Abstract
Schizophrenia is a serious psychiatric disorder that is most frequently treated with the administration of antipsychotics. Although onset of schizophrenia typically occurs in late adolescence, the majority of preclinical research on the behavioral effects of antipsychotics and their mechanism(s) of action has been conducted on adult male animals. In this study, the acute effects of haloperidol (0.03 – 0.3 mg/kg, i.p.) and clozapine (1 – 10 mg/kg, i.p.) on locomotor activity were examined in juvenile [postnatal day 22 (PN22)], adolescent (PN40), and adult (>PN70) rats of both sexes. Subsequently, in order to determine whether tolerance to the activity suppressive effects of these drugs would occur in adolescents, PN40 rats were dosed and assessed for an additional nine days. While all groups exhibited some degree of suppression following acute administration of both drugs, juvenile rats were considerably more sensitive to this effect. With sub-chronic administration during late adolescent development (PN40–PN49), tolerance failed to develop. These results emphasize the importance of age in pharmacological characterization of antipsychotics and suggest that pre-adolescents may have enhanced sensitivity to the motor effects of these drugs. Further, they suggest that, similar to adults, older adolescents may not develop tolerance to the activity suppression induced by these two antipsychotics.
Keywords: Adolescence, Clozapine, Haloperidol, Locomotion, Sex Differences, Tolerance
1. Introduction
Chronic administration of antipsychotics represents the most common pharmacotherapy for treatment of schizophrenia and other psychotic disorders. The primary mechanism of action of antipsychotic drugs is presumed to be dopamine antagonism, as all known antipsychotics block dopamine receptors with affinities that are positively correlated with their clinical potencies (McQuade et al., 1992; Schotte et al., 1996). Many antipsychotics also affect non-dopamine neurotransmission, which may contribute to their overall pharmacological profile and perhaps to their therapeutic effects. Since onset of schizophrenia (i.e., “first break” psychotic episode) typically occurs during late adolescence or young adulthood, adolescence is a frequent time of initial exposure to antipsychotics. Despite this fact, little research has been done to evaluate the effects of antipsychotics during adolescence. Given that mechanistic research in humans is restricted, most studies that have been done on the behavioral and neurochemical effects of antipsychotics have used animal models.
The majority of this preclinical research has focused on the effects of antipsychotics in adult male animals. Less is known about their effects in females and in adolescent animals. In rats, adolescence is characterized by observation of patterns of behavior that have been observed in young mammals across species (e.g., increased risk taking, novelty seeking, and increased orientation towards peers) and occurs from approximately postnatal day 28 to 42 (PN28–PN42) (Spear, 2000). While dopamine D1 and D2 receptors are functional prenatally (Moody et al., 1993) and during the pre-weanling period (Weihmuller and Bruno, 1989), substantial postnatal development of these receptors also occurs between weaning and adulthood. During the periadolescent period, significant pruning of D1 and D2 dopamine receptors has been observed in the nucleus accumbens and caudate putamen which is accompanied by increases in the densities of these receptors in rat cortical and hippocampal areas (Tarazi and Baldessarini, 2000). These differential developmental patterns of dopamine receptors across brain region result in a major shift of predominance of dopamine D1 and D2 receptor functioning from subcortical to cortical areas during early adolescence (for a review, see (Spear, 2000)).
Surprisingly, the effects of these pronounced developmental alterations in dopaminergic systems on the behavioral effects of antipsychotics have not been well-studied. Rather, most (but not all) developmental research with these drugs has focused primarily on delineation of their effects after pre- and perinatal administration (Coyle et al., 1985; Cuomo et al., 1985; Napier et al., 1985; Spear et al., 1980), as opposed to dosing during adolescence. Hence, the objective of this study was to investigate the behavioral effects of two prototypic antipsychotics, haloperidol and clozapine, during late adolescence. Older, traditional antipsychotics, such as haloperidol and chlorpromazine, are known to produce extrapyramidal motor effects that are associated with their blockade of dopamine D2 receptors in the basal ganglia. In contrast, second generation, atypical antipsychotics, such as clozapine and olanzapine, do not produce these motor effects. Preclinically, however, one of the most consistent effects observed with the acute administration of both typical and newer atypical antipsychotics is suppression of voluntary behavior, including motor activity (Simon et al., 2000; Wiley and Martin, 2003) and operant responding for food and water (Gramling and Fowler, 1985; Varvel et al., 2002). Because the ability of immature animals to acquire operant behavior quickly has not been demonstrated, motor activity was selected as the behavioral endpoint for this study.
2. Materials and methods
2.1 Subjects
Adult female Long-Evans rats (Harlan, Dublin, VA) were impregnated by adult male Long-Evans rats (Harlan) in our animal room facility. After breeding, dams were individually housed in clear plastic cages in a temperature-controlled (20–22°C) environment with a 12-hour light-dark cycle (lights on at 7 a.m.). Plenty of sawdust bedding was available in each cage for nesting. The dams were left undisturbed except for providing food, water, and fresh bedding until they gave birth (postnatal day 0, PN0). Pups were sexed and culled to no more than 10 pups per litter. Pups that were not used in this study were used in other independent studies. They remained with their dams until weaning at PN21. On PN21, pups were separated from the dam and were pair-housed with a same-sex rat from another litter that was assigned to receive the same treatment. Rats in the different dose groups for each drug were randomly chosen from different litters, with the exception that one male and one female from each litter may have been assigned to the same treatment condition. Adult male and female rats (Harlan, Dublin, VA) were aged PN70 or greater when tested. The studies reported in this manuscript were carried out in accordance with guidelines published in guide for the care and use of laboratory animals (National Research Council, 1996) and were approved by our Institutional Animal Care and Use Committee.
2.2 Apparatus
Clear plastic rat cages (22.5 cm width X 44 cm length X 20 cm height) were housed in sound-attenuating cabinets and were used as locomotor chambers. Each cabinet contained up to 12 chambers, with a maximum of 2 per shelf. Chambers did not contain bedding and were wiped with alcohol solution between sessions. Sessions occurred in darkness (i.e., with the cabinet doors closed). A cage rack system with 4 × 8 equally spaced photocell beams on the X- and Y-axes (Lafayette Instrument, Lafayette, IN) was placed around each chamber (4.5 cm from bottom of cage). Locomotor activity was assessed as total number of beam breaks during the entire 20-minute session.
2.3 Drugs
Haloperidol (McNeil Pharmaceutical, Spring House, PA) was diluted with saline from a commercially purchased 5 mg/ml stock solution. Clozapine (NIMH Chemical Synthesis and Drug Supply Program, Bethesda, MD) was mixed in purified distilled water. Physiological saline was used as a control treatment. All drugs were administered intraperitoneally in a volume of 1 ml/kg. Drugs and saline were administered one hour prior to testing.
2.4 Procedure
Male and female Long Evans rat pups were randomly assigned to receive one of dose of haloperidol (0.03, 0.1, or 0.3 mg/kg), clozapine (1, 3, or 10 mg/kg), or saline. On test days, rats were transported to the laboratory and were injected intraperitoneally with assigned doses of antipsychotic or saline. One hour later they were placed in locomotor chambers and activity was measured as total number of beam breaks during a 20-min session. After the session, each rat was returned to its home cage. Acute dosing tests for each pup occurred on either PN22 or PN40, dependent upon group assignment (i.e., between subjects design). Test conditions for adult rats were identical to those for the pups. In order to assess tolerance development during late adolescence / young adulthood, some of the rats that were aged PN40 on the day of the acute dosing experiment continued to receive daily injections of saline or their assigned dose of antipsychotic for an additional nine days. Throughout this repeated dosing regimen, an individual rat was always tested in the same locomotor chamber. Because the same rats were used in the acute and repeated dosing experiments, habituation to the locomotor chambers prior to drug administration was not included in the study design.
2.5 Data analysis
For the acute antipsychotic tests, mean (± S.E.M.) numbers of total locomotor counts during the entire 20-min session were calculated for each sex, dose, and age separately. Data for each drug were analyzed separately by sex with two-way factorial (age X dose) ANOVAs. Due to the sex differences in baseline (vehicle) activity (particularly in adults), direct comparisons between the sexes were not performed. For the older adolescents (PN40 – PN49), total number of locomotor counts for saline control tests for both sexes and both drugs and across the entire 10-day dosing period were subjected to a split-plot ANOVA (sex X drug X day). Subsequently, data for each drug were converted to percentage of saline control for each day and analyzed separately with split-plot ANOVA (sex X dose X day). For each ANOVA on the sub-chronic data, day was the repeated factor and sex and drug/dose were the between subject factor. When any ANOVA was significant, Tukey post hoc tests (α=0.05) were used to compare individual means.
3. Results
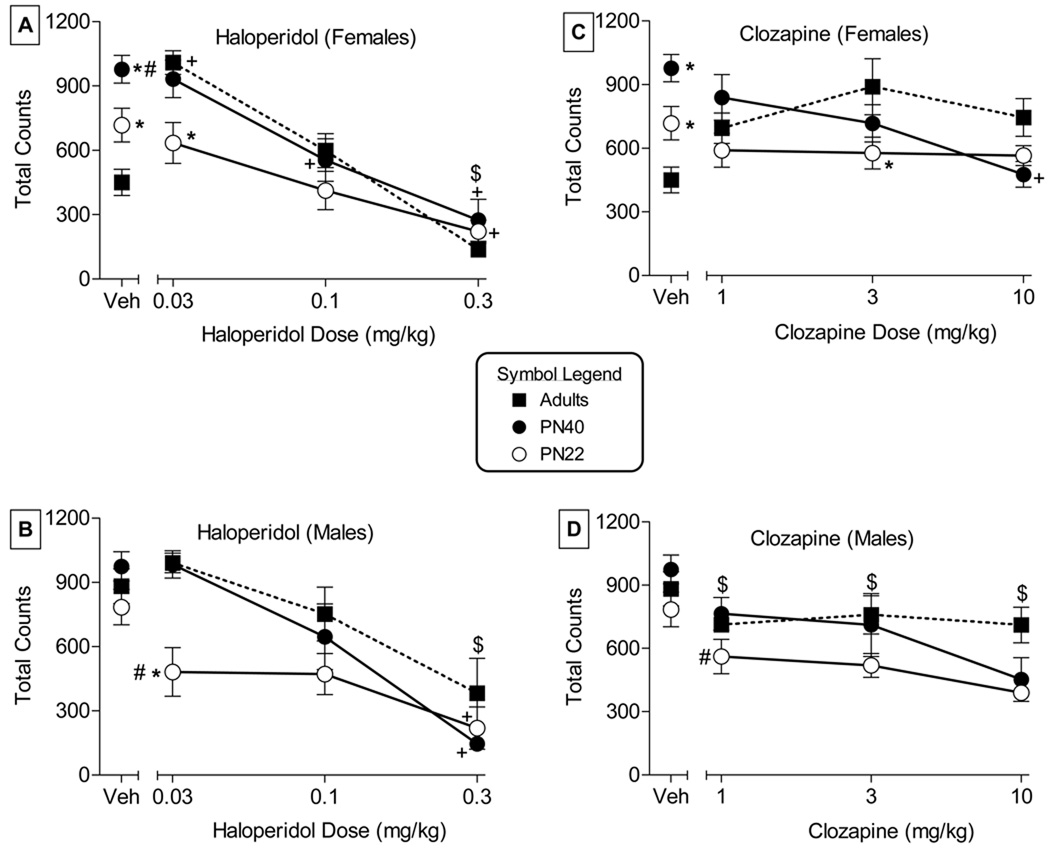
Figure 1 depicts the results of acute tests with haloperidol (left panels) and clozapine (right panels) in female (upper panels) and male (lower panels) rats aged PN22, PN40 or adult. Significant main effects for age [F(2,65)=7.3, P<0.05] and for dose [F(3,65)=28.0, P<0.05] and a significant age X dose interaction [F(6,65)=5.4, P<0.05] were obtained for haloperidol-treated female rats (Fig. 1, panel A). Tukey post hoc tests on the age and dose main effects revealed that overall activity of PN40 rats was significantly increased compared to both other age groups and that 0.3 mg/kg haloperidol decreased overall activity across age groups as compared to vehicle. Tukey post hoc analysis of the age X dose interaction showed that males were significantly more active after saline injection at ages PN22 and PN40 than were adults (Fig. 1, at left of panel A). In PN40 female rats, acute haloperidol doses of 0.1 and 0.3 mg/kg decreased activity whereas only 0.3 mg/kg haloperidol decreased activity in PN22 rats (comparisons to respective vehicle points) [Fig. 1, panel A]. Further, at the lowest (0.03 mg/kg) haloperidol dose activity in PN22 rats was also decreased compared to adults at this dose. In contrast, none of the acute doses of haloperidol significantly decreased activity in adult female rats, as compared to vehicle; rather, the 0.03 mg/kg dose of haloperidol increased activity in this group. Lower baseline activity in adult females, however, most likely contributed to these findings, as significant successive decreases in activity were observed in this age group as haloperidol dose increased (i.e., 0.1 mg/kg decreased activity compared to 0.03 mg/kg and 0.3 mg/kg decreased activity compared to both lower doses).
Figure 1.
Left panels show acute effects of haloperidol on spontaneous activity in female (panel A) and male (panel B) rats aged PN22, PN40 and adult. Right panels show acute effects of clozapine on spontaneous activity in female (panel C) and male (panel D) rats aged PN22, PN40 and adult. Baseline activity after vehicle (saline) injection is shown at the left of each panel and is the same for each drug. Each value represents the mean (± S.E.M.) of data from 6–10 rats, except for the vehicle condition, n=10–16 rats. # indicates significant main effect (P<0.05) for age as compared to adult. $ indicates significant main effect (P<0.05) for dose as compared to vehicle. * and + indicate significant age X dose interaction and that value is different from vehicle for that age (*) or from the adult group at the dose (+), respectively.
Similar to ANOVA results with females, significant main effects for age [F(2,70)=3.9, P<0.05] and for dose[F(3,70)=20.2, P<0.05] and a significant age X dose interaction [F(6,70)=2.4, P<0.05] were also seen for haloperidol-treated male rats (Fig. 1, panel B). Tukey post hoc tests on the age and dose main effects revealed that overall activity of PN22 rats was significantly decreased compared to both other age groups and that 0.3 mg/kg haloperidol decreased overall activity across age groups as compared to vehicle. Tukey post hoc analysis of the age X dose interaction showed that baseline activity under vehicle conditions was not significantly different across the three ages (Fig. 1, at left of panel B). In PN40 and PN22 male rats, 0.3 mg/k haloperidol administered acutely decreased activity compared to respective vehicle points for each age (Fig. 1, panel B). Further, at the lowest (0.03 mg/kg) haloperidol dose activity in PN22 rats was also decreased compared to both other ages at this dose. In contrast, none of the acute doses of haloperidol significantly decreased activity in adult male rats, as compared to vehicle, but activity after 0.3 mg/kg haloperidol was significantly decreased as compared to that seen after 0.03 mg/kg haloperidol.
Over the dose ranges tested, the effects of clozapine on locomotor activity were not as notable as those of haloperidol at any age (Fig. 1, panels C & D). In females, the main effects for clozapine dose [F(3,66)=1.5, P>0.05] and age [F(2,66)=2.0, P>0.05] were not significant; however, a significant age X dose interaction was obtained [F(6,66)=3.2, P<0.05] (Fig. 1, panel C). Post hoc analysis revealed that 10 mg/kg clozapine significantly decreased activity compared to vehicle only in PN40 rats, but there were no significant differences compared to vehicle in either of the other two age groups. Activity at the 3 mg/kg clozapine dose was significantly reduced in the PN22 rats compared to adults.
In males, the main effects for clozapine dose [F(3,63)=9.6, P<0.05] and age [F(2,63)=7.5, P<0.05] were significant; however, the age X dose interaction was not [F(6,63)=0.8, P>0.05] (Fig. 1, panel D). Post hoc analysis of the main effect for dose showed that each of the three doses of clozapine suppressed activity compared to vehicle (Fig. 1, panel D). As with haloperidol, male juvenile (PN22) rats were most sensitive to the locomotor suppression induced by clozapine and showed significantly greater reduction in activity across the clozapine dose range compared to both of the other age groups [main effect for age: F(2,63)=7.5, P<0.05].
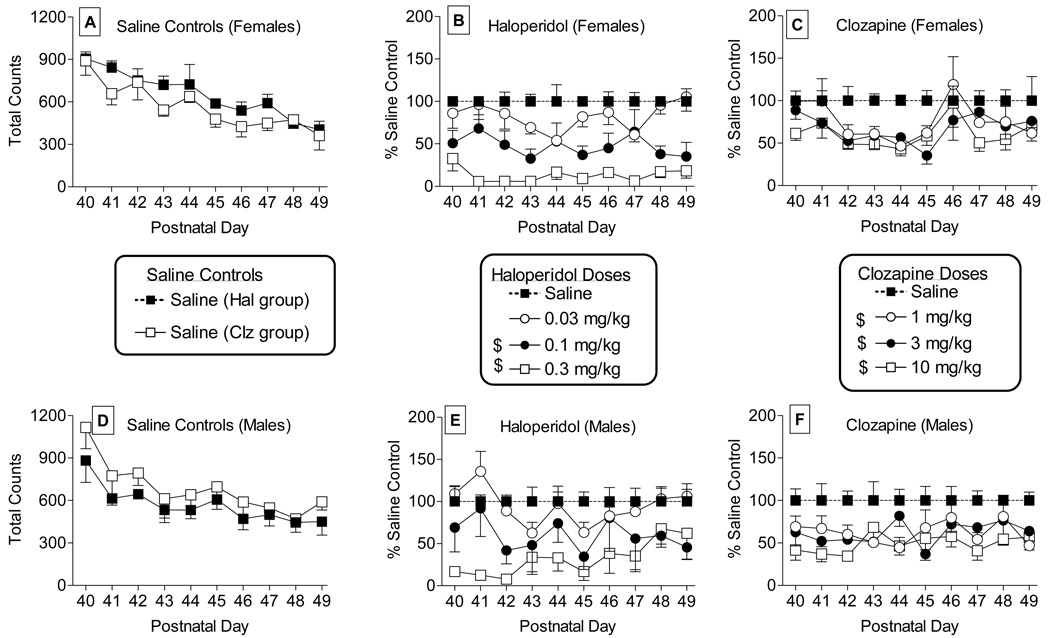
Figure 2 shows the effects of repeated injections of saline (left panels), haloperidol (middle panels) and clozapine (right panels) in female (top panels) and male (bottom panels) rats during 10 consecutive daily sessions starting at PN40. Repeated injections of saline starting at PN40 produced a significant and steady decrease in activity across the 10-day period (Fig. 2, females in panel A and males in panel D) [main effect for day: F(9,132)=16.6, P<0.05], suggesting development of habituation. In order to control for habituation effects, data for each drug dose were converted to percentage of daily saline control. Post hoc analysis of the significant main effect of haloperidol dose [F(3,13)=24.0, P<0.05] revealed that 0.1 and 0.3 mg/kg haloperidol significantly decreased overall activity in female and male rats across the dosing period (Fig. 2, females in panel B and males in panel E). Similarly, a significant main effect was observed for clozapine dose [F(3,12)=10.9, P<0.05]. Post hoc analysis showed that all three doses of clozapine (1, 3 and 10 mg/kg) significantly decreased locomotor activity over the course of the study (Fig. 2, females in panel C and males in panel F). When the habituation effect was controlled by conversion to percentage of control, tolerance or sensitization to the locomotor effects of these antipsychotics did not occur, as indicated by a non-significant dose X day interactions for both drugs[F(27,116)=1.4, P>0.05 for haloperidol and F(27,108)=0.9, P>0.05 for clozapine]. In addition, there were no significant differences from PN40 at any later day for either drug.
Figure 2.
Effects of repeated injections of saline on spontaneous activity (total number of photocell beam breaks) in female and male rats aged PN40–PN49 are shown in panels A and D, respectively. The middle set of panels show effects of haloperidol on spontaneous activity during the same time period in female (panel B) and male (panel E) rats. Values are expressed separately for each sex as a percentage of saline control for haloperidol for the day (as shown in panels A and D for females and males, respectively). Panels C and F show effects of clozapine on spontaneous activity in female and male rats, respectively. Values are expressed separately for each sex as a percentage of saline control for clozapine for the day (as shown in panels A and D for females and males, respectively). Each value represents the mean (± S.E.M.) of data from 4–7 rats. ANOVA indicated a significant main effect (P<0.05) for day for saline controls, with means for all days different from PN40. $ in the symbol legend indicates significant main effect (P<0.05) for the dose compared to saline.
4. Discussion
Consistent with a number of previous studies that have reported antipsychotic-induced suppression of behavior in adult male rodents (Gramling and Fowler, 1985; Simon et al., 2000; Wiley and Martin, 2003), haloperidol dose-dependently decreased locomotor activity by approximately the same magnitudes in adolescent and adult male rats over an identical dose range. In contrast, an overall greater sensitivity to the activity decreasing effects of haloperidol was observed in haloperidol-treated juvenile male rats (PN22), with this effect being most apparent at the lowest (0.03 mg/kg) haloperidol dose. A similar pattern of age-related effects was demonstrated in clozapine-treated male rats; i.e., activity was decreased in adolescents and adults to a similar extent and juvenile rats were more sensitive overall to the activity decreasing effects of clozapine. Of note, both the haloperidol and clozapine dose-response curves for male juvenile rats were considerably less steep than those seen in the other male adolescents and adults. Further, baseline levels of activity were not different in saline-treated male rats of each age (adult, PN40 and PN22), suggesting that these age differences in activity resulted from differential pharmacological effects of the antipsychotics across the three age groups. Results from previous studies showed that PN30 male rats were also more sensitive to haloperidol-induced suppression of locomotor activity than were adult (PN100) male rats (Campbell and Baldessarini, 1981; Campbell et al., 1988). Parallel dose-effect curve shifts to the right for this measure were observed with increasing age across several routes of administration, including p.o. and i.c.v., suggesting that potency differences across age were related to pharmacodynamic rather than to pharmacokinetic differences (Campbell et al., 1988). Further, similar effects were obtained with perphenazine (Campbell et al., 1988) and with clozapine (present study), both of which have chemical structures that differ radically from that of haloperidol. The present results are consistent with this pattern of decreasing sensitivity to the acute effects of antipsychotics on motor activity during early development (i.e., greater sensitivity at ages PN22 and PN30 with similar sensitivity at PN40 and adult).
The patterns of age-related effects produced by the two antipsychotics on motor activity in female rats were similar to those observed in males with the exception that female rats showed age-related differences in motor activity following saline treatment. Notably, saline-treated female adults were significantly less active than saline-treated PN22 and PN40 female rats. In addition, adolescent (PN40) female rats were more active than the younger juvenile (PN22) rats, an observation that is consistent with previous research showing an increase in exploratory activity with age in prepubertal Long-Evans rats (Renner et al., 1992). Hence, the finding in the present study that saline-treated female adult rats show substantially less activity than female rats of other ages is an anomaly that somewhat complicates interpretation of the effects of haloperidol across age in females. Although the patterns of activity as a function of haloperidol dose were essentially the same for female adolescent and adult rats, differences in baseline activity resulted in differential statistical findings of dose-dependent decreases in activity and a biphasic effect on activity (i.e., increases at 0.03 mg/kg and decreases at 0.3 mg/kg) in adolescent and adult rats, respectively. Similarly, only female adolescent rats (not female adults) exhibited decreased activity following administration of clozapine. One of the few previous studies examining the effects of antipsychotics on motor activity in adult female rats reported dose-dependent decreases similar to those obtained in males (Simon et al., 2000), findings that are consistent with the observed effects of antipsychotics in female adolescent rats in the present study, but in contrast with those for adult female rats. As noted, however, an abnormally low level of activity in saline-treated adult female rats is the most likely explanation of the observed effects rather than differential activity of haloperidol and/or clozapine per se in adult females. As with juvenile (PN22) male rats, juvenile female rats were notably more sensitive to decreases in locomotion produced by either haloperidol or clozapine, resulting in pronounced flattening of the dose-effect functions (compared to that observed at older ages).
Since patients with schizophrenia frequently have their first psychotic episode during late adolescence / young adulthood, the effects of sub-chronic administration of haloperidol and clozapine were assessed in the PN40 rats, as this age roughly corresponds to late adolescence in rats. In all groups of both sexes, substantial habituation to the locomotor chambers occurred across the 10-day dosing period under control conditions, as has been reported in previous studies (Carey et al., 2005; Leussis and Bolivar, 2006). When this habituation effect was minimized by converting the data to percentage of saline control, tolerance to the locomotor suppressing effects of either antipsychotic was not evident in male or female rats during this late adolescent period. Sub-chronic administration of 0.3 mg/kg haloperidol or 10 mg/kg clozapine, the highest antipsychotic doses used in the present study, also failed to produce adaptation to initial locomotor suppression in male or female rats when the 10-day dosing regimen was initiated at PN30 versus PN40 as in the present study [J.L. Wiley, unpublished observations]. Other previous studies of the behavioral effects of sub-chronic administration of antipsychotics during development have focused on assessment of its long-term consequences at later time points. For example, perinatal administration of haloperidol or clozapine impaired later acquisition of an operant task (Cuomo et al., 1981; Cuomo et al., 1983). In another series of studies, rat pups that received haloperidol from day 1 of gestation through weaning (PN21) exhibited hyperactivity in an open field and an increase in exploratory behavior during early adolescence (PN23–PN30) and after adolescence (PN47–PN54) and showed altered responsiveness to administration of dopamine agonists and antagonists (Shalaby and Spear, 1980; Spear et al., 1980). In contrast, rats that were perinatally dosed, but tested during the last part of adolescence (PN35–PN42), exhibited few differences from control rats (Spear et al., 1980). Collectively, these results emphasize the importance of examination of the effects of antipsychotics across a range of ages and tasks.
In summary, the present results demonstrate that the degree of sensitivity to the acute locomotor suppressant effects of two prototypic antipsychotics, haloperidol and clozapine, is age dependent, with juvenile (PN22) rats of both sexes being the most sensitive, although rats of all ages (PN22, PN40, and adult) showed decreases in locomotion at some dose(s). Further, rats entering late adolescence / early adulthood failed to show tolerance to either antipsychotic following sub-chronic dosing from PN40-PN49. These results, along with those of previous studies, emphasize the importance of considering age at dosing and at testing in pharmacological characterization of antipsychotics. They also suggest the need for caution in the use of antipsychotics in juveniles.
Acknowledgements
Research supported by National Institute of Mental Health grant MH-64771. Clozapine was generously provided by the NIMH Chemical Synthesis and Drug Supply Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Campbell A, Baldessarini RJ. Effects of maturation and aging on behavioral responses to haloperidol in the rat. Psychopharmacology (Berl) 1981;73:219–222. doi: 10.1007/BF00422406. [DOI] [PubMed] [Google Scholar]
- Campbell A, Baldessarini RJ, Teicher MH. Decreasing sensitivity to neuroleptic agents in developing rats; evidence for a pharmacodynamic factor. Psychopharmacology (Berl) 1988;94:46–51. doi: 10.1007/BF00735879. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav. Brain Res. 2005;158:321–330. doi: 10.1016/j.bbr.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Coyle S, Napier TC, Breese GR. Ontogeny of tolerance to haloperidol: behavioral and biochemical measures. Brain Res. 1985;355:27–38. doi: 10.1016/0165-3806(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Cuomo V, Cagiano R, Coen E, Mocchetti I, Cattabeni F, Racagni G. Enduring behavioural and biochemical effects in the adult rat after prolonged postnatal administration of haloperidol. Psychopharmacology (Berl) 1981;74:166–169. doi: 10.1007/BF00432686. [DOI] [PubMed] [Google Scholar]
- Cuomo V, Cagiano R, Mocchetti I, Coen E, Cattabeni F, Racagni G. Behavioural and biochemical effects in the adult rat after prolonged postnatal administration of clozapine. Psychopharmacology (Berl) 1983;81:239–243. doi: 10.1007/BF00427270. [DOI] [PubMed] [Google Scholar]
- Cuomo V, Cagiano R, Renna G, Serinelli A, Brunello N, Racagni G. Comparative evaluation of the behavioural consequences of prenatal and early postnatal exposure to haloperidol in rats. Neurobehav. Toxicol. Teratol. 1985;7:489–492. [PubMed] [Google Scholar]
- Gramling SE, Fowler SC. Effects of neuroleptics on rate and duration of operant versus reflexive licking in rats. Pharmacol. Biochem. Behav. 1985;22:541–545. doi: 10.1016/0091-3057(85)90272-2. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- McQuade RD, Duffy RA, Coffin VL, Barnett A. In vivo binding to dopamine receptors: a correlate of potential antipsychotic activity. Eur. J. Pharmacol. 1992;215:29–34. doi: 10.1016/0014-2999(92)90604-3. [DOI] [PubMed] [Google Scholar]
- Moody CA, Robinson SR, Spear LP, Smotherman WP. Fetal behavior and the dopamine system: activity effects of D1 and D2 receptor manipulations. Pharmacol. Biochem. Behav. 1993;44:843–850. doi: 10.1016/0091-3057(93)90015-l. [DOI] [PubMed] [Google Scholar]
- Napier TC, Coyle S, Breese GR. Ontogeny of striatal unit activity and effects of single or repeated haloperidol administration in rats. Brain Res. 1985;333:35–44. doi: 10.1016/0006-8993(85)90121-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Renner MJ, Bennett AJ, White JC. Age and sex as factors influencing spontaneous exploration and object investigation by preadult rats (Rattus norvegicus) J. Comp. Psychol. 1992;106:217–227. doi: 10.1037/0735-7036.106.3.217. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Shalaby IA, Spear LP. Chronic administration of haloperidol during development: later psychopharmacological responses to apomorphine and arecoline. Pharmacol. Biochem. Behav. 1980;13:685–690. doi: 10.1016/0091-3057(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Simon VM, Parra A, Minarro J, Arenas MC, Vinader-Caerols C, Aguilar MA. Predicting how equipotent doses of chlorpromazine, haloperidol, sulpiride, raclopride and clozapine reduce locomotor activity in mice. Eur. Neuropsychopharmacol. 2000;10:159–164. doi: 10.1016/s0924-977x(00)00070-5. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology (Berl) 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int. J. Dev. Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Vann RE, Wise LE, Philibin SD, Porter JH. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology (Berl) 2002;160:182–191. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- Weihmuller FB, Bruno JP. Age-dependent plasticity in the dopaminergic control of sensorimotor development. Behav. Brain Res. 1989;35:95–109. doi: 10.1016/s0166-4328(89)80110-x. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur. J. Pharmacol. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]