Abstract
Short or polyunsaturated lipid variants of the NKT cell antigen α-galactosylceramide (αGC) exhibit decreased potency and a Th2 bias in vivo despite conserved TCR contact residues and stable binding to CD1d at neutral and acidic pH. Using reagents to directly visualize lipids in their free or CD1d-bound form, we determined that, contrary to predictions, these lipids reached the lysosome better than αGC. However, in contrast with αGC, they loaded CD1d at the cell surface and underwent immediate pH-dependent dissociation upon recycling to the lysosome. In cell-free assays, ultrafast dissociation of preformed complexes could be induced at acidic pH only when free competitor lipids were added, suggesting active lipid displacement. These findings provide a common cell biological explanation for the decreased stimulatory properties of short and polyunsaturated αGC variants. They also suggest that direct lipid displacement is a potent mechanism underlying highly dynamic lipid exchange reactions in the lysosomal compartment that shape the repertoire of lipids associated with CD1d.
The presentation of lipid antigens to T lymphocytes is accomplished by various CD1 molecules and is involved in multiple immunological processes (reviewed in refs. 1–3). Although much is known about the intracellular trafficking of CD1 molecules and the mechanisms of lipid loading, the intracellular trafficking of lipid antigens and the location and dynamics of CD1-lipid complex formation and dissociation remain poorly understood.
This issue has gained considerable interest since recent observations that lipid variants of the synthetic NKT ligand αGC displayed different immunostimulatory properties (4–7). NKT cells are a conserved population of CD1d-restricted T cells expressing a semiinvariant TCR with a canonical Vα14-Jα18 (human Vα24-Jα18) alpha chain specific for microbial lipids. Their activation by administration of synthetic αGC unleashes explosive secretion of Th1 and Th2 cytokines and chemokines and CD40L-mediated activation of CD1d-expressing dendritic cells (reviewed in ref. 1). αGC has therefore emerged as an effective immunomodulator and a powerful adjuvant of adaptive immunity (reviewed in ref. 8). The ternary structure of the huVα24-Jα18 TCR complexed with CD1d-αGC revealed the major role of Jα18 in contacting the galactose residue emerging from the groove whereas the acyl and sphingosine chains were buried in hydrophobic channels shielded from direct TCR recognition (9).
It is surprising, therefore, that variants of the original αGC displaying truncated or unsaturated fatty acid chains generally exhibit decreased potency for NKT cell activation but also often selectively favor Th2 responses (4–7). In the case of OCH, a variant of αGC with a truncated phytosphingosine chain, the predominant Th2 response was linked to premature interruption of TCR signaling due to unstable association of the variant lipid with CD1d (10) but another study found stable binding of OCH and instead demonstrated altered interaction of the TCR with CD1d-OCH, possibly as a consequence of a conformational change linked to the ‘collapse’ of a semi-empty F' channel (11).
In contrast, truncated acyl variants (12) or a diunsaturated acyl variant (5, 13), which exhibited similarly altered functional properties, bound CD1d and engaged the TCR as stably like αGC, as shown by direct biophysical studies or by CD1d tetramer staining experiments. Further, the structure of the CD1d-αGC (ac C8:0) complex was identical to that of CD1d-αGC at the TCR interface (12). In fact, the analysis revealed the presence of a spacer lipid at the bottom of the A' channel filling in for the truncated acyl chain of αGC (ac C8:0). Other explanations must therefore be provided to account for the altered stimulatory properties of these lipids. Some studies suggested that short or polyunsaturated lipids failed to efficiently traffic to the lysosome (14, 15) but this has not been tested for αGC variants. While the variants could directly load CD1d molecules at the cell surface without trafficking to endosomal compartments (5, 12, 13), lysosomal loading might be required for access to membrane rafts and efficient T-cell stimulation (16).
To directly visualize the site and kinetics of CD1d loading by αGC variants, we generated fluorochrome-tagged lipids and exploited a recently described antibody specific for the CD1d-αGC complex (17). Here, we demonstrate that these variants efficiently reached the lysosome, but were immediately dissociated from CD1d due to their displacement by long endogenous lipids at acidic pH.
Results
Cellular Entry of Short and Long NKT Ligands.
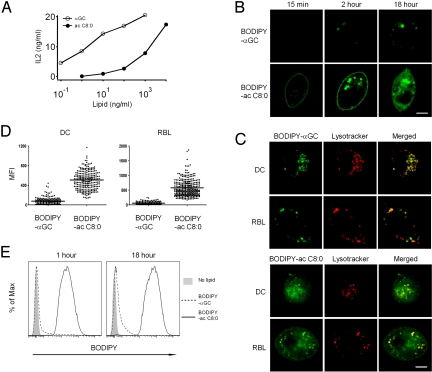
The parental compound αGC (ac C26:0, ps C18:0), known as KRN7000, its shorter or diunsaturated lipid variants, and the fluorescent conjugates with BODIPY linked to 6“ hydroxyl of the galactose ring (18) are depicted in Fig. S1. Previous biophysical and structural studies indicated that αGC and αGC (ac C8:0) bound CD1d and engaged the NKT cell TCR in a similar manner (12). However, Fig. 1A demonstrated that, when dendritic cells were pulsed overnight before stimulation of NKT cells, the dose response to the short acyl variant was markedly inferior and shifted >100-fold compared with αGC.
Fig. 1.
Cellular uptake and trafficking of αGC and αGC (ac C8:0). (A) BMDCs were incubated with indicated lipids at different concentrations and co-cultured with the NKT hybridoma DN32.D3 to measure IL-2 release. Representative of 3 experiments. (B) RBL.CD1d cells incubated with 1 μg/mL BODIPY-αGC or BODIPY-αGC (ac C8:0) for indicated times at 37 °C before confocal microscopy analysis. (Scale bar, 5 μm.) (C) BMDCs or RBL.CD1d cells incubated with 1 μg/mL BODIPY-αGC or BODIPY-αGC (ac C8:0) (green) for 18 h at 37 °C. Lysotracker (red) added in the last 15 min. (Scale bar, 5 μm.) (D) Scatter plot representation of the mean fluorescence intensity (MFI) of each Lysotracker positive vesicles in 12 to 50 individual cells analyzed by ImageJ. Data representative of 2 or 3 separate experiments. (E) Liposome-covered glass beads incubated at 37 °C in PBS with 10% FCS and 1 μg/mL BODIPY-αGC (dashed line) or 1 μg/mL BODIPY-αGC (ac C8:0) (solid line) for different times before FACS analysis. Shaded histograms are negative control (no BODIPY-lipid). Data representative of 2 separate experiments.
To investigate these differences, confocal microscopy studies using BODIPY conjugated lipids were performed using RBL.CD1d transfectants and mouse BMDCs. Paradoxically, the weaker antigen αGC (ac C8:0) exhibited a faster and augmented cellular uptake compared with αGC (Fig. 1B). Thus, BODIPY-αGC (ac C8:0) was found at the plasma membrane and in intracellular vesicles as soon as 15 min after incubation at 37 °C whereas BODIPY-αGC could only be detected at 2 h and in much lower amounts than BODIPY-αGC (ac C8:0). Furthermore, BODIPY-αGC was not detected at the plasma membrane and was only found in intracellular vesicles. At 18 h, while BODIPY-αGC (ac C8:0) exhibited a diffuse and intense staining pattern involving plasma membrane as well as intracellular vesicles, BODIPY-αGC was still concentrated in a vesicular compartment. Using conditions under which Lysotracker stains the LAMP-1-positive mannose 6-phosphate receptor-negative lysosomal compartment (19), we demonstrated that BODIPY-αGC was mostly contained within the lysosome after 18 h of incubation with RBL.CD1d or with BMDC, whereas BODIPY-αGC (ac C8:0) was detected in the lysosome as well as in non-lysosomal structures (Fig. 1C). The non-lysosomal structures included the Golgi apparatus. Quantitative analysis demonstrated 5–10 times more BODIPY-αGC (ac C8:0) than BODIPY-αGC in the Lysotracker-positive vesicles of DC or RBL (Fig. 1D).
Receptor-independent Uptake of Short Lipids.
The rapid staining of the cell membrane by BODIPY-αGC (ac C8:0) suggested direct, receptor-independent uptake. To directly test this possibility, liposomes were coated onto glass beads and incubated for various times with BODIPY-αGC or BODIPY-αGC (ac C8:0) prediluted in FCS-enriched PBS. After washes, the liposomes were analyzed by FACS to quantify lipid uptake. BODIPY-αGC (ac C8:0) demonstrated nearly maximal uptake by liposomes after only 1 h, whereas only trace amounts of BODIPY-αGC could be detected even after 18 h, demonstrating massive and efficient receptor-independent uptake of BODIPY-αGC (ac C8:0) by the lipid bilayer (Fig. 1E).
Opposite Impact of Endosomal Trafficking on CD1d Loading by Short or Long αGalCer Variants.
Using L363, an antibody specific for the mouse CD1d-αGalCer complex (17), we compared how αGC and αGC (ac C8:0) loaded CD1d at the cell surface and in intracellular compartments over time. Surprisingly, despite the massive differences in lipid uptake shown in Fig. 1, a relatively similar density of surface complexes was achieved for a range of lipid concentrations after 24 h (Fig. 2A). Thus, 1 μg/mL αGC generated equivalent surface loading as 5 μg/mL αGC (ac C8:0). These concentrations will be used throughout this study, unless indicated otherwise. The loading of αGC, however, was delayed by more than 6 h compared to that of αGC (ac C8:0) (Fig. 2B), reflecting a differential requirement for lysosomal loading as shown by the drastic reduction of CD1d-αGC complexes at the cell surface of RBL-TD cells expressing a truncated variant of CD1d lacking the endosomal recycling motif.
Fig. 2.
Different cellular sites and kinetics of CD1d binding for αGC and αGC (ac C8:0). (A) Mean fluorescence intensity (MFI) of surface staining with L363 (anti-CD1d-αGC complex) in RBL.CD1d cells incubated with different concentrations of αGC or αGC (ac C8:0) for 24 h. Representative of 2 experiments. (B) (Upper) L363 fluorescence histogram of RBL.CD1d cells incubated for 1 or 24 h with medium or lipids as indicated (αGC 1 μg/mL, dashed line; αGC (ac C8:0) 5 μg/mL, solid line). (Lower) Kinetics of CD1d loading (measured by surface staining with L363) in RBL.CD1d (circles) and RBL.CD1-TD (triangles) incubated with αGC or αGC (ac C8:0) as in Upper for various time periods. MFI at different time points is expressed as % MFI of RBL.CD1d cells at 24 h. Representative of 3 experiments. (C) RBL.CD1d cells incubated with αGC and αGC (ac C8:0) as above for 24 h were fixed, permeabilized, and stained with L363 (red) and anti-LAMP1 (green). Representative of 3 experiments. (Scale bar, 5 μm.)
Confocal microscopy showed that CD1d-αGC complexes were abundantly present in the lysosome, as expected (Fig. 2C Left). Furthermore, side-by-side kinetic analysis by confocal microscopy demonstrated that BODIPY- αGC reached the lysosome in a delayed manner, concomitant with the detection of CD1d-αGC complexes in this organelle (Fig. S2). However, CD1d-αGC (ac C8:0) complexes were quasi-absent in this compartment (Fig. 2C Right). This was notable because αGC (ac C8:0) efficiently reaches the lysosome and also because it can efficiently and stably load CD1d over a range of pH values in cell-free assays (12). Because CD1d-αGC (ac C8:0) complexes are readily formed at the plasma membrane and are expected to recycle to the lysosome, we hypothesized that these complexes were dissociated in the lysosomal compartment.
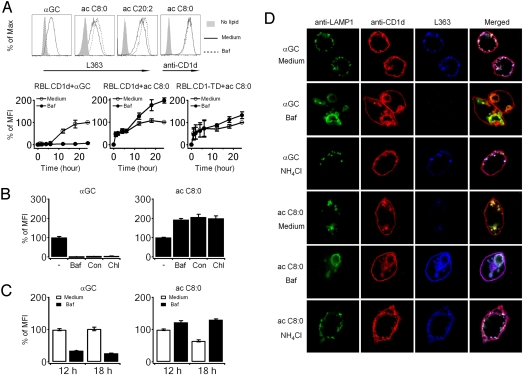
Role of Lysosomal Acidification.
Treatment of RBL.CD1d cells with the inhibitor of H+-ATPase bafilomycin A impaired the loading of αGC onto CD1d, as expected (Fig. 3A). The same treatment, however, had the opposite effect on the concentration of surface CD1d-αGC (ac C8:0) which increased approximately 2-fold. Another variant with a diunsaturated acyl chain αGC (ac C20:2) demonstrated the same increased loading after bafilomycin A. This increase occurred in a delayed manner after 6 h, consistent with the hypothesis that bafilomycin A prevented the dissociation of CD1d-αGC (ac C8:0) complexes formed at the cell surface as they recycled to the lysosome (Fig. 3A Lower). Bafilomycin A did not induce this augmenting effect in RBL-CD1-TD cells expressing the cytoplasmic tail truncated CD1d, further supporting the conclusion that the increase in surface CD1d-αGC (ac C8:0) depended on lysosomal recycling. Other lysosomal acidification inhibitors, including concanamycin and chloroquine, had identical effects (Fig. 3B). In other experiments, bafilomycin A was added after lipid antigen pulse to study the effect of lysosomal neutralization on existing CD1d-lipid complexes. The surface expression of CD1-αGC (ac C8:0) increased 2-fold over the next 18 h, consistent with cessation of the dissociation of recycling complexes (Fig. 3C). In contrast, the surface expression of CD1-αGC continued to decrease over the same period, presumably reflecting the interruption of lysosomal loading. We noted that, despite extensive washes after the pulse period, lipids seemed to be continuously secreted in the culture medium, possibly in the form of blebs or exosomes and subsequently served as a reservoir for prolonged loading of CD1d. This release was particularly abundant for αGC (ac C8:0) (Fig. S3) preventing strict ‘chase’ experiments.
Fig. 3.
Lysosomal recycling of CD1d regulates the presentation of αGC variants. (A) (Upper) L363 or anti-CD1d surface staining of RBL.CD1d cells incubated with αGC (1 μg/mL) or variants αGC (ac 8:0) or αGC (ac C20:2) (5 μg/mL) for 24 h in medium with (dashed line) or without (solid line) bafilomycin A. Shaded histogram is negative control with no lipid. (Lower) kinetics of surface appearance of CD1d-lipid complexes in RBL.CD1d or RBL.CD1-TD cells. MFI is expressed as % MFI of untreated cells at 24 h. Results represent mean of 2 to 3 experiments and error bars indicate SEM. (B) L363 surface staining of RBL.CD1d cells incubated with αGC or αGC (ac C8:0) as above for 24 h with or without lysosome inhibitors bafilomycin, concanamycin A, or chloroquine as indicated. L363 MFI is expressed as % MFI of untreated cells. Results represent mean of 5 experiments and error bars indicate SEM. (C) RBL.CD1d cells incubated with αGC or αGC (ac C8:0) as above for 12 h, washed, and chased in medium with (filled bars) or without (open bars) bafilomycin A for indicated times. Surface staining with L363 is expressed as % of MFI in untreated cells chased for 12 h. Error bars represent SEM of triplicate results. Representative of 2 experiments. (D) Confocal microscopy of RBL.CD1d incubated with αGC or αGC (ac C8:0) for 24 h. Bafilomycin was added at time 0, whereas NH4Cl was added at 24 h for only 2 min before fixation/permeabilization and staining as indicated. Representative of 3 experiments. (Scale bar, 5 μm.)
Confocal microscopy confirmed that bafilomycin A prevented lysosomal loading of αGC in the lysosome (Fig. 3D). The late endosomal/lysosomal compartment stained for LAMP-1 exhibited a distorted morphology with larger vesicles typical of bafilomycin A treatment. In contrast, the opposite effect was noted for αGC (ac C8:0), as bafilomycin A induced the de novo appearance of CD1d-αGC (ac C8:0) complexes in the late endosome/lysosome. Cells that had previously been pulsed with lipids were also treated with NH4Cl to induce immediate neutralization of the endosomal pH, as opposed to the progressive effect of bafilomycin A. Strikingly, CD1d-αGC (ac C8:0) complexes appeared in the late endosome/lysosome as early as 2 min after pH neutralization, whereas the same treatment did not alter the presence of CD1d-αGC complexes. Given that the CD1d-αGC (ac C8:0) complexes that form at the cell surface continuously recycle to the lysosome, they must be rapidly dissociated in the acidic environment of the lysosome. These results also provided independent confirmation of the presence of αGC (ac C8:0) in the lysosome as suggested by the experiment with fluorochrome-tagged lipids shown in Fig. 1.
Other Variants of αGC.
Experiments using phytosphingosine-truncated variants of αGC showed a similar bafilomycin A-induced increase in surface staining by L363 (Fig. S4A) and appearance of CD1d-lipid complexes in the late endosome/lysosome (Fig. S4B).
Influence of pH and Free Lipids on the Dissociation of CD1d-Lipid Complexes in Cell-Free Conditions.
To model the conditions leading to the rapid dissociation of CD1d-αGC (ac C8:0) complexes in the lysosome, we studied the loading and the dissociation of lipids in a cell-free assay using recombinant CD1d protein, lipid transfer proteins and a competitor lipid naturally present in the lysosome, glycosphingolipid GT1b, at variable pH conditions. Fig. 4A shows that, while αGC (ac C8:0) could load CD1d proteins independently of lipid transfer proteins at both acidic and neutral pH, αGC required both acidic pH and lipid transfer proteins for optimal loading. Preformed complexes of both CD1d-αGC and CD1d-αGC (ac C8:0) were stable at either neutral or acidic pH (Fig. 4B Left) as previously shown. However, in presence of the free GT1b, most αGC (ac C8:0) molecules were displaced within less than 1 min, whereas αGC remained associated with CD1d (Fig. 4B Right). Importantly, the rapid displacement of αGC (ac C8:0) was observed at acidic but not neutral pH. Similar findings were observed with the sphingosine-shortened or the diunsaturated acyl variants of αGC (Fig. 4C). These findings demonstrate the extraordinary synergy of pH and free lipids in dissociating, within seconds, preformed complexes made of CD1d and short or polyunsaturated variants of αGC.
Fig. 4.
Cell-free assay for lipid binding to CD1d. (A) Plate bound CD1d was incubated with αGC variants (1 μg/mL) at different pH with or without LTP (1 μg GM2a + 1.7 μg saposin B per well) for another 9 h at 37 °C, as indicated. After washes, DN32.D3 cells were added and IL-2 release measured by ELISA. Results represent the mean of 3 experiments and error bars indicate SEM. (B and C) Plate-bound CD1d loaded with indicated lipids (1 μg/mL) was washed with pH 4.5 or pH 7 PBS with or without competitor GT1b (5 μg/mL) for 5, 30, 60, 90, and 180 s. After the washes, DN32.D3 cells were added and IL-2 measured by ELISA. Results represent the mean of 3 to 5 experiments and error bars indicate SEM.
Pulse-Chase Assays In Vivo.
After overnight pulse with αGC or αGC (ac C8:0), RBL.CD1d cells were washed and chased in various culture conditions to monitor the decay of surface CD1d-αGC complexes over time. When examined immediately after a 24-h pulse with a broad range of concentrations of αGC or αGC (ac C8:0), the densities of CD1d-αGC and CD1d-αGC (ac C8:0) complexes at the surface of RBL.CD1d cells are roughly comparable (Fig. 2A). Given the similar recognition of these complexes by the NKT cell TCR, the markedly decreased potency of αGC (ac C8:0) might be due in part to the rapid dissociation of CD1d-αGC (ac C8:0) complexes after the pulse, during the overnight incubation with the NKT hybridoma. To directly test this hypothesis, we lipid-pulsed RBL.CD1d cells for 24 h and monitored the decay of surface complexes over a 18-h chase period. A shift of 10-fold in the surface concentration of CD1d-lipid complexes could be observed between αGC and αGC (ac C8:0) after only 4 h of chase and >100-fold after 18 h (Fig. 5A). Note that, as mentioned previously, a strict chase is not possible due to prolonged storage of lipids inside the cells and their release in the culture supernatant, even after thorough washes (Fig. S3). Nevertheless, the findings directly demonstrate the relatively faster decay of CD1d-αGC (ac C8:0) complexes at the cell surface, providing a clear explanation for the decreased stimulatory potency of αGC (ac C8:0) when this antigen is present only transiently during a pulse. To directly test the contribution of endosomal recycling to the dissociation of surface CD1d-lipid complexes, we pulsed RBL.CD1d and RBL.CD1-TD with αGC (ac C8:0) in serum-containing medium before a chase in serum-free medium (to minimize lipid displacement at the cell surface). As mentioned before, despite multiple washes, RBL cells continuously release the αGC variant lipids in the culture medium well after the pulse. These lipids can reload surface CD1d molecules, explaining the apparent absence of decay of CD1d-lipid complexes in Fig. 5B. However, RBL cells expressing CD1-TD, the non-recycling form of CD1d, exhibited a relative increase in CD1d-lipid complexes compared with RBL.CD1d cells, demonstrating that CD1d lysosomal recycling significantly contributes to the dissociation of CD1d-αGC (ac C8:0) complexes formed at the cell surface.
Fig. 5.
Accelerated dissociation of CD1d-αGC (ac C8:0) compared with CD1d-αGC in vivo. (A) RBL.CD1d cells pulsed with αGC or αGC (ac C8:0) for 24 h at various concentrations were washed and co-cultured with DN32.D3 cells for 0, 4, or 18 h as indicated before surface staining of RBL.CD1d cells with L363. Data representative of 3 independent experiments. (B) RBL.CD1d (open circles) and RBL.CD1-TD (open triangles) were incubated with αGC (ac C8:0) at 5 μg/mL in serum-containing medium for 18 h, washed, and chased in serum-free medium for indicated times before surface staining with L363 (expressed as % MFI at time 0). Results represent the mean of 4 experiments and error bars indicate SEM.
Discussion
This study defines a set of biochemical and cell biological properties that distinguish short or diunsaturated lipid variants of αGC from αGC itself, beyond recent reports that some of these variants can load CD1d directly at the cell surface (5, 12, 13). They clarify conflicting assessments of the stability of association of CD1d with some αGC variants (10, 11) and reveal highly dynamic lipid exchange mechanisms that operate within cells in vivo but eluded prior studies using cell-free assays in vitro.
The short and diunsaturated lipid variants showed immediate, receptor-independent binding to lipid bilayers, explaining their direct colonization of the plasma membrane at the cell surface. In contrast, αGC entry was delayed and appeared largely dependent on uptake by receptors, likely lipoprotein receptors (20). LDL receptor-mediated uptake specifically directs lipids such as αGC to the endosomal compartment, which is well equipped to load lipids onto CD1d with assistance of lipid transfer proteins (21, 22), whereas the short and diunsaturated variants directly loaded CD1d at the cell surface. The increased solubility of short and diunsaturated variants in aqueous solution might explain their different behavior, including the binding to CD1d in the absence of lipid transfer proteins or detergents. Through direct binding to the plasma membrane, the lipid variants participated in vesicular trafficking, ultimately reaching lysosomal concentrations 5–10 times greater than αGC. In this respect, these short lipids differed markedly from a short variant of glucomonomycolate (C32 GMM) and from short DiL lipid analogues, which showed impaired access to the late endosome/lysosome when compared with their longer versions (14, 15). The dependence of αGC on surface lipoprotein receptors might preferentially direct its entry to cells expressing high levels of these receptors, such as dendritic cells and macrophages (Fig. S5), whereas the short variants may be more indiscriminately captured and presented by various types of CD1d-expressing cells, including nonprofessional antigen-presenting cells. These relative differences in antigen-presenting cell types might in turn influence the magnitude and the type of responses in vivo. For example, a more sustained IFN-γ response could be achieved in vivo when αGC was selectively presented by DC (23, 24).
The major finding of this study is the demonstration of the intensely dynamic nature of the lipid exchange mechanisms that govern CD1d-mediated antigen presentation, specifically the process of lipid displacement and its regulation by lipid structure and acidic pH. Displacement, that is, the increased dissociation rate of some CD1-lipid complexes in the presence of free lipids, was previously reported in the CD1b system where a short form of GM1 could be displaced by longer natural glycosphingolipids during overnight incubation (25). Although its precise mechanisms remain to be elucidated, displacement might occur after a free lipid inserts a fatty acid chain into the CD1d channel transiently vacated by the shorter arm of a bound lipid, gaining a foothold to subsequently dislodge the second lipid chain. Likewise, acidic pH was recently shown to increase the rate of dissociation of C32 GMM from human CD1b with comparatively little effect on C80 GMM, a phenomenon ascribed to the disruption of ionic tethers between molecular domains of CD1b (26). While neither acidic pH nor the presence of a competing lipid exhibited strong effects by themselves, our studies directly show that it is the combination of these 2 mechanisms that accounts for the dissociation within seconds of CD1d complexed with short or diunsaturated αGC variants. These results resolve the discrepancies between the long lifespan of these complexes when measured in cell-free assays over a range of pH values (5, 11, 12) and their drastically reduced lifespan in living systems.
Because premature interruption of antigen presentation to NKT cells preserved IL-4 but markedly reduced IFN-γ secretion (10), these findings may also have implications for our understanding of the Th2 bias commonly observed with short or diunsaturated αGC variants in vivo. Indeed, rapid dissociation from CD1d may increase the frequency of abortive signaling events sufficient to induce IL-4 but not IFN-γ, further preventing the activation of NK cells to release more IFN-γ (27, 28). However, multiple mechanisms may contribute to the functional properties of αGC variants, including indirect alteration of the CD1d structure recognized by the TCR in the case of αGC (ps C9:0) (11) and differential uptake and presentation by different cell types (12, 29). Another intriguing possibility is that the preferential loading of αGC in the lysosome might result in the increased presence of CD1d-αGC complexes on lipid rafts at the cell surface, as previously suggested for MHC class II antigen presentation (16). Nevertheless, the common physical, cell biological, and functional properties shared by the short or diunsaturated variants of αGC in this study suggest that they must share fundamental mechanisms impacting their immune stimulatory properties.
Intense and highly dynamic lipid exchange mechanisms such as reported here constitute powerful mechanisms to shape the repertoire of natural self or foreign lipids bound to CD1 isotypes based simply on lipid structure and local pH. Therefore, these findings provide insights into the cell biological mechanisms that, over evolutionary time, directed different CD1 isotypes to optimize the structure of their lipid-binding groove to target specific types of lipids in different cellular compartments (2).
Materials and Methods
Cell Culture and Lysosomal Inhibitors.
Antigen-presenting cells, including bone marrow derived dendritic cells, stable RBL transfectants RBL.CD1d (mouse CD1d) and RBL.CD1-TD (tail truncated CD1d), and NKT hybridoma DN32.D3 were obtained and cultured in medium supplemented with 10% fetal calf serum (FCS) as described (30). Fifty millimolar NH4Cl buffer contained NaCl 79 mM, KCl 4.7 mM, NH4Cl 50 mM, KH2PO4 1.2 mM, NaHCO3 5 mM, HEPES 10 mM, glucose 3 mM, bovine serum albumin (BSA) 0.1%, MgSO4 1.2 mM, and CaCl2 2.5 mM. bafilomycin A, concanamycin A, and chloroquine (Sigma-Aldrich) were dissolved in DMSO and added to cell cultures at final concentrations of 50 nM, 20 nM, and 100 μM, respectively, 5 min before adding lipid antigens.
NKT Ligands.
αGC (ac 26:0, ps 18:0) (denoted αGC) was from Alexis Biochemicals and its variants αGC (ac C8:0), αGC (ac 24:0, ps C6:0), αGC (ac 24:0, ps C9:0), αGC (ac 24:0, ps C13:0), αGC (ac C20:2), BODIPY-αGC, and BODIPY- αGC (ac C8:0) were produced as described previously (4, 18). All lipids were dissolved in DMSO at 1 mg/mL and kept aliquoted at −20 °C.
Flow Cytometry.
RBL.CD1d and RBL.CD1-TD cells were pulsed with αGC or its variants at different concentrations for various periods of times before staining with purified antibody L363 (1 μg/mL) at room temperature for 30 min followed by fluorescent Cy5 conjugated-donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch) diluted 1:200. Flow cytometry was performed with BD LSR II (BD Biosciences) and FlowJo 8.2 software.
Liposomes.
Liposomes (60% phosphatidylcholine and 40% cholesterol) and liposome-covered glass beads were made as described previously (31). The liposome-covered beads were incubated with BODIPY-αGC or BODIPY-αGC (ac C8:0) diluted at 1 μg/mL in PBS buffer with 10% FCS at 37 °C.
Confocal Microscopy.
To study lipid trafficking, cells were adhered to polyL-lysine coated coverslip and incubated with 1 μg/mL BODIPY-αGC or 1 μg/mL BODIPY-αGC (ac C8:0) at 37 °C for various time. Lysosome marker LysoTracker (Red DND-99, Molecular Probes) was added at 1 μM for the last 15 min. The distribution of fluorescent lipids in the Lysotracker+ compartment was quantitated by measuring the fluorescence intensity of individual Lysotracker+ vesicles using software ImageJ. For CD1d-αGC complex staining, cells were incubated with αGC or variants for 24 h, then fixed with 4% PFA for 20 min and permeabilized with 0.05% saponin and 0.2% BSA in PBS for 20 min. Cells were blocked with 10% donkey serum at room temperature, then stained with mouse antibody L363 (5 μg/mL) and rabbit polyclonal anti-LAMP1 (10 μg/mL, Abcam) at room temperature followed by Cy5-conjugated donkey anti-mouse IgG (H+L) and FITC-conjugated donkey anti-rabbit IgG (H+L) (Jackson ImmunoResearch) diluted 1:200. CD1d was stained with 10 μg/mL 20H2 ((32)) conjugated with DyLight 549 (Pierce). Samples were mounted with Prolong Gold antifade reagent (Molecular Probes) overnight and examined by confocal microscopy (FV1000, Olympus) with a 100× oil objective. Data were analyzed using Adobe Photoshop and ImageJ.
Hybridoma Stimulation Assay.
BMDCs or RBL transfectants (5 × 104 cells per microwell) were pulsed with various concentrations of lipid antigens overnight, washed, and cocultured with the NKT hybridoma DN32.D3 (5 × 104 cells per well) overnight. IL-2 release was measured by ELISA (R&D Systems).
Cell-free Assay for CD1d-lipid Binding.
For the loading assay, 96-well microplates (ultra high binding polystyrene microtiter plate, Thermo) were coated with mouse CD1d protein (1 μg/well) in PBS at 37 °C for 17 h. NKT ligands (1 μg/mL) were added in PBS adjusted for acid or neutral pH with or without recombinant lipid transport proteins (LTP) (22) (1 μg/well mouse GM2a + 1.7 μg/well mouse saposin B) for another 9 h at 37 °C. For the lipid exchange assay, plate-bound CD1d was loaded with lipids at 1 μg/mL and washed with PBS at acidic or neutral pH in the presence or absence of 5 μg/mL GT1b (purified from bovine brain, Sigma-Aldrich) for various time periods. DN32.D3 hybridoma cells (5 × 104 per well) were then added and plates incubated overnight before IL-2 quantification by ELISA.
Supplementary Material
Acknowledgments.
We thank members of the Bendelac, Teyton, and Savage laboratories for help and advice. Research was supported by the National Institutes of Health PO1 AI053725 to AB, LT and PBS; AI45889 to SAP; the University of Chicago Digestive Diseases Research Core Center (P30 DK42086). AB is an investigator of the Howard Hughes Medical Institute, and YS was supported by a fellowship from the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901228106/DCSupplemental.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol. 2003;3:11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 3.De Libero G, Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol. 2005;5:485–496. doi: 10.1038/nri1631. [DOI] [PubMed] [Google Scholar]
- 4.Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 5.Yu KO, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 7.Schmieg J, et al. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh VV, Wilson MT, LVan Kaer iNKT-cell responses to glycolipids. Crit Rev Immunol. 2005;25:183–213. doi: 10.1615/critrevimmunol.v25.i3.20. [DOI] [PubMed] [Google Scholar]
- 9.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 10.Oki S, et al. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J Clin Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy C, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–1144. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant NKT cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkberg G, et al. Phage display-derived recombinant antibodies with TCR-like specificity against alpha-galactosylceramide and its analogues in complex with human CD1d molecules. Eur J Immunol. 2008;38:829–840. doi: 10.1002/eji.200737518. [DOI] [PubMed] [Google Scholar]
- 14.Moody DB, et al. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol. 2002;3:435–442. doi: 10.1038/ni780. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J Cell Biol. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turley SJ, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 17.Yu KO, et al. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou XT, et al. Synthesis and NKT cell stimulating properties of fluorophore- and biotin-appended 6“-amino-6”-deoxy-galactosylceramides. Org Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 19.Sagiv Y, et al. Cutting Edge: Impaired glycosphingolipid trafficking and NKT cell development in mice lacking Niemann-Pick Type C1 protein. J Immunol. 2006;177:26–30. doi: 10.4049/jimmunol.177.1.26. [DOI] [PubMed] [Google Scholar]
- 20.van den Elzen P, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrantz N, et al. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007;204:841–852. doi: 10.1084/jem.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii S, et al. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 24.Chun T, et al. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shamshiev A, et al. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000;13:255–264. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 26.Relloso M, et al. pH-dependent interdomain tethers of CD1b regulate its antigen capture. Immunit. 2008;28:774–786. doi: 10.1016/j.immuni.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnaud C, et al. Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol [Cutting Edge] 1999;163:4647–4650. [PubMed] [Google Scholar]
- 28.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Bezbradica JS, et al. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 30.Jayawardena-Wolf J, et al. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 31.Mallet-Designe VI, et al. Detection of low-avidity CD4+ T cells using recombinant artificial APC: following the antiovalbumin immune response. J Immunol. 2003;170:123–131. doi: 10.4049/jimmunol.170.1.123. [DOI] [PubMed] [Google Scholar]
- 32.Roark JH, et al. CD1.1 expression by mouse antigen presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.