Abstract
Stomatal pores are microscopic structures on the epidermis of leaves formed by 2 specialized guard cells that control the exchange of water vapor and CO2 between plants and the atmosphere. Stomatal size (S) and density (D) determine maximum leaf diffusive (stomatal) conductance of CO2 (gcmax) to sites of assimilation. Although large variations in D observed in the fossil record have been correlated with atmospheric CO2, the crucial significance of similarly large variations in S has been overlooked. Here, we use physical diffusion theory to explain why large changes in S necessarily accompanied the changes in D and atmospheric CO2 over the last 400 million years. In particular, we show that high densities of small stomata are the only way to attain the highest gcmax values required to counter CO2“starvation” at low atmospheric CO2 concentrations. This explains cycles of increasing D and decreasing S evident in the fossil history of stomata under the CO2 impoverished atmospheres of the Permo-Carboniferous and Cenozoic glaciations. The pattern was reversed under rising atmospheric CO2 regimes. Selection for small S was crucial for attaining high gcmax under falling atmospheric CO2 and, therefore, may represent a mechanism linking CO2 and the increasing gas-exchange capacity of land plants over geologic time.
Keywords: Phanerozoic, photosynthesis, plant evolution, transpiration, xylem
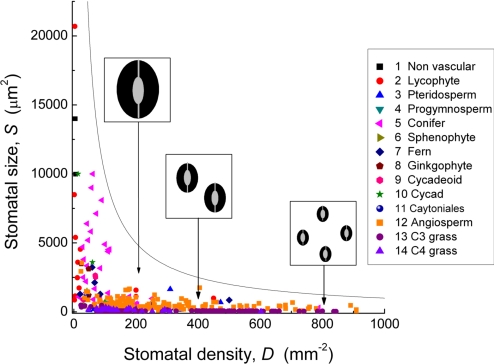
Stomata crucially permit plants to regulate transpirational water loss from leaves during the simultaneous uptake of CO2 for photosynthesis (1, 2). Originating more than 400 Ma, their evolutionary appearance was pivotal to the colonization of the land by plants, permitting the survival and ecological radiation of floras throughout diverse terrestrial habitats experiencing widely fluctuating environmental conditions (2–4). Remarkably, the fundamental design of stomata has remained unchanged over the 400-million year (Myr) history of vascular plants (3, 4) but has been fine-tuned through increases in the operational efficiency of guard cell function (5). Nevertheless, major changes in stomatal size (S) and density (D) are evident in the fossil record (2, 6) (Fig. 1; Table S1), but their significance as land-plant evolution progressed, in parallel with advancements in gas-exchange capacity, is not yet understood. Here, we use diffusion theory and Earth's global atmospheric CO2 history to provide a unifying functional explanation for these changes in stomatal geometry over evolutionary time scales.
Fig. 1.
The relationship between stomatal size S and density D across the Phanerozoic. Each point is an individual species (see Table S1). The curved line is the estimated upper theoretical limit to stomatal size at a given density, or the upper limit of density at a given size. All combinations of size and density must fall below this line. Diagrams in boxes are comparative representations of possible combinations of stomatal size and density to assist visualization of the scaling of these dimensions in real leaves.
One basic feature of leaves that should be highly conserved is the relationship between maximum pore area and leaf gas-exchange capacity, which is governed by the physics of diffusion through pores. The maximum area of the open stomatal pore (amax) and its depth (l) are set by stomatal size S (defined here as guard cell length, L, multiplied by total width, W, of the closed guard cell pair). Taken together, amax, l, and D determine maximum diffusive conductance to water vapor or CO2 (gwmax or gcmax, respectively) according to ref. 7:
where d is the diffusivity of water vapor in air (m2·s−1), v is the molar volume of air (m3·mol−1), and gcmax = gwmax/1.6 (1). Critically, it can be shown mathematically that for the same total pore area, amax, smaller stomata result in higher gwmax compared with larger stomata (Fig. 2). This is because gwmax is inversely proportional to the distance that gas molecules have to diffuse through the stomatal pore (l), which increases with S as guard cells inflate to be approximately circular in cross-section (5). It can be further shown, by using Eq. 1, that for a given gwmax, smaller stomata allow more epidermal space to be allocated to other structures such as subsidiary cells, trichomes, or oil cells (Fig. 3). Stomatal size is important not only to gwmax and gcmax but also to the overall structure and broader ecophysiological function of leaf surfaces.
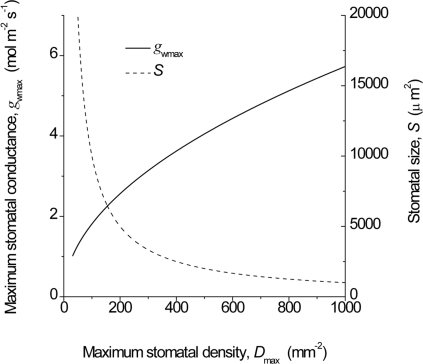
Fig. 2.
Smaller stomata provide higher stomatal conductance for the same total pore area because of the shorter diffusion path length, l (see Eq. 1). For any stomatal size S there is a theoretical maximum stomatal density Dmax based on simple geometric packing limitations. Here the total stomatal pore area per unit leaf area remains constant at the theoretical maximum for any given stomatal size S, but because that pore area is made up of ever-smaller and more numerous stomata, the stomatal conductance (gwmax) increases. See Data and Methods for details.
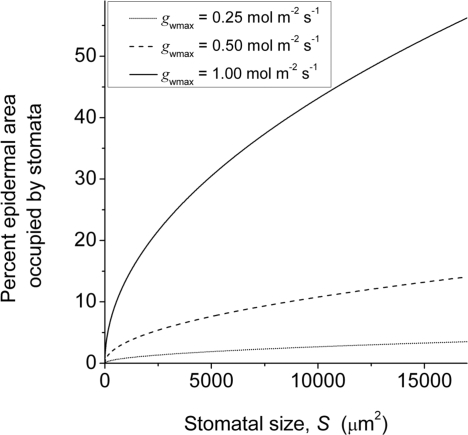
Fig. 3.
For any given gwmax, smaller stomata free up more epidermal space for other cell types and functions. Shown are percentages of epidermal surface occupied by stomata as a function of variable stomatal size S for 3 fixed values of gwmax. CO2-forcing of changes in gwmax via changes in S and D have implications for leaf attributes unrelated to gwmax. See Data and Methods for details.
The 400-Myr fossil history of stomata reveals that although S and D have both varied by several orders of magnitude (Fig. 1), all reported combinations are located within the biophysical envelope defined by maximum packing of stomata on the leaf surface (i.e., the maximum number of stomata of a given size, S, that can fit within a unit area) (Fig. 1; Table S1). Indeed, combinations of S and D must lie somewhat below this upper packing limit to accommodate several developmental, physiological, and mechanical requirements for spacing of stomata (5, 8).
Irrespective of the size, shape, or number of epidermal cells filling the space between individual stomata on a leaf surface, S and D are virtually the only epidermal characteristics determining gcmax, an important feature constraining maximum leaf gas-exchange capacity. At the time of first appearance of vascular plants in the fossil record, atmospheric CO2 concentration was several times higher than current ambient CO2 (9), but subsequent periods of falling atmospheric CO2 challenged plants with diminished CO2 availability. Adaptation of gcmax to these conditions required a change in S and/or D, and we hypothesize from principles of stomatal packing on leaf surfaces (Figs. 2 and 3) and gas-exchange diffusion theory (Eq. 1) that selection for higher gcmax by falling atmospheric CO2 is characterized by a trend toward smaller S and higher D.
Results
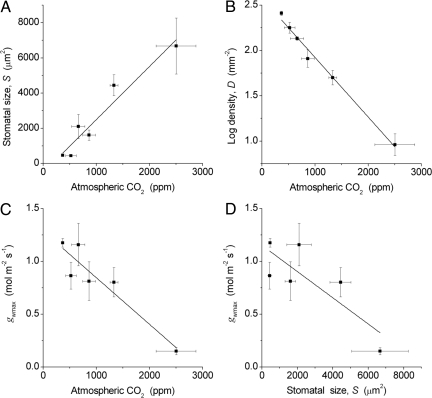
Consistent with our hypothesis we report that a positive correlation between S and Earth's global atmospheric CO2 history (Fig. 4A) is mirrored by a negative correlation between D and CO2 over hundreds of millions of years of plant evolution (Fig. 4B). The magnitude of change in S is far greater than, but consistent with, that observed in short-term CO2 experiments (Table 1). These short-term changes are presumably reflecting phenotypic plasticity (i.e., the limited range in magnitude of S resulting from the effect of changes in the environment on stomatal development within a single generation). The strong negative correlation of D with CO2 over 400 Myr (Fig. 4B) is consistent with observations on Quaternary fossil leaves over documented glacial–interglacial CO2 changes (10, 11), trees experiencing the rapid rise in atmospheric CO2 over the past century (12), and experiments (13).
Fig. 4.
Relationships between fossil stomatal traits and atmospheric CO2. Each point is the mean for either a 50- or 100-Myr time slice of the Phanerozoic, beginning at −400 Myr, or the mean for modern times (0 Myr). (A) Stomatal size is positively correlated with atmospheric CO2 concentration (y = 3.02x − 528; r2 = 0.92; P < 0.002). (B) Stomatal density D decreases exponentially with atmospheric CO2 concentration, hence log10D is linearly correlated with CO2 (y = −0.000657x + 2.58; r2 = 0.98; P < 0.0001). (C) Maximum stomatal conductance to water vapor, gwmax, is negatively correlated with atmospheric CO2 concentration (y = −0.000441x + 1.29; r2 = 0.84; P < 0.006). (D) gwmax is negatively correlated with stomatal size S (y = −0.000124x + 1.15; r2 = 0.60; P = 0.04).
Table 1.
Increase in stomatal size, S, in response to growth at elevated atmospheric CO2 (short-term experiments)
| Species | S at low CO2, μm2 | S at high CO2, μm2 | S increase, % | Ref. |
|---|---|---|---|---|
| Zea mays | 918 | 1,072 | 16.78 | 18 |
| Oryza sativa cv. Pusa Basmati-1 | 209 | 231 | 10.53 | 31 |
| O. sativa cv. P-677 | 155 | 281 | 81.29 | 31 |
| O. sativa cv. P-834 | 185 | 251 | 35.68 | 31 |
| O. sativa cv. P-2503-6-693 | 160 | 222 | 38.75 | 31 |
| Betula pubescens | 618 | 705 | 14.08 | 32 |
Low CO2 treatment: 350 ppm; high CO2 treatment: 700 ppm.
The functional consequence of the tradeoff between S and D allows gwmax and gcmax to increase as global atmospheric CO2 declines (Fig. 4C). The requirement for large numbers of small stomata to maximize gaseous CO2 diffusion into leaves for higher rates of photosynthesis (Fig. 2) is indicated by the negative correlation between gwmax and S (Fig. 4D). Large numbers of small stomata, linked to a decrease in atmospheric CO2, could potentially also allow transpiration rates to rise. However, on the multi-million year time scale involved, the climate would also be expected to cool through a weakening of the atmospheric greenhouse effect and thereby diminish the driving force for evaporation from leaves.
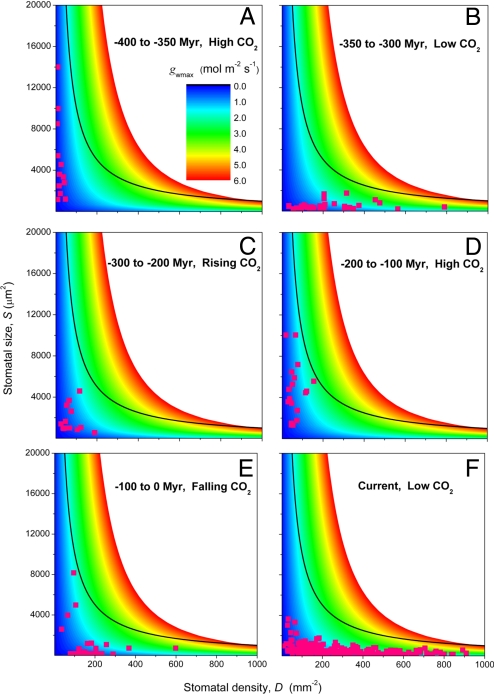
The inability of large stomata to sustain high gwmax is illustrated in Fig. 5. Color contours representing constant gwmax for various combinations of S and D show that as S decreases, higher values of gwmax become possible, as delineated by the increasing range of colors falling below the theoretical maximum (black line). Throughout the entire 400-Myr history of land-plant evolution, 2 distinct modes of change in stomatal S and D occur repeatedly in the fossil history of stomata, as revealed by S versus D cross-plots for five 50- or 100-Myr intervals (symbols in Fig. 5). Coordinated changes in S and D occur against a consistent pattern of CO2 change or regime, as estimated by geochemical carbon cycle modeling (9) that is validated against independent proxy estimates (14).
Fig. 5.
Distinct modes of stomatal trait evolution. Graduations in gwmax for multiple combinations of S and D are represented by color contours. The curved black line represents the theoretical upper limit of S versus D. Note that gwmax at this limit increases with D, even though S decreases. Stomatal conductances attainable at small S and high D are theoretically unattainable at much larger S and lower D (red area), even though total pore area per unit leaf area is constant. Overlaid on this surface are data (pink symbols) for S and D from fossil plants, showing the predominance of smaller S and higher D in times of falling atmospheric CO2 concentration.
The first mode emerges for plants experiencing a relatively stable, high-CO2 atmosphere with nonglacial climates and is characterized by low densities of stomata (D), but with 10-fold variations in size (S) (Fig. 5 A, C, and D). These combinations of S and D, characterized by predominantly large S and small D, restrict gwmax to low values. The pattern is consistent across a wide range of evolutionary life histories, from homosporous early vascular plants, such as Cooksonia in the CO2-rich atmosphere of the Silurian (Fig. 5A), through to advanced gymnosperm and angiosperm trees experiencing “greenhouse” conditions in the Jurassic and Cretaceous (Fig. 5D). Similar responses of S and D are observed in modern plants under drought treatment, whereby S often becomes smaller, with minor increases in D (15–17). Conditions of simulated water stress via treatment with abscisic acid, a plant hormone released under water stress, induce a similar response (7). Ultimately in these circumstances, reductions in S tend to be proportionally greater than any increases in D, reducing gwmax and improving the water-use economies of leaves (7, 17).
The second distinct mode of change in stomatal traits emerges when atmospheric CO2 is falling and the Earth system is transitioning from a predominant “greenhouse” to “icehouse” state (Fig. 5 B, E, and F). Falling atmospheric CO2 levels during glacial inceptions are accompanied by large variations in fossil density (D), but generally small stomates (S), allowing plants to operate with a higher gwmax. This pattern occurs repeatedly as CO2 levels drop during the onset of the Permo-Carboniferous glaciation (Fig. 5B), the long-term cooling leading to the Cenozoic glaciation (Fig. 5E), and the current icehouse world characterized by a geological interval of low CO2 (Fig. 5F). It mirrors the effects of changes in atmospheric CO2 on the stomata of extant plants (12, 13, 17, 18) and holds even for grasses evolving in the low-CO2 atmosphere of the Miocene.
High gwmax values contribute significantly to alleviating the negative impact of diminishing CO2 availability on photosynthesis at these times by increasing CO2 diffusion into leaves and can only be attained with considerable reduction in S in combination with high D. Having fewer, larger stomata cannot achieve the same effect because of concomitant increases in the length of the diffusion pathway through the pore (Fig. 2). A possible additional advantage to having flexibility in D relates to energetic cost. The energetic requirements of running stomata are proportionally small in relation to the respiratory costs of the whole leaf (19) but locally, as a fraction of epidermal tissue costs, may be significant. The guard cell respiratory cost in high-conductance cotton varieties, for example, is about twice that of low-conductance varieties (20). The flexibility to reduce D when environmental conditions demand lower gwmax might, therefore, also constitute a metabolic cost-saving mechanism.
Discussion
Our analyses implicate long-term global atmospheric CO2 change as a continuous driver of increasing gwmax and gcmax throughout the entire evolutionary history of vascular plants (Fig. 4C). The analyses identify a previously unrealized effect of CO2 on stomatal size that is consistent with the direction of change observed in plant CO2-enrichment experiments for a grass, C4 herb, and an angiosperm C3 tree (Table 1). Our study highlights the functional interdependence of S and D, particularly the inability of large S to support high gcmax, while reinforcing the more widely recognized effect of CO2 on D (12, 21, 22). We are not suggesting that atmospheric CO2 has been the only driver on evolutionary time scales; other features of the climatic and ecological environment are also likely to be involved (2). However, the tight correspondence between changes in S and D with CO2 is consistent with the biophysical requirements for increasing gcmax under decreasing atmospheric CO2 concentration and with physiological (23) and molecular (24) mechanisms linking plasticity of S and D with sensitivity to CO2. If changing atmospheric CO2 forced S to change by several orders of magnitude, then this mechanism has profound implications for the evolution of plant function in relation to cell size-dependent plant attributes.
The major physiological significance of the correlation of guard cell size, stomatal density, and gcmax with atmospheric CO2 over millions of years suggests a mechanism connecting long-term CO2 change with the ecological radiation of land plants. Reconstructions of gwmax from S and D in the fossil record have shown a systematic increase over geologic time (25), a pattern that mirrors the rise in land-plant diversity (26) and the coevolution of vascular water-conducting capacity (27). The mechanistic linkage underpinning the similarity of these records remains to be understood, but with regard to stomatal size, there are additional dynamic properties inherent in small S that, coupled with the higher photosynthetic capacity accompanying high gwmax, would enhance plant fitness in a broader range of environments. Smaller stomata are capable of faster response times (2, 5), enabling improved water-use efficiency and optimization of long-term carbon gain with respect to water loss (28). Stomata of most plants reduce their apertures to counteract potentially high transpiration rates in dry air or high wind speeds (29). Smaller, faster stomata would, therefore, minimize exposure to excessive water-potential gradients through the plant and help protect plants from xylem embolisms. Taken together, these ecophysiological traits permitted plants to occupy broader ecological and environment niches and allowed diversity to increase.
We conclude that the coevolution of plants' gas-exchange capacity and the geochemical carbon cycle has, therefore, likely been a continuous feature of the Earth system for 400 Myr, entraining a suite of planetary-wide feedbacks (30) arising through the action of CO2 at the cellular scale of stomata. A better understanding of the processes involved will contribute to elucidating a mechanistic explanation for the rise in land-plant biodiversity over this geologic time period, as documented in the fossil record a quarter of a century ago (26).
Data and Methods
Stomatal Dimensions.
All measures of S and D were obtained from published articles, acknowledging potential sampling bias and incompleteness of the paleobotanical literature. Data and sources are given in Table S1. Values were taken either directly as reported in the text or tables or, in some cases, measured from photomicrographs. Stomatal size S was calculated as guard cell length L multiplied by the width W of the guard cell pair. When W was not available, it was estimated as L/2 for nongrasses or L/8 for grasses. The theoretical maximum stomatal density Dmax (in units of mm−2) for any given stomatal size, indicated by the hyperbolic black line in Figs. 1, 2, and 5, was calculated as 1/S, with S in units of mm2 (i.e., Dmax is 1 mm2 divided by the area, in mm2, of 1 stoma).
Calculating gwmax.
Maximum stomatal conductance to water vapor, gwmax, was calculated by using Eq. 1, with amax approximated as π(p/2)2, where p is stomatal pore length (Figs. 2, 4, and 5). When p was not available it was approximated as L/2 on the basis of information provided in ref. 5. Stomatal pore depth l for fully open stomata was taken as equal to guard cell width (i.e., W/2), assuming guard cells inflate to a circular cross-section. Values for standard gas constants d and v were those for 25°C. In Fig. 5, using S and D as inputs and p as L/2 (5), a matrix of gwmax was generated and plotted as a graduated-color contour map (Fig. 5). Plots of S versus D for individual species in respective geological time periods were overlaid on this surface.
Calculating S for Fixed gwmax and Variable D.
(Fig. 3.) With respect to Eq. 1, amax was taken as 0.12S, with 0.12 being midway between the range of pore-area/stoma-area ratios for fully open stomata reported in ref. 5. Rearranging Eq. 1 then gives L ≈ 6(gwmax·v)/(d·D), from which S was approximated as S = (L2)/2.
Statistical Analysis.
The significance of correlations was tested by using linear regression, with P values of <0.05 considered statistically significant. Means were compared by using one-way analysis of variance and post hoc means comparison (Scheffé Test). All data analysis and plotting were performed with OriginPro 8.0 data analysis software (OriginLab Corporation, Northampton, MA).
Supplementary Material
Acknowledgments.
We thank Joe Berry, Mark Chase, Ian Cowan, Jeremy Beaulieu, Alistair Hetherington, Robert Berner, Ian Woodward, and Graham Farquhar for comments and discussion on this work. D.J.B. gratefully acknowledges receipt of an Edward P. Bass Environmental Scholarship at the Yale Institute of Biospheric Studies, Yale University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904209106/DCSupplemental.
References
- 1.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:17–45. [Google Scholar]
- 2.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 3.Edwards D, Kerp H, Hass H. Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot. 1998;49:255–278. [Google Scholar]
- 4.Raven JA. Selection pressures on stomatal evolution. New Phytol. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Franks PJ, Farquhar GD. The mechanical diversity of stomata and its significance in gas exchange control. Plant Physiol. 2007;143:78–87. doi: 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beerling DJ, Woodward FI. Changes in land plant function over the Phanerozoic: reconstructions based on the fossil record. Bot J Linn Soc. 1997;124:137–153. [Google Scholar]
- 7.Franks PJ, Farquhar GD. The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 2001;125:935–942. doi: 10.1104/pp.125.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann DC, Sack FD. Stomatal development. Annu Rev Plant Biol. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- 9.Berner RA. Inclusion of the weathering of volcanic rocks in the GEOCARBSULF model. Am J Sci. 2006;306:295–302. [Google Scholar]
- 10.van de Water PK, Leavitt SW, Betancourt JL. Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial-interglacial cycle. Science. 1994;264:239–243. doi: 10.1126/science.264.5156.239. [DOI] [PubMed] [Google Scholar]
- 11.Beerling DJ, Chaloner WG, Huntley B, Pearson JA, Tooley MJ. Stomatal density responds to the glacial cycle of environmental-change. Proc R Soc London Ser B. 1993;251:133–138. [Google Scholar]
- 12.Woodward FI. Stomatal numbers are sensitive to increases in CO2 from preindustrial levels. Nature. 1987;327:617–618. [Google Scholar]
- 13.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher BJ, Brentnall SJ, Anderson CW, Berner RA, Beerling DJ. Atmospheric carbon dioxide linked with Mesozoic and early Cenozoic climate change. Nat Geosci. 2008;1:43–48. [Google Scholar]
- 15.Salisbury EJ. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philos Trans R Soc London Ser B. 1928;216:1–65. [Google Scholar]
- 16.Gindel I. Stomatal number and size as related to soil moisture in tree xerophytes in Israel. Ecology. 1969;50:263–267. [Google Scholar]
- 17.Clifford SC, et al. The effect of elevated atmospheric CO2 and drought on stomatal frequency in groundnut (Arachis hypogaea L. ) J Exp Bot. 1995;46:847–852. [Google Scholar]
- 18.Driscoll SP, Prins A, Olmos E, Kunert KJ, Foyer CH. Specification of adaxial and abaxial stomata, epidermal structure and photosynthesis to CO2 enrichment in maize leaves. J Exp Bot. 2006;57:381–390. doi: 10.1093/jxb/erj030. [DOI] [PubMed] [Google Scholar]
- 19.Assmann SM, Zeiger E. Guard cell bioenergetics. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal Function. Palo Alto, CA: Stanford Univ Press; 1987. pp. 163–193. [Google Scholar]
- 20.Srivastava A, Lu Z, Zeiger E. Modification of guard cell properties in advanced lines of Pima cotton bred for higher yields and heat resistance. Plant Sci. 1995;108:125–131. [Google Scholar]
- 21.Woodward FI, Kelly CK. The influence of CO2 concentration on stomatal density. New Phytol. 1995;131:311–327. [Google Scholar]
- 22.Royer DL. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev Palaeobot Palynol. 2001;114:1–28. doi: 10.1016/s0034-6667(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 23.Lake JA, Woodward FI. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and stomatal numbers. New Phytol. 2008;179:397–404. doi: 10.1111/j.1469-8137.2008.02485.x. [DOI] [PubMed] [Google Scholar]
- 24.Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- 25.Franks PJ, Beerling DJ. CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology. 2009;7:227–236. doi: 10.1111/j.1472-4669.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 26.Niklas KJ, Tiffney BH, Knoll AH. Patterns in vascular land plant diversification. Nature. 1983;303:614–616. [Google Scholar]
- 27.Sperry JS. Evolution of water transport and xylem structure. Int J Plant Sci. 2003;164:S115–S127. [Google Scholar]
- 28.Cowan IR, Farquhar GD. Stomatal function in relation to leaf metabolism and environment. In: Jennings DH, editor. Integration of Activity in the Higher Plant. Cambridge, UK: Cambridge Univ Press; 1977. pp. 471–505. [PubMed] [Google Scholar]
- 29.Franks PJ, Farquhar GD. A relationship between humidity response, growth form and photosynthetic operating point in C3 plants. Plant Cell Environ. 1999;22:1337–1349. [Google Scholar]
- 30.Beerling DJ, Berner RA. Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci USA. 2005;102:1302–1305. doi: 10.1073/pnas.0408724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uprety DC, Dwivedi N, Jain V, Mohan R. Effect of elevated carbon dioxide concentration on the stomatal parameters of rice cultivars. Photosynthetica. 2002;40:315–319. [Google Scholar]
- 32.Vanhatalo R, Huttunen S, Back J. Effects of elevated [CO2] and O3 on stomatal and surface wax characteristics in leaves of pubescent birch growth under field conditions. Trees–Structures and Function. 2001;15:304–313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.