sex, Hispanic status, and maternal race. For each case subject, we identified four or more potential control subjects from the California birth registry and then randomized the control subjects for selection. If the first-choice control subject could not be located, was ineligible, or refused to participate, we then contacted the next randomly ordered control subject until an eligible control subject agreed to participate.8
As of December 1, 2002, we had obtained data from 866 individuals, including 283 leukemia case-control pairs matched 1:1 and 100 case-control triplets matched 1:2. The overall participation rate was 86% for cases (83% in phase 1 and 89% in phase 2) and 56% for controls (49% in phase 1 and 64% in phase 2). The reasons for nonparticipation among control subjects included refusal (26%) and unable to contact (18%). We excluded a total of 190 participants for the following reasons: leukemia diagnosis was not ALL (n=127), there were no dietary data (n=4), a case or control respondent was not the biological mother (n=14), and a case and/or control food frequency questionnaire (FFQ) contained questionable data (i.e., more than 10 foods were skipped, the mother consumed fewer than two or more than 17 solid foods per day, or the energy estimate was greater than 4,500 kilocalories, n=45). Therefore, the final analytic sample included 282 matched case-control sets of children comprising 205 case-control pairs and 77 case-control triplets.
Data collection
We obtained maternal dietary intake by interview, using a modified version of the Block FFQ.9–11 The time frame covered was the 12 months before the index pregnancy. We chose this period rather than diet during pregnancy because it represents the probable state of nutritional adequacy at the time of conception and during early pregnancy. The FFQ contained 76 food items and questions on vitamin supplement usage. Food items were selected by identifying the top population contributors of each nutrient among white, African American, and Hispanic populations in the Third National Health and Nutrition Examination Survey (NHANES III) and the Hispanic Health and Nutrition Examination Survey (HHANES).12–14 Frequency of consumption was reported in nine categories, ranging from never or less than once per month to greater than twice per day. We obtained portion size for each food using three-dimensional abstract models. We selected 76 food items to be representative of a wide range of dietary factors including total calories, macronutrients, fiber, vitamins, minerals, and other dietary factors such as carotenoids and phytoestrogens. In addition, we included cured meats and other foods to address prior hypotheses. The vitamin supplement questions asked about two types of multiple vitamins and nine single vitamins, and obtained information on frequency and duration of vitamin use, as well as usual daily dose for vitamins C and E.
We developed a Spanish version of the FFQ to include culturally appropriate translations of the English version and additional foods important in the diets of the Latina population. To identify these foods, we again examined NHANES III and HHANES,12–14 and also used information from focus groups of Latinas in the San Francisco Bay area. This process resulted in the addition of seven foods (evaporated/condensed milk, green peppers/chile rellenos, avocados/guacamole, chili peppers, mole/sofrito sauces, corn tortillas, and flour tortillas). These items were added only to the Spanish version of the FFQ. Bilingual interviewers administered the questionnaire.
We used the BlockSys program11 to calculate dietary nutrients from food by multiplying frequency of consumption of each food by its nutrient content and reported portion size, and then summing over all foods. We estimated nutrients obtained from vitamin supplements by multiplying the frequency of consumption of each type (multiple vitamins and specific single vitamins) times the amount of the nutrient in typical compositions of each type. For vitamins E and C, we obtained the usual daily dose from subjects who took those vitamins. We calculated total nutrient intake as the sum of nutrients from foods and supplements, unless otherwise noted. We calculated frequency of consumption of food groups—vegetables, fruits, dairy foods, beans, protein sources, grains, alcoholic beverages, and a group consisting of fats, oils, sweets, and snacks—by summing the reported frequency for all foods in a food group. Food groups corresponded to the food groups found in the U.S. Department of Agriculture Food Guide Pyramid,15 and their component foods are listed in the Figure. Macro- and micronutrient estimates from Block questionnaires have been subjected to numerous validation studies and found to produce good point estimates and rankings in relation to a variety of reference data.16–18 Bunin et al. conducted a validation of retrospective assessment of diet before and during pregnancy, and found correlations of the Willett FFQ with reference data to be similar to those obtained for current diet.19
We obtained dietary data on each child from the biological mother or primary caregiver using a self-administered questionnaire completed prior to the in-home interview in phase 1 and after the in-home interview in phase 2.6 The biological mother provided the information in 95% of cases. The wording of the dietary questions did not differ between the two phases of the study. The questionnaire asked about the child's weaning history and the frequency of consumption of nine foods/food groups (hot dogs/lunch meats, beef/hamburger, vegetables, oranges/bananas, apples/grapes, orange juice, other fruit juice, milk, and soda) and vitamin supplements during the child's first and second years of life. Frequency of consumption was reported in six categories, ranging from “rarely or never” to “three or more times a day”. In addition, we assessed whether the child consumed vitamins during the first two years of life. To summarize food consumption during the first two years of life, we created new categories of “rare/no consumption”, “occasional consumption”, and “regular consumption” based on the original categories.
Statistical analysis
We used the Pearson chi-square test to compare selected characteristics of cases and controls. To assess the association between maternal diet and childhood ALL risk, we constructed conditional logistic regression models. We considered odds ratios (ORs) representing dietary consumption on a continuous scale to be statistically significant if the 95% confidence interval (CI) excluded 1.00. Before running the statistical models, we used log transformation for variables as needed to improve normality and reduce skewness. Potential confounding variables considered a priori were birthweight of the index child, whether the child was breastfed (yes or no), maternal age, maternal education (≤high school graduate, some college, or college graduate), and smoking during pregnancy (yes or no). However, we observed no evidence of confounding for these covariates, as the change in OR when adding each of these variables to the main model was not considered substantial (greater than 10%). We included total energy consumption, the proportion of foods consumed as large or extra-large portion size, household income, and maternal exposure to indoor insecticides during pregnancy20 in the final model because these variables explained a significant proportion of the variability in the outcome and, therefore, allowed for a more sensitive test of the relationship between maternal diet and ALL. We also examined the relationship between maternal diet and ALL risk while controlling for the child's diet early in life (intake of hot dogs/lunch meats, beef/hamburger, vegetables, oranges/bananas, apples/grapes, orange juice, other fruit juice, milk, soda, and vitamin supplements; intake was coded as “rarely/none,” “occasional”, or “regular”).
RESULTS
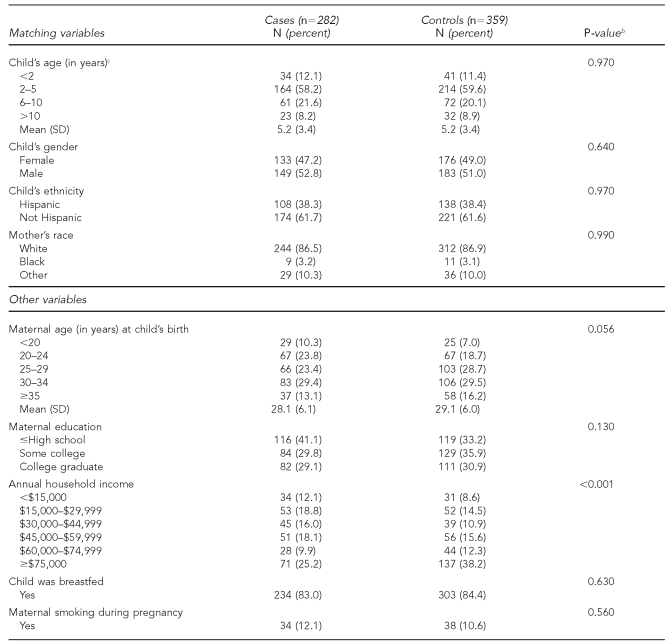
The mean age at ALL diagnosis was 5.2 years, 58% of case children were aged 2 to 5 years at time of diagnosis, and 53% were male (Table 1). Case and control children were similar with respect to birthweight, breastfeeding, and maternal smoking. In terms of race, the study sample was 87% white, 3% African American, and 10% other. Ethnically, approximately 38% of mothers described themselves as Hispanic. Compared with matched control subjects, case children were born to younger mothers (p=0.06) with fewer years of education (p=0.13), and came from families with lower household incomes (p<0.001).
Table 1.
Case and matched-control maternal and child characteristics, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bPearson Chi-square test, two-sided
cAge at diagnosis for cases and age at the corresponding date for controls
SD = standard deviation
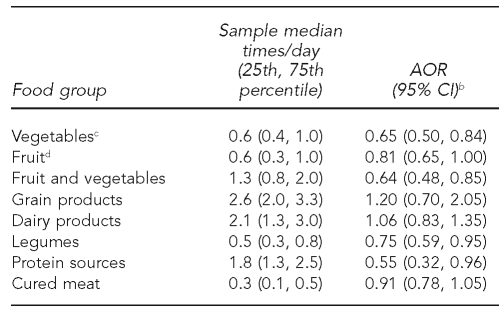
The relationships between maternal consumption of food groups (times/day) and offspring ALL risk for combined phase 1 and 2 data are shown in Table 2. We noted inverse relationships between maternal consumption and childhood ALL risk for the vegetable (adjusted OR [AOR] = 0.65, 95% CI 0.50, 0.84), fruit and vegetable (AOR=0.64, 95% CI 0.48, 0.85), legume (AOR=0.75, 95% CI 0.59, 0.95), and protein sources food groups (AOR=0.55, 95% CI 0.32, 0.96). Fruit consumption was also inversely associated with ALL, but was borderline statistically significant (AOR=0.81, 95% CI 0.65, 1.00). We did not find consumption of cured meats, grains, and dairy products to be significantly related to disease risk. When we included maternal consumption of the vegetable and protein sources food groups together in the model, consumption of both vegetables (AOR=0.65, 95% CI 0.50, 0.84) and protein sources (AOR=0.57, 95% CI 0.32, 0.99) remained associated with a statistically significant decreased risk of ALL.
Table 2.
Associations between frequency of maternal consumption of food groups and risk of ALL among offspring, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bSeparate models for each food group as a continuous variable with odds ratios and 95% CIs calculated using log-transformed values of consumption; adjusted for total energy intake, household income, indoor insecticide exposure during pregnancy, and proportion of foods reported as large or extra-large portion size
cGarden vegetables only (excludes salad, potatoes, soup, and stew)
dFruit only (excludes fruit juice)
ALL = acute lymphoblastic leukemia
AOR = adjusted odds ratio
CI = confidence interval
We also examined the relationship between maternal intake of either the vegetable or protein sources food group and offspring ALL risk while controlling for each of the childhood diet variables individually. In all models, maternal vegetable intake remained statistically significant (p<0.05), whereas maternal consumption of protein sources remained statistically significant when controlling for children's intake of beef/hamburger, hot dogs/lunch meats, oranges/bananas, apples/grapes, soda, orange juice, and vitamins, and was borderline statistically significant (0.05>p<0.09) when controlling for children's intake of vegetables, fruit juice, and milk. In no case did the childhood diet variables reach significance, although several approached significance (p<0.10 for intake of vegetables, oranges/bananas, and orange juice) (data not shown).
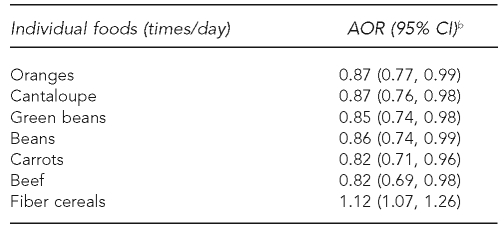
Additionally, we examined the maternal consumption of individual foods and found that six foods were inversely related to offspring risk of ALL: carrots, cantaloupe, oranges, green beans, other beans, and beef (Table 3). Regular use of any dietary supplement was not associated with disease risk (AOR=1.10, 95% CI 0.76, 1.60) (data not shown).
Table 3.
Associations between maternal consumption of individual foods and risk of ALL among offspring, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bSeparate models for each food group as a continuous variable with odds ratios and 95% CIs calculated using log-transformed values of consumption; adjusted for total energy intake, household income, indoor insecticide exposure during pregnancy, and proportion of foods reported as large or extra-large portion size
ALL = acute lymphoblastic leukemia
AOR = adjusted odds ratio
CI = confidence interval
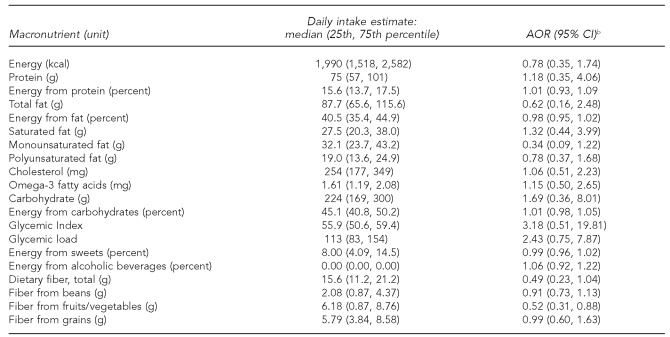
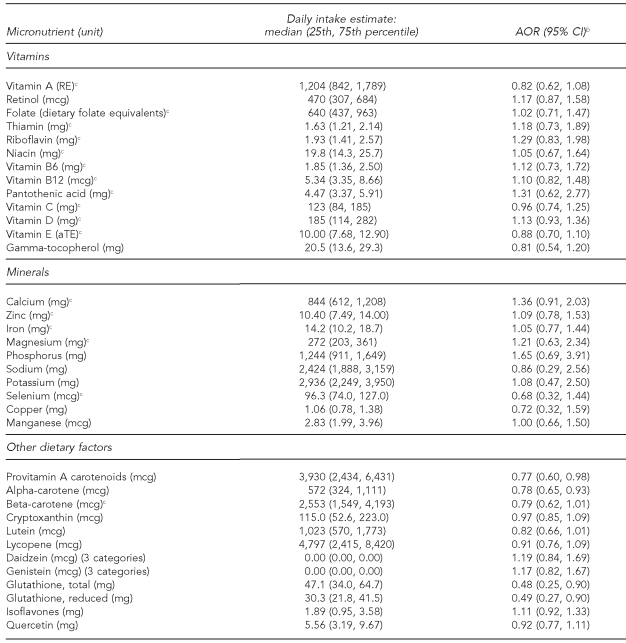
Among the macronutrients (Table 4) and micronutrients (Table 5), maternal consumption was inversely associated with offspring ALL for fiber from fruits and vegetables (AOR=0.52, 95% CI 0.31, 0.88), provitamin A carotenoids (AOR=0.77, 95% CI 0.60, 0.98), alpha-carotene (AOR=0.78, 95% CI 0.65, 0.93), total glutathione (AOR=0.48, 95% CI 0.25, 0.90), and reduced glutathione (AOR=0.49, 95% CI 0.27, 0.90). We found borderline significant inverse associations for maternal consumption of beta-carotene (AOR=0.79, 95% CI 0.62, 1.01) and lutein (AOR=0.82, 95% CI 0.66, 1.01).
Table 4.
Associations between maternal consumption of macronutrients and risk of ALL among offspring, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bSeparate models for each nutrient as a continuous variable with odds ratios and 95% CIs calculated using log-transformed values of consumption; adjusted for total energy intake, household income, indoor insecticide exposure during pregnancy, and proportion of foods reported as large or extra-large portion size
ALL = acute lymphoblastic leukemia
AOR = adjusted odds ratio
CI = confidence interval
kcal = kilocalorie
g = gram
mg = milligram
Table 5.
Associations between maternal consumption of micronutrients and risk of ALL among offspring, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bSeparate models for each nutrient as a continuous variable with odds ratios and 95% CIs calculated using log-transformed values of consumption; adjusted for total energy intake, household income, indoor insecticide exposure during pregnancy, and proportion of foods reported as large or extra-large portion size
cNutrient estimates represent the sum of food and supplement sources. Nutrient estimates for variables without a footnote c reflect nutrients only from foods.
ALL = acute lymphoblastic leukemia
AOR = adjusted odds ratio
CI = confidence interval
RE = retinol equivalents
mcg = microgram
mg = milligram
aTE = alpha-tocopherol equivalents
The direction of the relationships between offspring ALL and maternal consumption of food groups did not change when we examined only case-control sets for case children aged 2 to 5 years at diagnosis (the peak incidence of childhood ALL) and when we restricted the analysis to 81 income-concordant case-control sets, although the magnitude of the relationships was attenuated somewhat and CIs were wider (data not shown).
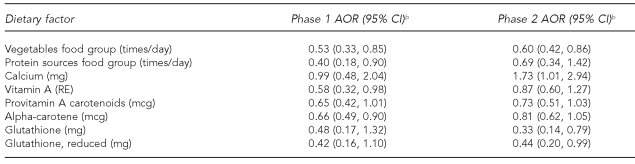
The data collected during phases 1 and 2 of the NCCLS represented replications of the study with independent samples and virtually identical methods. Maternal intake of the vegetable and protein sources food groups, and the dietary factors calcium, vitamin A, provitamin A carotenoids, alpha-carotene, total glutathione, and reduced glutathione were significantly related to offspring disease risk in phase 1 and/or phase 2 (Table 6). Of these variables, only consumption of the vegetable food group was statistically significant in both phase 1 and 2 data.
Table 6.
Maternal food groups and dietary factors significantly associated with ALL among offspring in either phase 1 or phase 2, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 138 case-control sets from phase 1 and 144 sets from phase 2
bSeparate models for each food group or dietary factor as a continuous variable with odds ratios and 95% CIs calculated using log-transformed values of consumption; adjusted for total energy intake, household income, indoor insecticide exposure during pregnancy, and proportion of foods reported as large or extra-large portion size
ALL = acute lymphoblastic leukemia
AOR = adjusted odds ratio
CI = confidence interval
mg = milligram
RE = retinol equivalents
mcg = microgram
DISCUSSION
In the combined phase 1 and 2 data from the NCCLS, the largest case-control study to date on maternal diet and childhood ALL, we found that intake of the vegetable, fruit, legume, and protein sources food groups in the 12 months before the index pregnancy (and by inference during early pregnancy) was inversely associated with offspring disease risk when adjusted for household income, maternal total energy intake, proportion of foods reported as large or extra-large portion size, and maternal exposure to indoor insecticides during pregnancy. The inverse relationships were strongest for intake of the protein sources and vegetable food groups, suggesting that two distinct maternal dietary patterns are independently and inversely associated with offspring ALL risk.
Maternal vegetable and protein sources intake remained significant and borderline statistically significant, respectively, after further adjustment for the child's diet early in life. In no case did early childhood intake of foods/food groups or vitamin supplements reach significance, although intake of vegetables, oranges/bananas, and orange juice approached statistical significance. Thus, childhood diet may contribute to a reduced risk of ALL as previously reported,6 but the stronger effect appears to be due to maternal diet.
When examining the diet-disease relationship in the combined data at the level of individual foods, maternal consumption of foods in the fruit and vegetable food groups (i.e., carrots, cantaloupe, and oranges) and the protein sources food group (i.e., green beans, beans, and beef) was inversely associated with offspring ALL risk. At the nutrient level, consumption of provitamin A carotenoids (found in fruits and vegetables) and reduced glutathione (found in protein-containing foods) was inversely related to ALL risk. The study findings may have etiologic relevance in light of evidence that the initiating genetic event in the development of leukemia frequently occurs in utero.4 Fetal exposure to bioactive compounds in vegetables, fruit, and protein foods such as vitamins and minerals, fiber, peptides and amino acids, and other factors may contribute to the ability of these foods to reduce cancer risk.21–27
Using virtually identical data collection methods and independent samples from phases 1 and 2 of the NCCLS to examine the reproducibility of our findings, we found that maternal intake of the vegetables food group and its related component, provitamin A carotenoids, was statistically or borderline-statistically associated with decreased offspring ALL risk in both phases of the study. Thus, the inverse diet-disease relationship appears to be strongest for maternal consumption of the vegetable food group.
Our results are generally in agreement with those of a recently published case-control study of maternal diet and offspring ALL in Greece involving 131 case-control sets.28 In this study, investigators reported that maternal consumption of the vegetable and fruit food groups was inversely associated with risk of childhood ALL, which is generally consistent with our findings from combined phase 1 and 2 data. While consumption of fish and seafood as a food group was also reportedly associated with reduced risk of disease, consumption of meats and meat products was related to a moderate increase in disease risk,28 in contrast to our findings.
We observed no evidence of increased offspring ALL risk associated with maternal consumption of cured meats as a food group or individual cured meats, which is consistent with previous reports.29,30 We also found no evidence of significant risk reduction associated with maternal prepregnancy use of dietary supplements. This is in contrast to previous reports where vitamins A and D from cod liver oil31 and maternal use of folic acid supplements with or without iron32 have been reported to be associated with reduced risk of childhood ALL.
Strengths of our study included the use of population-based birth certificate controls that have been shown to be representative of the source population.8 Matching on race and Hispanic ethnicity reduced some of the biases that can be a problem in other recruitment designs. We obtained the maternal dietary data using a previously validated FFQ, and we designed the extensive food list to assess a wide range of food groups, foods, and nutrients, thus enabling us to control for total energy intake. The child's diet questionnaire, although not validated, was composed of foods that covered a broad spectrum of a typical young child's diet such that we were able to examine its impact on the potential association between maternal diet and ALL risk. To our knowledge, this type of comprehensive dietary analysis has not been conducted before in the childhood leukemia literature.
In addition, we obtained nondietary data, which allowed us to evaluate and control for other potential confounding variables. We were able to observe the relationships between maternal diet and offspring ALL risk for all cases, as well as in the subgroup of cases where children were aged 2 to 5 years at diagnosis, thereby increasing our confidence that the findings are generalizable to the majority of childhood ALL cases. Furthermore, the consistent findings of an inverse association between maternal vegetable consumption and childhood ALL risk with independent samples from phases 1 and 2 of the NCCLS support the validity of the findings, although robust replication would involve different investigators and methods, and a different population.
Limitations
Several limitations of these data should be considered. Our data collection focused on maternal diet in the 12 months before the index pregnancy and, therefore, may have the greatest implications for maternal nutritional status immediately before and during early pregnancy. Recall bias is a concern in case-control studies, and although no widely known hypotheses exist about the possible role of maternal dietary factors in childhood leukemia that might lead to recall bias, general hypotheses about a healthy diet could have led case mothers to underreport their intake of perceived healthy foods such as vegetables and fruits. However, it is difficult to imagine that this would hold true for reported consumption of protein sources. Measurement error is a problem in research requiring self-reports; however, data collection methods did not differ between cases and controls in this study. Thus, misclassification from measurement error would be expected to be nondifferential and would likely, although not always, bias the results toward the null.
The participation rate of controls was modest, and controls tended to have higher incomes and more education than cases; as a result, selection bias must be considered as a factor in interpreting our results. A selection that favored controls with higher income and education might produce an inverse association between disease risk and vegetable consumption, as higher socioeconomic status is associated with higher consumption of fruits and vegetables.33,34 However, it would be unlikely to result in an inverse association with consumption of protein sources (meats and legumes), which are less frequently consumed among more affluent or well-educated groups.35 In addition, such a selection bias should also have produced an apparently protective effect of dietary supplements, as the more affluent and well educated do take more vitamin supplements.36,37 However, we did not find an inverse association with dietary supplement use. Also, to account for differences in socioeconomic status, we controlled for household income, matched on Hispanic ethnicity, and repeated the analysis in income-concordant pairs, with no material change in our findings. Finally, dietary factors are often correlated with one another. Thus, caution is warranted in directly attributing risk or benefit to any particular food or nutrient.
CONCLUSION
Maternal intake of the vegetable, fruit, legume, and protein sources food groups was found to be inversely associated with offspring ALL risk. The associations were strongest for consumption of protein sources and vegetable food groups, and their relationships with disease risk were independent of one another and the child's diet early in life. Although causality cannot be inferred from this study, the data suggest that it may be prudent for women to consume a diet rich in vegetables and adequate in protein prior to and during pregnancy as a possible means of reducing the risk of ALL in their offspring.
Figure.
Foods/food groups represented on the food frequency questionnaire, Northern California Childhood Leukemia Study, Berkeley, California, 1995–2002a
aIncludes 282 case-control sets comprising 205 pairs and 77 triplets
bIncluded on Spanish version of food frequency questionnaire only
USDA = U.S. Department of Agriculture
Acknowledgments
The authors thank Dr. Jim Feusner of Children's Hospital – Research Center Oakland; Dr. Gary Dahl of Lucile Packard Children's Hospital in Palo Alto; Drs. Katherine Matthay and Mignon Loh of the University of California, San Francisco; Dr. Vonda Crouse of Children's Hospital Central California in Madera; Dr. Kenneth Leung of Kaiser Permanente San Francisco Medical Center; Drs. Carolyn Russo and Alan Wong of Kaiser Permanente Santa Clara Medical Center; Dr. Jonathan Ducore of the University of California, Davis; and Dr. Vincent Kiley of Kaiser Permanente Sacramento Medical Center for assistance with recruiting patients. The authors also thank Dr. Youqing Hu for analytic assistance; Monique Does for supervising fieldwork and interviews; Dr. Catherine Metayer for data management; and all of the University of California, Berkeley, as well as the study staff for their hard work and dedication.
Footnotes
This research was supported by two U.S. Public Health Service grants, #R01ES09137 (Environmental Exposures and Childhood Leukemia) and #P42ES04705 (Superfund, Molecular Epidemiology of Childhood Leukemia), and a grant from the Paul O'Gorman Foundation for Children with Leukaemia.
REFERENCES
- 1.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Bethesda (MD): National Cancer Institute, SEER Program; 1999. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995 [NIH publication no. 99-4649] [Google Scholar]
- 2.Buffler PA, Kwan ML, Reynolds P, Urayama KY. Environmental and genetic risk factors for childhood leukemia: appraising the evidence. Cancer Invest. 2005;23:60–75. [PubMed] [Google Scholar]
- 3.Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect. 2007;115:138–45. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maia AT, Koechling J, Corbett R, Metzler M, Wiemels JL, Greaves M. Protracted postnatal natural histories in childhood leukemia. Genes Chromosomes Cancer. 2004;39:335–40. doi: 10.1002/gcc.20003. [DOI] [PubMed] [Google Scholar]
- 5.Jensen CD, Block G, Buffler P, Ma X, Selvin S, Month S. Maternal dietary factors in childhood acute lymphoblastic leukemia (United States) Cancer Causes Control. 2004;15:559–70. doi: 10.1023/B:CACO.0000036161.98734.17. [DOI] [PubMed] [Google Scholar]
- 6.Kwan ML, Block G, Selvin S, Month S, Buffler PA. Food consumption by children and the risk of childhood acute leukemia. Am J Epidemiol. 2004;160:1098–107. doi: 10.1093/aje/kwh317. [DOI] [PubMed] [Google Scholar]
- 7.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al., editors. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 8.Ma X, Buffler PA, Layefsky M, Does MB, Reynolds P. Control selection strategies in case-control studies of childhood diseases. Am J Epidemiol. 2004;159:915–21. doi: 10.1093/aje/kwh136. [DOI] [PubMed] [Google Scholar]
- 9.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 10.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 11.NutritionQuest. Block dietary data systems. [cited 2008 Jan 30]. Available from: URL: http://www.nutritionquest.com.
- 12.Centers for Disease Control and Prevention, National Center for Health Statistics (US) Hyattsville (MD): NCHS; 1998. Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III [CD-ROM Series 11, No. 2A] [Google Scholar]
- 13.The Hispanic Health and Nutrition Examination Survey. Nutr Rev. 1991;49:156–8. doi: 10.1111/j.1753-4887.1991.tb03010.x. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Norris JC, Mandel RM, DiSogra C. Sources of energy and six nutrients in diets of low-income Hispanic-American women and their children: quantitative data from HHANES, 1982–1984. J Am Diet Assoc. 1995;95:195–208. doi: 10.1016/S0002-8223(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 15.Department of Agriculture (US), Center for Nutrition Policy and Promotion. The food guide pyramid [Home and Garden Bulletin Number 252] Washington: USDA; 1992. [Google Scholar]
- 16.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–93. [PubMed] [Google Scholar]
- 17.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 18.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123:489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 19.Bunin GR, Gyllstrom ME, Brown JE, Kahn EB, Kushi LH. Recall of diet during a past pregnancy. Am J Epidemiol. 2001;154:1136–42. doi: 10.1093/aje/154.12.1136. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110:955–60. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003;24:511–5. doi: 10.1093/carcin/24.3.511. [DOI] [PubMed] [Google Scholar]
- 22.Astley SB, Elliott RM, Archer DB, Southon S. Increased cellular carotenoid levels reduce the persistence of DNA single-strand breaks after oxidative challenge. Nutr Cancer. 2002;43:202–13. doi: 10.1207/S15327914NC432_11. [DOI] [PubMed] [Google Scholar]
- 23.Torbergsen AC, Collins AR. Recovery of human lymphocytes from oxidative DNA damage; the apparent enhancement of DNA repair by carotenoids is probably simply an antioxidant effect. Eur J Nutr. 2000;39:80–5. doi: 10.1007/s003940050006. [DOI] [PubMed] [Google Scholar]
- 24.Fillion L, Collins A, Southon S. Beta-carotene enhances the recovery of lymphocytes from oxidative DNA damage. Acta Biochim Pol. 1998;45:183–90. [PubMed] [Google Scholar]
- 25.Wolterbeek AP, Roggeband R, van Moorsel CJ, Baan RA, Koeman JH, Feron VJ, et al. Vitamin A and beta-carotene influence the level of benzo[a]pyrene-induced DNA adducts and DNA-repair activities in hamster tracheal epithelium in organ culture. Cancer Lett. 1995;91:205–14. doi: 10.1016/0304-3835(95)03740-n. [DOI] [PubMed] [Google Scholar]
- 26.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 2001;37:948–65. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 27.Meister A. Strategies for increasing cellular glutathione. In: Packer L, Cadenas E, editors. Biothiols in health and disease. New York: Marcel Dekker, Inc.; 1995. pp. 165–89. [Google Scholar]
- 28.the Childhood Hematology-Oncology Group. Petridou E, Ntouvelis E, Dessypris N, Terzidis A, Trichopoulos D. Maternal diet and acute lymphoblastic leukemia in young children. Cancer Epidemiol Biomarkers Prev. 2005;14:1935–9. doi: 10.1158/1055-9965.EPI-05-0090. [DOI] [PubMed] [Google Scholar]
- 29.Sarasua S, Savitz DA. Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer Causes Control. 1994;5:141–8. doi: 10.1007/BF01830260. [DOI] [PubMed] [Google Scholar]
- 30.Ross JA, Potter JD, Reaman GH, Pendergrass TW, Robison LL. Maternal exposure to potential inhibitors of DNA topoisomerase II and infant leukemia (United States): a report from the Children's Cancer Group. Cancer Causes Control. 1996;7:581–90. doi: 10.1007/BF00051700. [DOI] [PubMed] [Google Scholar]
- 31.Shu XO, Gao YT, Brinton LA, Linet MS, Tu JT, Zheng W, et al. A population-based case-control study of childhood leukemia in Shanghai. Cancer. 1988;62:635–44. doi: 10.1002/1097-0142(19880801)62:3<635::aid-cncr2820620332>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case-control study. Lancet. 2001;358:1935–40. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 33.Patterson BH, Block G, Rosenberger WF, Pee D, Kahle LL. Fruit and vegetables in the American diet: data from the NHANES II survey. Am J Public Health. 1990;80:1443–9. doi: 10.2105/ajph.80.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.California Department of Health Services. California dietary practices survey: overall trends in healthy eating among adults, 1989–1997, a call to action, part 2. Sacramento (CA): California Department of Health Services; 1999. [Google Scholar]
- 35.Patterson BH, Block G. Food choices and the cancer guidelines [published erratum appears in Am J Public Health 1988;78:620] Am J Public Health. 1988;78:282–6. doi: 10.2105/ajph.78.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block G, Cox C, Madans J, Schreiber GB, Licitra L, Melia N. Vitamin supplement use, by demographic characteristics. Am J Epidemiol. 1988;127:297–309. doi: 10.1093/oxfordjournals.aje.a114805. [DOI] [PubMed] [Google Scholar]
- 37.Subar AF, Block G. Use of vitamin and mineral supplements: demographics and amounts of nutrients consumed. The 1987 Health Interview Survey. Am J Epidemiol. 1990;132:1091–101. doi: 10.1093/oxfordjournals.aje.a115752. [DOI] [PubMed] [Google Scholar]