SYNOPSIS
Objective
We examined use of colorectal cancer (CRC) screening exam modalities among county health centers and private physician offices, where both were located in the same geographic area.
Methods
We surveyed 500 county health center registrants and 570 private physician patients, aged 52–75 years. We administered telephone surveys during 2004 to examine relationships among sociodemographic characteristics; perceived barriers to screening with fecal occult blood test (FOBT), sigmoidoscopy, and colonoscopy; and self-reported receipt of each exam.
Results
FOBT was more frequent among county health center registrants; sigmoidoscopy and colonoscopy were more frequent among private physician patients (p<0.001). County health center registrants less frequently cited no physician recommendation as a barrier to FOBT, but more frequently cited no recommendation as a barrier to sigmoidoscopy and colonoscopy, compared with private physician patients (p≤0.02). Among county health center registrants, better health insurance coverage was associated with lower odds of FOBT and higher odds of screening endoscopy; perceived barriers were associated with lower odds of screening (p<0.02). Among private physician patients, we noted an association between perceived barriers to screening and lower odds of any screening (p<0.001).
Conclusions
Overall, CRC screening among county health center and private physician patient samples compared favorably with overall New York and U.S. rates. Although prior studies using national data suggested that screening rates were equivalent in county health center and private physician primary care settings, we found exam-specific differences in patient-reported screening endoscopy among our two patient samples. Understanding factors that contribute to differences in CRC screening between primary care settings is important for ensuring equal access to CRC screening options for all patients.
Early detection of colorectal cancer (CRC) through screening is associated with reduced mortality due to this disease.1,2 While CRC screening utilization has improved since 2000, compliance with screening recommendations remain suboptimal3–7 and fall short of Healthy People 20108 goals and American Cancer Society (ACS) objectives for 2015.9
Underuse of CRC screening is frequently associated with socioeconomic disadvantage, which is characterized by less education and income, barriers to health-care access (e.g., no or inadequate medical insurance coverage and no regular source of health care),7,10–13 and racial/ethnic minority characteristics.12,14,15
Health centers serve a greater proportion of patients who are at risk for underuse of CRC screening7,10–13 than private physician practices.16 By providing CRC referrals and screening to low-income, underinsured patients (many are not age-eligible for Medicare17), health centers reduce CRC screening disparities associated with socioeconomic disadvantage and racial/ethnic minority status—an objective of Healthy People 2010.8 Evidence provided by the few studies that have compared CRC screening rates at health centers with rates at private physician practices has suggested that screening among these primary care settings is equivalent. The results of one study of 1998 National Health Interview Survey (NHIS) data found similar rates of self-reported guaiac-based fecal occult blood test (FOBT) in the past year and endoscopy in the past five years among adults using community health centers and those using private physician offices/health maintenance organizations (HMOs).18 Another comparison found that self-reported rates of any CRC screening exam for eight Florida community health centers during 2002 were equivalent to national rates from the 1998 NHIS.19 However, in both studies, CRC screening rates for both primary care sites were lower than recommended.18,19
In its recently updated consensus guidelines for detecting adenomatous polyps and CRC in asymptomatic adults, the ACS, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology underscored the importance of prevention as the goal of CRC screening.20 Screening exams that detect CRC as well as precursor polyps (e.g., colonoscopy, flexible sigmoidoscopy, computed tomographic colonography, and double-contrast barium enema) are more likely to influence prevention of CRC than those that largely detect CRC (e.g., FOBT, fecal immunochemical test, and the stool DNA test).20 However, lack of coverage for the full range of screening options may contribute to disparities in the use of CRC screening exams associated with the prevention rather than detection of CRC.21
Uptake of a particular CRC screening exam may be influenced by patient, provider, and health-care system factors.22 Therefore, we examined factors associated with use of FOBT, sigmoidoscopy, and colonoscopy among two patient groups with access to primary care: users of county health centers aged 52–75 years and users of private physician practices of the same age, where both groups were located in the same geographic area.
METHODS
Settings and participants
County health center sample.
We obtained data as part of our National Cancer Institute (NCI)-funded CRC screening performance-improvement collaboration between the Department of Preventive Medicine at Stony Brook University, Stony Brook, New York, and the Suffolk County Department of Health Services (SCDHS), Long Island, New York, from October 2004 to January 2005. The SCDHS county-funded health center network includes 10 centers delivering primary care services in Suffolk County.
We selected participants randomly from among adults who were age-eligible for CRC screening and who received primary care at the county health centers. We stratified random (probability) sample selection by county health center to balance proportions of registrants selected from each. Registrants received an advance letter, in English and Spanish, describing the study, inviting their participation, and alerting them that they would be contacted by telephone to complete a survey. To be eligible, county health center registrants had to (1) be without a prior diagnosis of CRC, colonic polyps, or other colorectal diagnoses requiring surveillance rather than screening in the general population (e.g., ulcerative colitis or Crohn's Disease); (2) not be too impaired to answer questions; (3) consent to the telephone survey; and (4) speak either English or Spanish. We included individuals with a family history of CRC in the sample. Five hundred eligible county health center registrants completed the survey (52% response rate).
Private physician patient sample.
We obtained data for the random (probability) population-based private physician patient sample (aged 50–64 years and 65–75 years) as part of our NCI-funded Reducing Barriers to Colorectal Cancer Screening Project. We oversampled adults aged 65–75 years and conducted random -selection in blocks stratified on gender and age group to ensure equal proportions of women and men in both age groups. Eligibility criteria were similar to criteria for county health center participants (described previously), except that all private physician participants spoke English; we did not include non-English speakers (2%). Details regarding this project, sample selection, and recruitment procedures have been published elsewhere.23
We obtained private physician patient data from April to July 2004 using a telephone survey, and achieved a 47% response rate.23 The analysis sample (n=570) included participants who resided in Suffolk County and who received medical care from private family practitioners' and internists' practices located in Suffolk County.
Surveys
Survey of county health center registrants and the private physician patient sample.
The Stony Brook Center for Survey Research conducted telephone surveys of county health center registrants and the private physician patient sample using a computer-assisted telephone interviewing system. A Spanish version was available to the county health center participants. Surveys contained validated items from the NHIS. We developed and piloted survey items addressing CRC screening behaviors and attitudes with a prior sample; details about the survey construction and administration have been published elsewhere.23
The Stony Brook and SCDHS institutional review boards (IRBs) approved the research protocol for the county health center project; the Stony Brook IRB approved the Reducing Barriers to Colorectal Cancer Screening Project protocol.
Study measures
CRC screening.
We determined use of CRC screening exams by responses to questions asking whether the respondent had ever had FOBT, sigmoidoscopy, or colonoscopy, and the date of each most recent exam. We based screening intervals for FOBT and sigmoidoscopy on guidelines from the ACS,24 the Interdisciplinary Task Force (in association with gastroenterological organizations25), and the U.S. Preventive Services Task Force2 for average risk of adults aged ≥50 years, issued during the study period (2004). A recent FOBT occurred within 12 months of the interview date, a recent sigmoidoscopy occurred within five years of the interview date, and a recent colonoscopy occurred within 10 years of the interview date.24,25
Barriers to CRC screening.
Open-ended questions derived from the NHIS 2000 Cancer Module26 addressed participants' perceived barriers to screening with FOBT, sigmoidoscopy, and colonoscopy. We asked those who did not have a recent FOBT, sigmoidoscopy, or colonoscopy: “Why haven't you had a[n] [FOBT/sigmoidosopy/colonoscopy] recently?” We asked those who had a recent exam but did not intend to have another, “Why don't you intend on having [regular FOBTs/sigmoidoscopy/colonoscopy exams] in the future?” We asked participants who recently screened with FOBT, sigmoidoscopy, or colonoscopy, “What could keep you from having [regular FOBTs/sigmoidoscopy/colonoscopy exams] in the future?”
Participant characteristics.
We obtained information on gender, age, race/ethnicity, medical insurance coverage, and health status. Because race/ethnicity is not a surrogate for socioeconomic status (SES),27–29 we included education and annual household income as measures of SES.27,30 We ascertained usual source of health care for county health center and private physician patient samples by asking where the respondent usually went if sick, needing a routine checkup, or seeking advice about health.
Statistical analysis
We conducted analyses using SPSS® software.31 We examined frequency distributions for all study variables, which were categorical. We used bivariate cross-tabular analyses and Chi-square tests of association to examine relationships between the source of primary care (county health center vs. private physician), participant characteristics, CRC screening, and barriers to screening. We identified covariates of recent screening FOBT, sigmoidoscopy, and colonoscopy, as well as potential interaction effects, using bivariate analyses stratified by age group (52–64 vs. 65–75 years) to control for potential confounding by age differences between the two samples. Overall, rates of missing data for study variables were low, ranging from <1%–2%. However, the proportion of missing data for annual household income reached 27% for the county health center sample and 17% for the private physician patient sample. To maximize the number of cases included in the multivariate logistic regression analyses (described later), we represented those with missing income data by creating a “missing” category for the income variable11 (data not shown).
We conducted logistic regression analyses evaluating the probability of reporting recent FOBT, sigmoidoscopy, and colonoscopy (yes/no) for each exam separately for the county health center and private physician patient samples. County health center models included a variable denoting the 10 health centers to adjust for screening differences by health center (data not shown) and were adjusted for survey language (English/Spanish) to control for influence of language on CRC screening.32
For both samples, we entered participant characteristics (gender, race/ethnicity, education, income, medical insurance, and health status) as a block, regardless of whether they were significantly related to the dependent variables in the cross-tabular analyses. This was because independent variables, which may not be significantly associated with a dependent variable at the bivariate level, may become significantly related to the dependent variable when considered together in a multivariate model. We also wanted to avoid biasing estimates of other potential covariate effects.33 We added the variable denoting screening barriers (e.g., no provider recommendation, other barrier vs. no barrier) to the last step because of its importance as a predictor of CRC screening.34 Other barriers included healthy, exam not needed, fear, put it off, forgot, and exam embarrassing, among others. Relatively small proportions of respondents cited one of these as other barriers to screening with FOBT, sigmoidoscopy, and colonoscopy. Thus, we collapsed responses to create the “other barriers” category.
For all covariates, we made the category with the largest number the referent. We computed odds ratios from maximum-likelihood parameter estimates, and calculated 95% confidence intervals. We reported the Nagelkerke R2 as an indicator of the usefulness of the explanatory variables to predict CRC screening.35 We used two-sided significance tests, evaluated at the p<0.05 level and based on the Wald Chi-square statistic.36
RESULTS
Demographic characteristics
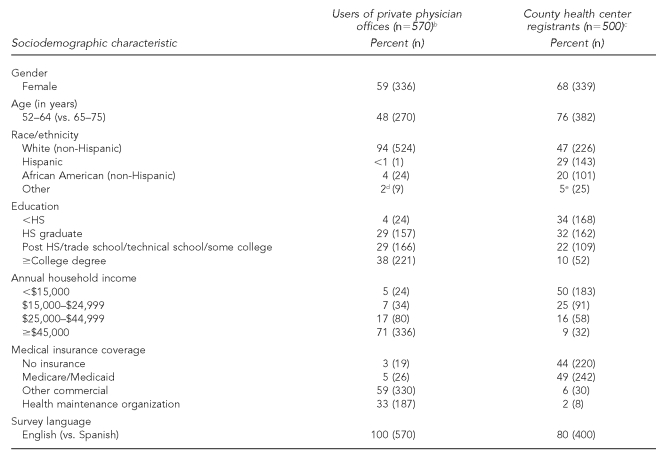
Women comprised the majority of county health center registrants (Table 1) and less than half were white. Private physician patients were primarily white. Compared with the private physician patient sample, county health center registrants were younger, had less education, lower income, and less medical insurance coverage. All county health center and private physician patients had a usual source of health care (data not shown).
Table 1.
Comparison of sociodemographic characteristics of county health center registrants and users of private physician offices in New Yorka
NOTE: Sample may not sum to total n for all characteristics due to missing data for some categories.
aAll comparisons, p<0.001
bData were from April 2004 to July 2004.
cData were from October 2004 to January 2005.
dIncludes Asian (1.0%), American Indian/Alaska Native (0.4%), and other ethnic (0.4%) categories
eIncludes Asian (1.2%), American Indian/Alaska Native (1.4%), and other ethnic (2.4%) categories
HS = high school
Health behavior characteristics
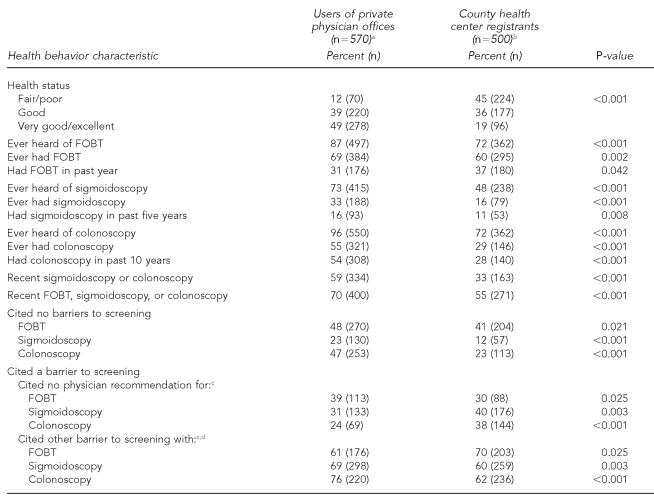
Fewer county health center registrants reported very good/excellent health status compared with the private physician patient sample (Table 2). Overall, the private physician patient sample reported higher CRC screening than the county health center registrants. While a greater proportion of county health center registrants reported an FOBT in the past year, private patients were more likely to report a recent endoscopy.
Table 2.
Comparison of health behavior characteristics of the county health center registrants and users of private physician offices in New York
NOTE: Sample may not sum to total n for all characteristics due to missing data for some categories.
aData were from April 2004 to July 2004.
bData were from October 2004 to January 2005.
cAmong respondents who cited a barrier
dOther barriers included healthy, exam not needed, fear, put it off, forgot, exam embarrassing, and others.
FOBT = fecal occult blood test
Overall, fewer county health center registrants than private physician patients cited no barriers to CRC screening. Although fewer county health center registrants cited no physician recommendation as a barrier to screening FOBT, greater proportions cited this as a barrier to screening sigmoidoscopy and colonoscopy than private physician patients.
Characteristics associated with CRC screening
County health center sample.
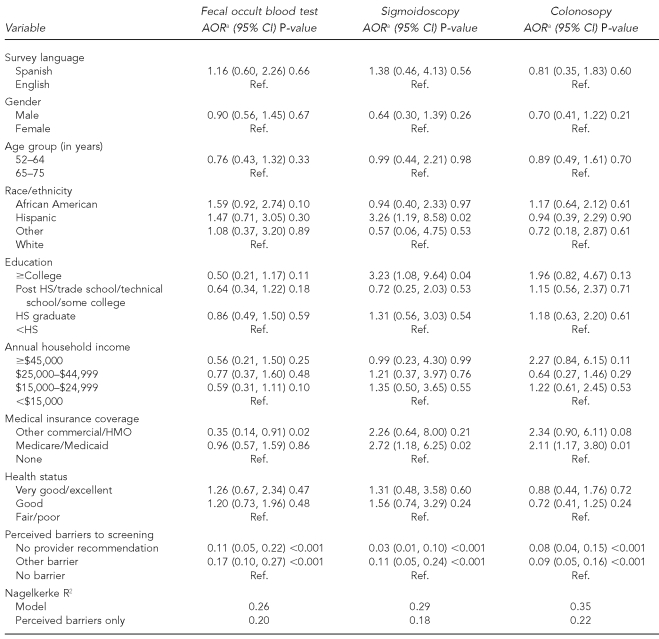
In the bivariate analyses (not shown), fewer Spanish- than English-speaking (16% vs. 31%, p=0.002) and fewer younger than older (25% vs. 38%, p=0.01) registrants had a colonoscopy. As shown in Table 3, these relationships were no longer statistically significant after adjusting the multivariate models for other demographic characteristics. Race/ethnicity, which was significantly associated with FOBT initially (49% of African American, 37% of Hispanic, 33% of other race/ethnicity, and 33% of white registrants had recent FOBT [p=0.04]), was no longer significant after adjusting for perceived barriers. Colonoscopy did not vary with race/ethnicity, but Hispanic registrants were significantly more likely than white registrants to report recent sigmoidoscopy.
Table 3.
Results of multivariate logistic regression models describing characteristics associated with recent CRC screening among county health center registrants, New York, October 2004 to January 2005 (n=500)
aModels adjusted for health center (not shown) and all other variables
CRC = colorectal cancer
AOR = adjusted odds ratio
CI = confidence interval
Ref. = referent group
HS = high school
HMO = health maintenance organization
County health center registrants with a college education were significantly more likely to report recent sigmoidoscopy than those with less than a college education. We associated other commercial/HMO insurance coverage significantly with lower odds of FOBT, while we associated Medicare/Medicaid significantly with greater odds of endoscopy, compared with no coverage. We found a significant association between no physician recommendation or other perceived barrier to screening with FOBT, sigmoidoscopy, or colonoscopy and lower odds of having one recently, compared with no perceived barriers. The magnitude of the Nagelkerke R2 indicated the usefulness of perceived barriers for predicting CRC screening use among county health center registrants.
Private patient sample.
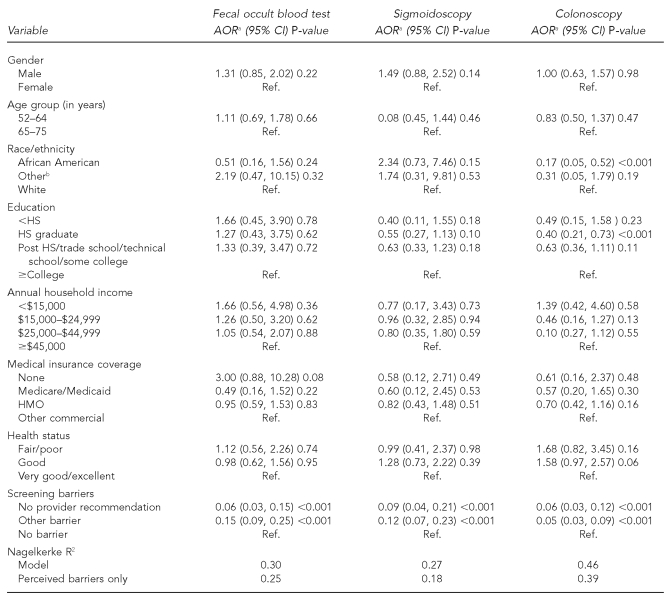
In the bivariate analyses, men more frequently had screening FOBT, sigmoidoscopy, and colonoscopy than women (p=0.01, p<0.001, and p=0.02, respectively [data not shown]). These p-values were no longer statistically significant after adjusting the multivariate models for perceived barriers (Table 4). African American (vs. white) adults and those with a high school education (vs. those with at least a college education) were significantly less likely to report recent colonoscopy. Respondents citing no physician recommendation or another barrier to recent FOBT, sigmoidoscopy, or colonoscopy screening were significantly less likely than those citing no barriers to report recent screening with these exams. The magnitude of the Nagelkerke R2 indicated the usefulness of perceived barriers for predicting CRC screening use among private physician patients.
Table 4.
Results of multivariate logistic regression models describing characteristics associated with recent CRC screening among users of private physician offices, New York, April–July 2004 (n=570)
aModels adjusted for all other variables
bIncludes <1% Hispanic
CRC = colorectal cancer
AOR = adjusted odds ratio
CI = confidence interval
Ref. = referent group
HS = high school
HMO = health maintenance organization
DISCUSSION
In general, self-reports of any recent CRC screening exam, during 2004, among our county health center and private physician patient samples compared favorably with overall rates of any CRC screening in New York (60%) and the U.S. (57%), as described by the 2004 Behavioral Risk Factor Surveillance System survey. We found that reported use of any screening exam by our private physician patient sample surpassed New York and U.S. rates by 20 percentage points. Reported FOBT in the past year also surpassed New York (18%) and U.S. (19%) rates for both primary care samples. However, screening disparities emerged when we considered endoscopy. Although recent endoscopy screening among our private physician patients was nearly 10 percentage points higher than the New York and U.S. rates (54% and 51%, respectively), recent endoscopy for the county health center sample was nearly 20 percentage points lower than private physician patient rates.6 This resulted in a 20-percentage point disparity in any screening exam (recent FOBT or endoscopy) among the county health center vs. private physician patient samples.
County health center registrants with adequate coverage for CRC screening (i.e., Medicare/Medicaid or other commercial/HMO) were more likely to report recent screening endoscopy and less likely to report recent FOBT than those with no insurance. Costs associated with screening endoscopy may influence physician referral and/or patient access to these exams, particularly for patients with no/inadequate insurance coverage.3,10,21,37,38 Underuse of CRC screening among socioeconomically disadvantaged populations that is associated with health-care system barriers such as no/inadequate insurance coverage22 may be a greater barrier to CRC screening than individual patient characteristics.39 In a previous comparison of mammography screening by county health centers and private community physicians conducted by the author (D.S. Lane) prior to New York and Medicare legislation providing coverage for screening mammography, mammography screening by low-income/underinsured women was higher among county health centers than among socioeconomically disadvantaged patients using private physician practices.40 The contrast to CRC screening may have been due to greater differences in access to breast imaging as compared with private endoscopists. In the current study, we found no relation between medical insurance and CRC screening for the private physician patient sample, in which only 3% were uninsured.
We did not find CRC screening disparities generally associated with age, gender, race/ethnicity, and education in other samples in our county health center sample. African American and white county health center registrants were equally likely to report recent FOBT, sigmoidoscopy, or colonoscopy. Although CRC screening among African Americans is generally lower than among white men and women,41,42 others have reported similar findings.19,43,44 For example, an analysis of 2000 NHIS Cancer Control Supplement data found African Americans as likely as their white counterparts to self-report screening FOBT, sigmoidoscopy, and colonoscopy, after controlling for sociodemographic and health-care access covariates.13 Results for the private physician patient sample suggesting that African Americans were less likely than white men and women to report recent colonoscopy were based on data obtained from a relatively small number of African American participants (n=24), and should be interpreted with caution. The finding that Hispanic county health center registrants were more likely than white registrants to report recent sigmoidoscopy was unexpected because the literature largely reports lower screening among Hispanic people13,34 and may be due to sigmoidoscopy screening provided at one county health center with a large Hispanic population.
Language (i.e., English vs. Spanish) did not influence CRC screening among county health center registrants in the multivariate analyses. Although Spanish-speaking registrants reported less colonoscopy screening compared with English-speaking registrants in the unadjusted comparisons, controlling for other patient characteristics (age, gender, race/ethnicity, and insurance coverage) attenuated this relationship. This finding contrasts results from the 2000 NHIS, which found an association among Hispanic people between low English proficiency and lower odds of cancer screening, after adjusting for sociodemographic and access variables.45 However, an analysis of the 2001 California Health Interview Survey data also found no association between English proficiency and cancer screening among Hispanic people, after controlling for sociodemographic/access variables.46 This finding and ours may reflect regional and local efforts to provide culturally sensitive health care for low English-proficient groups, such as language-concordant county health center providers, which is associated with improved medical comprehension47 and satisfaction with medical care communications,48 especially among Spanish-speaking Hispanic people.
No physician recommendation and other perceived barriers to screening contributed to decreased CRC screening among county health center registrants and users of private physician offices. These findings reiterate the importance of physician recommendation as a facilitator of CRC screening, regardless of primary care setting and participant demographics, as documented in the literature.13,49,50 Our findings also underscore the importance of other types of patient barriers, such as the perception that the respondent is healthy, the exam is not needed, fear, putting it off, forgetting, exam embarrassing, and others as correlates of screening.51 Patient perceptions of barriers to screening have implications for barrier-specific, targeted health-promotion efforts, which may effectively motivate screening in different patient populations.
Limitations
This study had several limitations. For one, we relied on patient recall to ascertain use of CRC screening. Although self-reports may have overestimated actual screening, evidence supports moderate to good correspondence (agreement of 70% or better) between self-reports and medical record rates.52–54 Recall of invasive tests was more accurate54 and overestimation of CRC screening was similar among African American, Hispanic, and non-Hispanic white registrants.54,55 Findings may not be generalized beyond the geographic setting of our project. However, our random population-based sample represented patients using private physician practices within Suffolk County. Our county health center registrant sample, randomly selected from all health centers serving disadvantaged Suffolk County residents, represented the population using these centers and populations targeted by Healthy People 2010 for reducing health-care disparities related to socioeconomic disadvantage.8 The cross-sectional study design precluded inferences about causal relationships among variables because findings were correlational and the direction of relationships was unknown. We conducted multiple comparisons to increase the likelihood that some statistical associations were obtained by chance.
CONCLUSIONS
Our findings highlight the importance of county health centers for reducing CRC screening disparities. Nonetheless, endoscopy screening by our private physician patient sample, but not our county health center sample, more closely approached goals set by Healthy People 20108 and ACS 2015.9 Thus, limited access to endoscopy associated with no/inadequate health insurance among county health center registrants remains a barrier to achieving equitable use of this screening modality for county health center and private physician patients. Although the recommendation of one test over another is not currently supported,1,3 there is growing support for the prevention of CRC among patients and physicians as evidenced by the increased use of colonoscopy.5 Mandatory insurance coverage and public financing for breast cancer screening has contributed to increased mammography screening by disadvantaged populations. Although screening for CRC is more complex than screening for breast cancer, because of different exam options, similar funding provisions would help increase CRC screening and broaden access to screening options among disadvantaged populations.
Footnotes
This research was supported by National Cancer Institute grant #R01 CA1010206-1-10435, and National Cancer Institute and Agency for Healthcare Research and Quality grant #R21 CA 1035717-1-30427.
REFERENCES
- 1.U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 2.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–41. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, Cokkinides VE, Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin. 2008;58:161–79. doi: 10.3322/CA.2007.0017. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 5.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer uptake among women and men in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 6.Seeff LC, King J, Pollack LA, Williams KN. Increased use of colorectal cancer tests—United States, 2002 and 2004. Morb Mortal Wkly Rep. 2006;55(11):308–11. [PubMed] [Google Scholar]
- 7.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, et al. Patterns and predictors of colorectal cancer test use in the U.S. population. Cancer. 2004;100:2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services (US) Healthy People 2010. 2nd ed. vol I and vol II. Washington: U.S. Government Printing Office; 2000. [Google Scholar]
- 9.American Cancer Society. Cancer prevention and early detection facts and figures, 2005. Atlanta: American Cancer Society; 2005. [Google Scholar]
- 10.Cokkinides VE, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among U.S. adults, age 50 years and older. Prev Med. 2003;36:85–91. doi: 10.1006/pmed.2002.1127. [DOI] [PubMed] [Google Scholar]
- 11.Hsia J, Kemper E, Kiefe C, Zapka J, Sofaer S, Pettinger M, et al. The importance of health insurance as a determinant of cancer screening: evidence from the Women's Health Initiative. Prev Med. 2000;31:261–70. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. National Health Interview Survey: linked mortality public-use data file, 2000. Atlanta: Centers for Disease Control and Prevention (US); 2002. [Google Scholar]
- 13.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–9. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A, et al. Effects of heath insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 15.Goel MS, Wee CC, McCarthy EP, Davis RB, Ngo-Metzger Q, Phillips RS. Racial and ethnic disparities in cancer screening: the importance of foreign birth as a barrier to care. J Gen Intern Med. 2003;18:1028–35. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine. America's health care safety net: intact but endangered. Washington: National Academy Press; 2000. [PubMed] [Google Scholar]
- 17.Frick KD, Regan J. Whether and where community health center users obtain screening services. J Health Care Poor Underserved. 2001;12:429–45. doi: 10.1353/hpu.2010.0787. [DOI] [PubMed] [Google Scholar]
- 18.O'Malley AS, Mandelblatt J. Delivery of preventive services for low-income persons over age 50: a comparison of community health clinics to private doctors' offices. J Community Health. 2003;28:185–97. doi: 10.1023/a:1022956223774. [DOI] [PubMed] [Google Scholar]
- 19.Christman LK, Abdulla R, Jacobsen PB, Cantor AB, Mayhew DY, Thompson KS, et al. Colorectal cancer screening among a sample of community health center attendees. J Health Care Poor Underserved. 2004;15:281–93. doi: 10.1353/hpu.2004.0021. [DOI] [PubMed] [Google Scholar]
- 20.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 21.Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and health insurance status (United States) Cancer Causes Control. 2005;16:735–42. doi: 10.1007/s10552-005-1228-z. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–44. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 23.Messina CR, Lane DS, Grimson R. Colorectal cancer-screening attitudes and practices preferences for decision making. Am J Prev Med. 2005;28:439–46. doi: 10.1016/j.amepre.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancer [published erratum appears in CA Cancer J Clin 2001;51:150] CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics. National Health Interview Survey public-use data file, 2000: cancer control module. Atlanta: Centers for Disease Control and Prevention (US); 2002. [Google Scholar]
- 27.Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA. 2003;289:2709–16. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]
- 28.Lillie-Blanton M, LaVeist M. Race/ethnicity, the social environment, and health. Soc Sci Med. 1996;43:83–91. doi: 10.1016/0277-9536(95)00337-1. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Jackson JS. Race/ethnicity and the 2000 census: recommendations for African American and other black populations in the United States. Am J Public Health. 2000;90:1728–30. doi: 10.2105/ajph.90.11.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braverman P, Cubbin C, Marchi KS, Egerter S. An approach to policy-oriented monitoring of equity in health. Paper presented at the 130th Annual Meeting of the American Public Health Association; 2002 Nov 8–14; Philadelphia. [Google Scholar]
- 31.SPSS Inc. SPSS: Version 15.01 for Windows. Chicago: SPSS Inc; 2007. [Google Scholar]
- 32.Woloshin S, Schwartz LM, Katz SJ, Welch HG. Is language a barrier to the use of preventive services? J Gen Intern Med. 1997;12:472–7. doi: 10.1046/j.1525-1497.1997.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley and Sons; 1999. [Google Scholar]
- 34.Walsh JM, Kaplan CP, Nguyen B, Gildengorin G, McPhee SJ, Perez-Stable EJ. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. J Gen Intern Med. 2004;19:156–66. doi: 10.1111/j.1525-1497.2004.30263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bewick V, Cheek L, Ball J. Statistics review 14: logistic regression. Crit Care. 2005;9:112–8. doi: 10.1186/cc3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlesselman JJ. Case-control studies: design, conduct, analysis. New York: Oxford University Press; 1982. [Google Scholar]
- 37.Chao A, Connell CJ, Cokkinides V, Jacobs EJ, Calle EE, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of U.S. adults. Am J Public Health. 2004;94:1775–81. doi: 10.2105/ajph.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, et al. Predictors of nonadherance to screening colonoscopy. J Gen Intern Med. 2005;20:989–95. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Malley AS, Beaton E, Yabroff KR, Abramson R, Mandelblatt J. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med. 2004;39:56–63. doi: 10.1016/j.ypmed.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Lane DS, Polednak AP, Burg MA. Breast cancer screening practices among users of county-funded health centers vs. women in the entire community. Am J Public Health. 1992;82:199–203. doi: 10.2105/ajph.82.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agrawal S, Bhupinderjit A, Bhutani MS, Boardman L, Nguyen C, Romero Y, et al. Colorectal cancer in African Americans [published erratum appears in Am J Gastroenerol 2005;100:1432] Am J Gastroenterol. 2005;100:515–23. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- 42.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among U.S. whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) program population-based study. Arch Intern Med. 2002;162:1985–93. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 43.Dolan NC, Ferreira MR, Fitzgibbon ML, Davis TC, Rademaker AW, Liu D, et al. Colorectal cancer screening among African-American and white male veterans. Am J Prev Med. 2005;28:479–82. doi: 10.1016/j.amepre.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Morales LS, Rogowski J, Freedman VA, Wickstrom SL, Adams JL, Escarce JJ. Sociodemographic differences in use of preventive services by women enrolled in Medicare+Choice plans. Prev Med. 2004;39:738–45. doi: 10.1016/j.ypmed.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 45.De Alba I, Sweningson JM, Chandy C, Hubbell FA. Impact of English language proficiency on receipt of pap smears among Hispanics. J Gen Intern Med. 2004;19:967–70. doi: 10.1007/s11606-004-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Alba I, Hubbell FA, McMullin JM, Sweningson JM, Saitz R. Impact of U.S. citizenship status on cancer screening among immigrant women. J Gen Intern Med. 2005;20:290–6. doi: 10.1111/j.1525-1497.2005.40158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson E, Chen AH, Grumbach K, Wang F, Fernandez A. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800–6. doi: 10.1111/j.1525-1497.2005.0174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez A, Schillinger D, Grumbach K, Rosenthal A, Stewart AL, Wang F, et al. Physician language ability and cultural competence: an exploratory study of communication with Spanish-speaking patients. J Gen Intern Med. 2004;19:167–74. doi: 10.1111/j.1525-1497.2004.30266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogedegbe G, Cassells AN, Robinson CM, DuHamel K, Tobin JN, Sox CH, et al. Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc. 2005;97:162–70. [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor V, Lessler D, Mertens K, Tu SP, Hart A, Chan N, et al. Colorectal cancer screening among African Americans: the importance of physician recommendation. J Natl Med Assoc. 2003;95:806–12. [PMC free article] [PubMed] [Google Scholar]
- 51.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 52.Baier M, Calonge N, Cutter G, McClatchey M, Schoentgen S, Hines S, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol, Biomarkers Prev. 2000;9:229–32. [PubMed] [Google Scholar]
- 53.Gordon NP, Hiatt RA, Lampert DI. Concordance of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993;85:566–70. doi: 10.1093/jnci/85.7.566. [DOI] [PubMed] [Google Scholar]
- 54.Hall HI, Van Den Eeden SK, Tolsma DD, Rardin K, Thompson T, Hughes Sinclair A, et al. Testing for prostate and colorectal cancer: comparison of self-report and medical record audit. Prev Med. 2004;39:27–35. doi: 10.1016/j.ypmed.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Hiatt RA, Perez-Stable EJ, Quesenberry C, Sabrogal F, Otero-Sabrogal R, McPhee SJ. Agreement between self-reported early cancer detection practices and medical audits among Hispanic and non-Hispanic white health plan members in Northern California. Prev Med. 1995;24:278–85. doi: 10.1006/pmed.1995.1045. [DOI] [PubMed] [Google Scholar]