Abstract
Sensorimotor gating disruptions are seen in various psychiatric illnesses with putatively different pathologies, including schizophrenia and bipolar disorder. Interestingly, mice lacking the dopamine (DA) transporter (DAT) gene display markedly increased levels of DA, deficits in sensorimotor gating, and hyperactivity relative to wild-type mice. Atypical antipsychotics are effective treatments of schizophrenia and manic symptoms, presumably in part by antagonizing DA receptors. Here we report that treatment with clozapine (3 mg/kg) or quetiapine (2.5 mg/kg) attenuated prepulse inhibition deficits in male DAT knockout mice. Thus male DAT knockout mice may provide a useful animal model for predicting the efficacy of novel drugs in treating psychiatric illnesses characterized by a dysregulated DA system.
Keywords: antipsychotics, bipolar disorder, clozapine, dopamine transporter, knockout, mouse, prepulse inhibition, quetiapine, schizophrenia
Introduction
Hyperdopaminergia has been implicated in numerous neuropsychiatric disorders such as schizophrenia and bipolar disorder. Dopaminergic homeostasis is maintained by the dopamine (DA) transporter (DAT), a Na+/Cl− - dependent transmembrane transporter containing 12 putative transmembrane domains (Volz and Schenk, 2005), which functions to take up released DA from the synaptic cleft (Zhuang et al., 2001). DAT abnormalities have been implicated in schizophrenia, bipolar disorder, and attention deficit hyperactivity disorder (Cook et al., 1995; Inada et al., 1996; Kelsoe et al., 1996; Fujiwara et al., 1997; Greenwood et al., 2001; Friedel et al., 2007).
DAT knockout (KO) mice lack the gene coding for the DAT and exhibit chronic hyperdopaminergia compared with wild-type (WT) mice (Giros et al., 1996). Despite compensatory changes in DA release and receptor expression, DAT KO mice are dramatically hyperactive in a novel environment (Giros et al., 1996; Gainetdinov et al., 1999; Spielewoy et al., 2001), have impairments in spatial cognitive function (Weiss et al., 2007a), and display deficits in prepulse inhibition (PPI) of startle (Ralph et al., 2001; Barr et al., 2004; Yamashita et al., 2006).
PPI is a form of startle plasticity in which presentation of a weak stimulus (prepulse) preceding an intense startling stimulus (pulse) by 30–500 ms reduces the startle response (Graham et al., 1975). Deficits in PPI have been reported repeatedly in patients with schizophrenia (Braff et al., 2001), as well as in patients with bipolar disorder during the manic phase (Perry et al., 2001), and in a PPI attendance task in attention deficit hyperactivity disorder sufferers (Hawk et al., 2003). PPI has also shown good predictive validity as a screen for antipsychotic drugs (Swerdlow et al., 1994; Geyer et al., 2001).
Second-generation atypical antipsychotic drugs are the main line of treatment for both schizophrenia and bipolar disorder. In fact, 77–89% of bipolar patients are on antipsychotics at time of discharge (Yatham, 2003). Atypical antipsychotics may be more effective at normalizing PPI deficits in patients with schizophrenia than are typical antipsychotics (Kumari et al., 1999). Although both DA D2 and 5-HT2A antagonists reverse the PPI deficit in DAT KO mice (Ralph et al., 2001; Barr et al., 2004), there have been no reports as to whether atypical antipsychotic drugs reverse the deficient PPI phenotype in DAT KO mice. To further assess the validity of this model to screen putative antipsychotic medications, we examined whether or not two clinically effective atypical antipsychotic drugs, clozapine and quetiapine, reverse PPI deficits in DAT KO mice.
Methods
Subjects
The DAT mutant mice used in these experiments were derived from a breeding colony from parental DAT (+/−) mice on a C57BL/6 × 129SvJ hybrid background, originally received from Duke University (Giros et al., 1996). Mice from each strain were group housed (n = 4/cage) in a climate-controlled animal colony with a 12-h reversed day/night cycle (lights on at 20.00 h). Food and water were freely available, except during behavioral testing. Animal facilities were AAALAC-approved, and protocols were in accordance with the ‘Guiding Principles in the Care and Use of Animals’ from the American Physiological Society and guidelines from the National Institutes of Health.
Apparatus and procedure
Startle and PPI testing were performed in SR-LAB startle chambers (San Diego Instruments, San Diego, California, USA), using an experimental session [background noise level (65 dB), prepulse trials (69, 73, and 81 dB), pulse alone trials (120 dB), etc.] that has been specified previously (Barr et al., 2004). Mice (3–6 months old) were first tested in a characterization session to confirm PPI deficits (data not shown) in DAT KO mice reported earlier by our group (Ralph et al., 2001). Four weeks later, mice were tested with clozapine in a within-subjects crossover design, with 1 week between drug treatments. Following a 5-week washout period, the same mice were retested with quetiapine or vehicle in a similar crossover design. Mean startle magnitude for each trial type presentation, the dependent measure, was determined by averaging 65 one-ms readings taken from the onset of the startle P120 stimulus.
Drugs
Clozapine (3.0 mg/kg, intraperitoneally; Tocris, Eillisville, Missouri, USA) was dissolved in tartaric acid (5% volume) and 0.9% saline and brought to a pH of about 5–6 with 0.1N NaOH. Quetiapine (2.5 mg/kg; subcutaneously; gift from Astra Zeneca, Wilmington, Delaware, USA) was dissolved in a small amount of 0.1N HCl and distilled water and brought to a pH of about 5–6 with 0.1N NaOH. All injections were given 20 min before behavioral testing at a volume of 5-ml/kg body weight. The doses of antipsychotics chosen were based on preliminary dose–response studies in vendor-supplied C57BL/6 and 129SvEv mice.
Statistical analysis
Startle reactivity, mean startle magnitudes within the test session, and percentage of PPI were each analyzed using two-factor analyses of variance (ANOVAs) with drug as a within-subjects factor and genotype as between-subjects factor.
Results
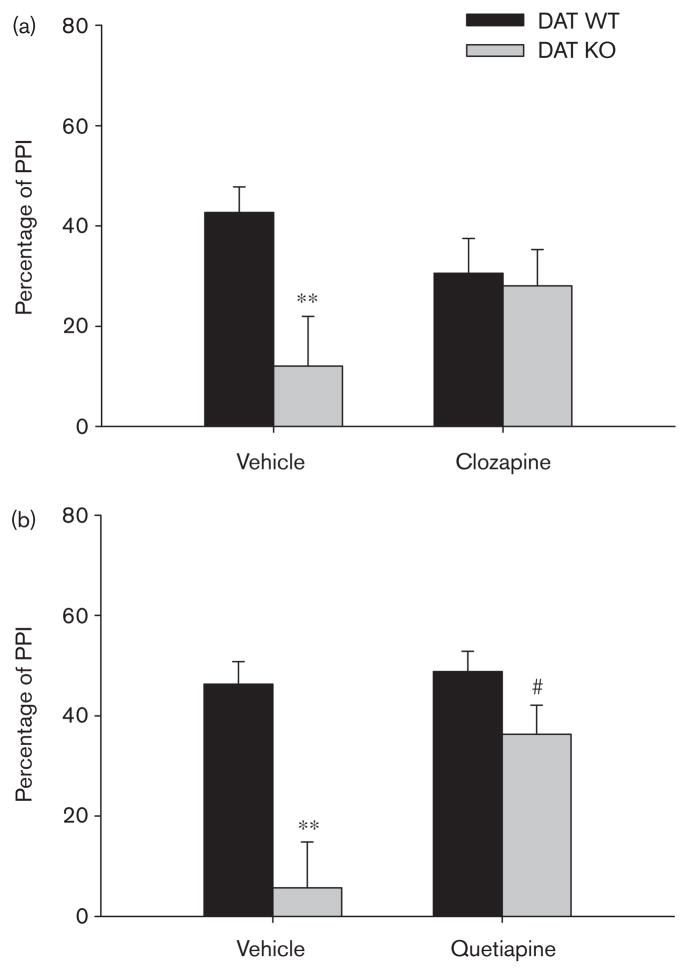
In the clozapine experiment, there was a significant overall interaction between genotype and clozapine treatment on PPI [F(1,34) = 5.59, P < 0.05] as well as a significant main effect of genotype [F(1,34) = 9.13, P < 0.01; Fig. 1a]. Similarly, in the quetiapine study there was a significant overall quetiapine × genotype interaction on PPI [F(1,33) = 8.23, P < 0.01] as well as a significant main effect of genotype [F(1,33) = 21.38, P < 0.001; Fig. 1b]. On the basis of an inconsistent PPI deficit in female DAT KO mice (data not shown), only data from male mice are presented and included in subsequent statistical analyses.
Fig. 1.
(a) Effect of clozapine (3.0 mg/kg, intraperitoneally) on average prepulse inhibition (PPI) in male dopamine transporter (DAT) wild-type (WT) and knockout (KO) mice. Vehicle-treated DAT KO mice (n=7) showed PPI deficits compared with vehicle-treated DAT WT mice (n=9), **P<0.01. PPI deficits in DAT KO mice were attenuated by clozapine treatment, as indicated by a lack of a significant genotype effect in clozapine-treated mice. (b) Effect of quetiapine (2.5 mg/kg, subcutaneously) on average PPI in male DATWT and KO mice. Vehicle-treated DAT KO mice (n=6) showed PPI deficits compared with vehicle-treated DAT WT mice (n=9), **P<0.01. PPI deficits in DAT KO mice were attenuated by quetiapine treatment, as indicated by a lack of a significant effect of genotype in quetiapine-treated mice and a significant effect of quetiapine in DAT KO mice, #P<0.05. Data were expressed as mean ± SEM.
Separate post-hoc ANOVAs were conducted in male vehicle-treated and clozapine-treated mice to determine the nature of the genotype × drug interaction. Male KO mice administered vehicle had significantly lower PPI levels compared with their WTcounterparts administered vehicle [genotype; F(1,14) = 9.89, P < 0.01; Fig. 1a]. The percentage of PPI of the DAT KO mice administered clozapine, however, did not differ significantly from that of the DAT WT mice administered clozapine, suggesting that clozapine attenuated the PPI deficit in male DAT KO mice. Pairwise ANOVAs revealed that clozapine did not increase PPI significantly in either DAT KO or DAT WT mice.
Separate post-hoc ANOVAs conducted in male vehicle-treated and quetiapine-treated mice revealed that male KO mice administered vehicle had significantly lower PPI levels compared with WT littermates administered vehicle [genotype; F(1,13) = 23.11, P < 0.001; Fig. 1b]. In contrast, the percentage of PPI of male DAT KO mice administered quetiapine did not differ significantly from that of WT mice administered quetiapine, suggesting that quetiapine restored PPI to normal levels in DAT KO mice. Pairwise ANOVAs revealed that in DAT KO mice, quetiapine increased PPI [drug; F(1,5) = 10.49, P < 0.025]; whereas, in DAT WT mice, quetiapine had no effect on PPI.
No significant main effect of genotype on startle magnitude in either the clozapine or quetiapine experiments (Table 1) was observed. Clozapine decreased startle magnitude [main effect of drug; F(1,14) = 10.09, P < 0.01] and did not interact with genotype. A significant interaction, however, between quetiapine and genotype [F(1,13) = 7.37, P < 0.025] on startle magnitude was observed. Pairwise ANOVA revealed that quetiapine decreased startle magnitude in DAT WT mice [F(1,8) = 5.70, P < 0.05].
Table 1.
Mean (SEM) startle magnitude in DAT WT and KO mice treated with vehicle and clozapine or quetiapine
| Startle magnitude | DAT WT | DAT KO |
|---|---|---|
| Vehicle | 81.2 (28.1) | 147.4 (58.4) |
| Clozapine* | 41.0 (12.5) | 66.1 (20.3) |
| Vehicle | 111.9 (31.4) | 91.4 (21.9) |
| Quetiapine | 61.0 (26.4)* | 134.3 (48.9) |
DAT KO mice, dopamine transporter knockout mice; DAT WT mice, dopamine transporter wild-type mice
P < 0.05 vs. vehicle.
Discussion
In these studies, DAT KO mice displayed PPI deficits, corroborating previous findings from our group (Ralph et al., 2001; Barr et al., 2004) and others (Yamashita et al., 2006). The present data demonstrate that the PPI deficits in male DAT KO mice were attenuated by acute administration of the atypical antipsychotic drugs clozapine and quetiapine. Thus, greater predictive validity is afforded to the use of these mice as a model for the prediction of antipsychotic drugs that are effective in treating schizophrenia and bipolar disorder.
Despite compensatory changes in neurotransmission in these mice (Trinh et al., 2003; Weiss et al., 2007b), the PPI deficits observed are likely to be a consequence of their lack of DATs (Giros et al., 1996). Pharmacological blockade of the DAT with the selective DAT inhibitor GBR-12909 produces similar deficits in PPI in mice (Young et al., unpublished observations). Pharmacological or genetically reduced function of the DAT results in a hyperdopaminergic state (Giros et al., 1996), and thus DA receptors are activated in these mice at a greater rate than in WT mice (Trinh et al., 2003). Both clozapine and quetiapine exhibit high affinities for the serotonin 5-HT2A receptor as well as the DA D2 receptor (Schotte et al., 1996; Richelson and Souder, 2000). Consistent with these studies, both selective D2 receptor and 5-HT2A receptor antagonists have been shown to attenuate PPI deficits in DAT KO mice (Ralph et al., 2001; Barr et al., 2004). Antipsychotics are not the only drug class that has been shown to reverse or attenuate PPI deficits in DAT KO mice, however. For example, the norepinephrine transporter inhibitor nisoxetine, the serotonin transporter inhibitor fluoxetine, the monoamine transporter inhibitor methylphenidate, and cocaine all reversed PPI deficits in DAT KO mice, whereas citalopram did not (Yamashita et al., 2006). Hence, general monoamine transporter inhibition seems to attenuate PPI deficits in DAT KO mice, and as such this model may produce false positives when using it as a screen for antipsychotic medications.
In summary, we describe the reversal of PPI deficits in male DAT KO mice by the atypical antipsychotics clozapine and quetiapine. Quetiapine was more effective than clozapine at reversing the PPI deficit in DAT KO mice. These studies support the use of DAT KO mice as a model for assessing the efficacy of putative antipsychotics.
Acknowledgments
The authors thank Virginia Masten for her excellent technical assistance. These studies were supported by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center, and grants from the National Institute of Mental Health MH071916 (M.A.G.), MH42228 (M.A.G.), MH60451 (M.G.C.), and the National Institute for Neurological Disorders and Stroke NS19576 (M.G.C.). Mark A. Geyer holds an equity interest in San Diego Instruments.
References
- Barr AM, Lehmann-Masten V, Paulus M, Gainetdinov RR, Caron MG, Geyer MA. The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology. 2004;29:221–228. doi: 10.1038/sj.npp.1300343. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Friedel S, Saar K, Sauer S, Dempfle A, Walitza S, Renner T, et al. Association and linkage of allelic variants of the dopamine transporter gene in ADHD. Mol Psychiatry. 2007;12:923–933. doi: 10.1038/sj.mp.4001986. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Yamaguchi K, Tanaka Y, Tomita H, Shiro Y, Kashihara K, et al. Polymorphism of dopamine receptors and transporter genes in neuropsychiatric diseases. Eur Neurol. 1997;38 (Suppl 1):6–10. doi: 10.1159/000113436. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Graham FK, Putnam LE, Leavitt LA. Lead-stimulation effects of human cardiac orienting and blink reflexes. J Exp Psychol Hum Percept Perform. 1975;104:175–182. [PubMed] [Google Scholar]
- Greenwood TA, Alexander M, Keck PE, McElroy S, Sadovnick AD, Remick RA, et al. Evidence for linkage disequilibrium between the dopamine transporter and bipolar disorder. Am J Med Genet. 2001;105:145–151. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1161>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr, Yartz AR, Pelham WE, Jr, Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology (Berl) 2003;165:118–127. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Inada T, Sugita T, Dobashi I, Inagaki A, Kitao Y, Matsuda G, et al. Dopamine transporter gene polymorphism and psychiatric symptoms seen in schizophrenic patients at their first episode. Am J Med Genet. 1996;67:406–408. doi: 10.1002/(SICI)1096-8628(19960726)67:4<406::AID-AJMG15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Sadovnick AD, Kristbjarnarson H, Bergesch P, Mroczkowski-Parker Z, Drennan M, et al. Possible locus for bipolar disorder near the dopamine transporter on chromosome 5. Am J Med Genet. 1996;67:533–540. doi: 10.1002/(SICI)1096-8628(19961122)67:6<533::AID-AJMG4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry. 1999;156:1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Feifel D, Braff DL. Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry. 2001;50:418–424. doi: 10.1016/s0006-3223(01)01184-2. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. J Neurosci. 2001;21:305–313. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Biala G, Roubert C, Hamon M, Betancur C, Giros B. Hypolocomotor effects of acute and daily D-amphetamine in mice lacking the dopamine transporter. Psychopharmacology (Berl) 2001;159:2–9. doi: 10.1007/s002130100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Schenk JO. A comprehensive atlas of the topography of functional groups of the dopamine transporter. Synapse. 2005;58:72–94. doi: 10.1002/syn.20183. [DOI] [PubMed] [Google Scholar]
- Weiss S, Nosten-Bertrand M, McIntosh JM, Giros B, Martres MP. Nicotine improves cognitive deficits of dopamine transporter knockout mice without longterm tolerance. Neuropsychopharmacology. 2007a;32:2465–2478. doi: 10.1038/sj.npp.1301385. [DOI] [PubMed] [Google Scholar]
- Weiss S, Tzavara ET, Davis RJ, Nomikos GG, Michael McIntosh J, Giros B, et al. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007b;52:1496–1508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Fukushima S, Shen HW, Hall FS, Uhl GR, Numachi Y, et al. Norepinephrine transporter blockade can normalize the prepulse inhibition deficits found in dopamine transporter knockout mice. Neuropsychopharmacology. 2006;31:2132–2139. doi: 10.1038/sj.npp.1301009. [DOI] [PubMed] [Google Scholar]
- Yatham LN. Acute and maintenance treatment of bipolar mania: the role of atypical antipsychotics. Bipolar Disord. 2003;5 (Suppl 2):7–19. doi: 10.1111/j.1399-2406.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]