Abstract
Neurons extend axonal processes over long distances, necessitating efficient transport mechanisms to convey target-derived neurotrophic survival signals from remote distal axons to cell bodies. Retrograde transport, powered by dynein motors, supplies cell bodies with survival signals in the form of “signaling endosomes”. In this review, we will discuss new advances in our understanding of the motor proteins that bind to and move signaling components in a retrograde direction, and discuss mechanisms that might specify distinct neuronal responses to spatially restricted neurotrophin signals. Disruption of retrograde transport leads to a variety of neurodegenerative diseases, highlighting the role of retrograde transport of signaling endosomes for axonal maintenance and the importance of efficient transport for neuronal survival and function.
Retrograde axonal transport mechanisms
Neurons extend long axons to contact post-synaptic targets far removed from the neuronal cell body. Hence long-range communication between the nerve ending and the cell body is essential for axonal pathfinding, target innervation, plasticity and survival. The neurotrophin family, including nerve growth factor (NGF), brain derived neurotrophic factor (BDNF) and neurotrophin 3 or 4 (NT-3 and NT-4), are well-characterized growth factors that are often produced in post-synaptic cells and initiate signals at axon terminals by binding specific Trk receptors [1,2]. How are these signals conveyed to the cell body in order to alter gene expression? Several lines of experiments indicate that transport by microtubule-dependent dynein motors transmit these long-range signals [3–6]. Although there exist alternative models [4,7,8], it is widely accepted that vesicular-based transport of a “signaling endosome” is required for many, if not most, aspects of retrograde signaling mechanisms and will be the focus of this review.
In compartmentalized cultures of sensory and sympathetic neurons, activated phosphorylated Trks (P-Trks) accumulate in cell bodies upon distal axon exposure to neurotrophins [9–11]. The signaling endosome model proposes that upon ligand binding and activation, Trk receptors at the distal axon are internalized via clathrin-mediated [12–14] or pincher-dependent endocytosis [15,16]. The resultant vesicles containing the ligand-receptor complex are retrogradely transported to the cell body in order to mediate a survival response [3,7–11,17,18].
A major challenge in the field has been to define the essential components of the signaling endosome. Transport of activated receptors can occur even when minimal amounts of ligand are transported; indeed a single NGF dimer molecule is sufficient to maintain Trk activity during retrograde transport [19]. Vesicles containing neurotrophins and Trks have also been found to include activated components of the Ras-MAP kinase, PLCγ and PI3 kinase pathways [12,13,20,21]. These vesicles could therefore carry entire signaling complexes, capable of recapitulating in their entirety the signaling events that occurred at the nerve terminal [22].
It has long been thought that components of the Ras-MAP kinase, PLCγ and PI3 kinase pathways, are activated either in association with signaling endosomes or in the cell body upon arrival of the signaling endosome. In the cell body, critical kinases such as Rsks and Erk5, translocate to the nucleus, where they phosphorylate and activate transcription factors such as CREB to mediate neurotrophin-induced gene expression. However, a recent study has proposed instead that the transcription factor CREB itself is a component of the signaling endosome. Jaffrey and colleagues demonstrated that mRNA encoding CREB is located in the axon and is locally translated in response to distal axon stimulation with NGF. Since newly synthesized CREB in the axons co-localizes with both P-Trk and P-Erk immuno-reactivity, the investigators suggest that axonally synthesized CREB is transported through the axon together with the TrkA-signaling complexes [23]. The idea that transcription factors themselves might be co-transported with retrograde signaling endosomes provides enticing new possibilities for retrograde signaling.
Motor proteins and mechanisms of cargo recognition
Intracellular transport in axons relies on the microtubule-dependent motors, dyneins and kinesins, which mediate retrograde and anterograde transport respectively [24]. Thus dyneins are the motor responsible for transporting signaling endosomes [4,5,25]. While kinesins are a large gene family with 45 members, allowing a high degree of specificity with regard to expression and cargo selectivity [26], the mechanism for specificity of dyneins is much less obvious. The motor domain of cytoplasmic dynein is encoded by a single gene, dnhc1. This motor-domain protein associates with intermediate chains, light intermediate chains, light chains, and a dynactin complex; any of these might provide specificity for dynein interactions with the signaling endosome. The functional roles of dynein variants, defined by different intermediate chain (IC) isoforms, were investigated with regard to their specific role during retrograde signaling [27]. A recent study has indicated that IC-1B, an isoform of the intermediate chain that is specifically expressed in the nervous system [28–31], binds to and transports neuronal TrkB signaling endosomes [27]. In contrast, the highly related, ubiquitously expressed IC-2C intermediate isoform, does not. These data indicate that the intermediate chain isoforms contribute to selective retrograde transport of signaling endosomes in the nervous system.
A second mechanism for specificity of dynein interactions involves post-translational modification of the cargo. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La [32]. La is anterogradely transported into axons by kinesin motors, and once in the axon, La is covalently modified by addition of small ubiquitin-like modifying polypeptides (SUMO). Sumoylated La binds exclusively to dynein allowing retrograde transport. It is interesting to consider that sumoylation, or perhaps other post-translational modifications, may likewise target signaling endosome components for retrograde transport.
Mechanisms of specificity of responses
The idea that the subcellular location of stimulation can act as a determinant of signaling specificity was addressed by Watson et al.. They proposed that differential use of individual MAP kinases could account for location-specific biological effects of neurotrophins [33]. Whilst neurotrophin stimulation at the cell body results in simultaneous activation of the classic Erk1/2 pathway and alternative Erk5 pathway, retrograde signaling by neurotrophin stimulation at the distal axon leads to preferential activation of Erk5 in the cell body. In this way, the location of neurotrophin stimulation could regulate differential gene expression patterns.
The recent study demonstrating that CREB is translated in the axon upon distal-axon NGF stimulation provides another possible mechanism for location-dependent specificity [23]. The authors indicate that axon-derived CREB is transported together with the signaling endosome, and is responsible for transcriptional responses that mediate NGF-dependent survival. Translation and retrograde transport of this distinct pool of axonally localized mRNAs provides a possible new mechanism by which signaling at the distal axon may selectively regulate gene expression.
While much of the initial emphasis in studies of retrograde signaling has focused on neuronal survival, transcriptional events that promote NGF-dependent axon growth, branching and target innervation of sensory neurons must also involve retrograde signaling [35]. A recent study highlighted the role of the transcription factor serum response factor (SRF) in NGF-dependent axonal outgrowth and terminal branching. Wickramasinghe and colleagues demonstrate that NGF stimulation of axon terminals regulates SRF-dependent gene expression through activation of the MEK/ERK and MAL signaling pathways. Although the sequence of events from initial NGF-Trk signaling to axonal outgrowth has not been fully elucidated, the idea that NGF might regulate axon growth through retrograde MEK/ERK and MAL signaling, demonstrates the potential for the signaling endosome to stimulate diverse cellular responses to distal axon signaling events.
The signaling endosome hypothesis also suggests a mechanism by which neurons can use sequential neurotrophin cues for long-range axon guidance. Postganglionic sympathetic neurons that express TrkA extend their axons past intermediate targets that express NT-3, such as the vasculature, but ultimately these neurons innervate NGF-expressing target organs. While both NT3 and NGF promote axonal outgrowth of these neurons, target-derived NGF is solely responsible for retrograde survival signaling [34]. Axonal NGF stimulation induces robust Trk phosphorylation in the axon together with subsequent transport of P-TrkA, P-Erk1/2 and P-Akt to the cell body; in contrast, NT-3 stimulation of axons is unable to initiate formation of signaling endosomes and so cannot support survival. Thus, in sympathetic neurons, TrkA stimulation without endocytosis is sufficient to promote axonal growth via local signaling independent of transcriptional events. However, to support transcriptional changes that promote axon growth and long-distance survival responses, stimulation plus endocytosis of TrkA is required. The differential control of TrkA internalization and consequent retrograde signaling by NT-3 and NGF provides a mechanism for axon guidance, instructing the neuron through the sequential stages of axon growth, terminal innervation and survival. In this way, signaling endosomes allow specific responses to intermediate and final target-derived factors, thereby ensuring only neurons that correctly innervate target organs survive [34].
Retrograde transport and neurodegeneration
Retrograde transport of neurotrophin signaling endosomes requires the activity of dynein and its associated protein complex dynactin. Disruption of dynein function has been shown to cause neurodegeneration in both mice and human diseases, and it has been suggested that this may reflect impaired transport of signalling endosomes. Interestingly, disruption of either retrograde or anterograde transport can block trafficking in both directions by causing a “traffic jam”, suggesting that even diseases caused by mutations in kinesins can be influenced by disruption in retrograde transport of signalling endosomes [36].
Several mutations in dynein lead to such degenerative processes. In two lines of mice, Legs at odd angles (Loa) and Cramping 1 (Cra1), mutations in the dynein heavy chain gene cause motor neuron disease, and neurons from these mice exhibit a defect in the fast component of retrograde transport in vitro [37]. The Loa mice also exhibit an early-onset sensory neuropathy [38]. A distinct mutation in the dynein heavy chain leads to a proprioceptive sensory neuropathy in Sprawling (Swl) mice [38]. In humans, a mutation in the p150Glued subunit of the dynactin complex has been discovered in a family with a progressive lower motor neuron disease. This mutation disrupts binding of p150Glued to microtubules, thereby reducing the efficiency of retrograde transport [39]. Mice with mutations in p150Glued also develop motor neuron disease, with pathological findings of muscle atrophy, axonal swellings, disrupted intracellular trafficking, and proliferation of degradative organelles, including lysosomes and autophagosomes [40,41]. As dynein is responsible for retrograde transport of signaling endosomes, disruption of these survival signals is likely to contribute to the neuronal dysfunction observed in diverse neurodegenerative disorders.
Several of the degenerative diseases linked to faulty axonal transport show a pattern of distal axon degeneration as the primary pathology. Hereditary spastic paraplegia (HSP) exemplifies the axonal “dying back” phenomenon, in which axons are affected with a distal to proximal progression of disease. Causative mutations for various forms of HSP occur in molecular motors themselves or in other proteins that indirectly affect axonal transport [42–44]. In some cases of ALS, denervation of the neuromuscular junction is followed by loss of ventral root fibers, and finally motor neuron cell body loss, demonstrating that a disease causing early abnormalities in transport results in motor neuron pathology originating in the distal axon [45]. Axo-terminal degeneration of motor neurons also occurs in mice with mutations in the p150Glued subunit of dynactin, although it is unclear whether axonopathy arises from impaired retrograde transport or an alteration in protein degradation [40,41]. The juvenile ALS-associated gene ALS2, encoding the protein Alsin, seems to play a role in transport and axonal maintenance, as several lines of Alsin-deficient mice show variable defects in endosomal trafficking of growth factor and neurotrophin receptors, including epidermal growth factor (EGF), insulin-like growth factor (IGF), and BDNF receptors [46,47], and some lines of Alsin-deficient mice also show axonal degeneration of motor neurons [48,49]. The pathology of these diseases suggests that retrograde transport may play a special role in maintaining axonal health. The signals transported from the distal axon may provide a “snapshot” of the local environment of the axon, including the supply of trophic support originating from target tissues. Therefore, when retrograde transport is defective, information about the local environment does not reach the cell body. This may initiate an axon-specific degenerative program designed to remove the axon from an incorrect or inhospitable environment. Diseases caused by defective retrograde transport may thus specifically affect axonal integrity as a primary event in disease progression. Future research will need to investigate why only a subset of diseases caused by faulty transport lead to axon-specific degeneration. The common denominator among these diseases may provide clues about the factors, environmental or otherwise, that are specifically necessary for maintaining axonal health.
Conclusions and future directions
These studies highlight the importance of functional axonal transport in projection neurons with long axons. Interrupted transport of survival signals, such as signaling endosomes containing activated neurotrophin receptors, is likely to play a role in the pathogenesis of neurodegenerative disease caused by axonal transport defects. A growing body of evidence suggests that retrograde neurotrophin signaling can elicit different effects than local cell body signaling, and the specific requirement of retrograde transport for maintaining axonal integrity may be a result of this spatially distinct neurotrophin signaling program.
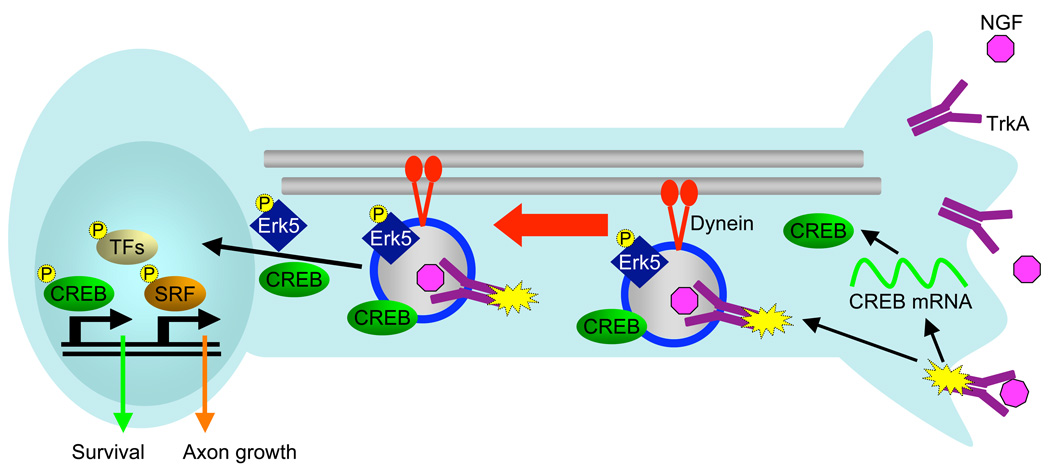
Figure 1.
Signaling endosomes are transported retrogradely from distal axons to neuronal cell bodies by the motor protein dynein along microtubules. NGF is released from target tissues, where it binds to and activates TrkA receptors at the nerve terminal. Ligand-receptor complexes are endocytosed into signaling endosomes and transported retrogradely in association with downstream signaling components. These downstream components help identify the spatial location of neurotrophin signaling events. Spatially selective signaling components include phosphorylated Erk5 and NGF-induced axonally translated CREB. Upon arrival at the cell body, translocation of signaling components to the nucleus activates a set of transcription factors to mediate target-derived NGF-dependent responses. CREB induces a set of genes necessary for survival, while SRF induces a set of genes to mediate axonal outgrowth and target innervation.
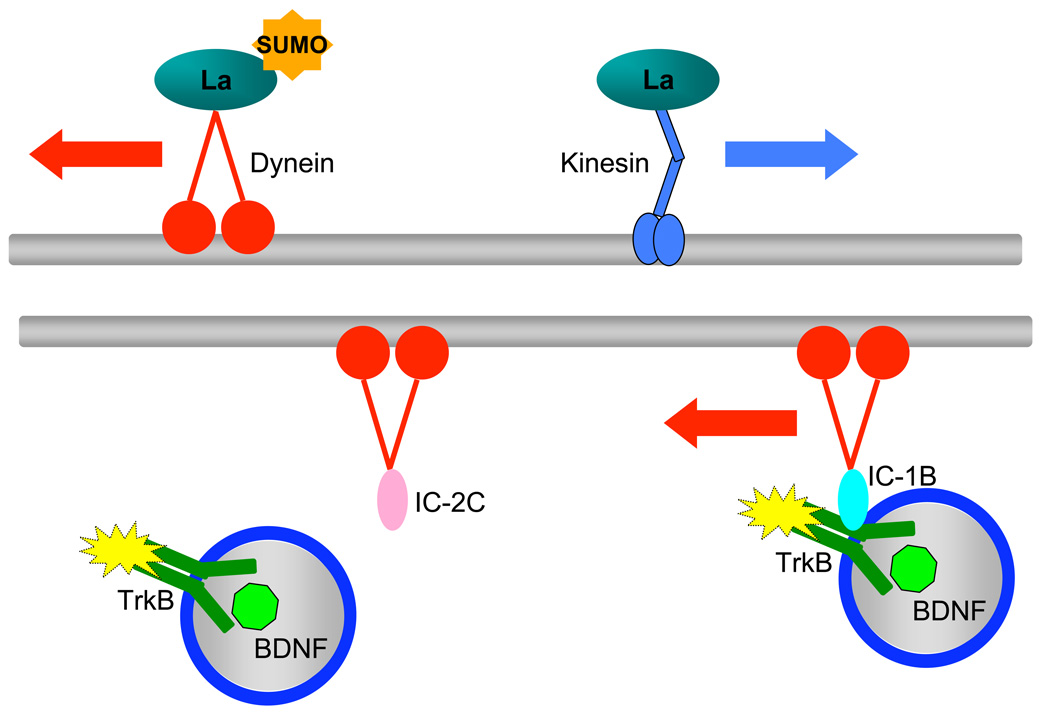
Figure 2.
Proteins are transported along the microtubules anterogradely and retrogradely by kinesins and dyneins, respectively. Post-translational modifications such as sumoylation can specify directionality of transport in the case of the RNA-binding protein La. La binds to kinesin and is anterogradely transported in the absence of sumoylation; when sumoylated, La binds to dynein and is retrogradely transported. Specificity of cargo binding for retrograde transport can be achieved through differential isoforms of dynein subunits. The neuron-specific intermediate chain IC-1B selectively binds to and transports TrkB-containing signaling endosomes, while the ubiquitously expressed IC-2C does not.
Acknowledgements
We would like to apologise to those authors whose work was not included in this review due to space constraints. Our thanks go to Maria Pazyra-Murphy for helpful comments and editing. We also thank the NINDS (NS050674) and the Stuart H.Q & Victoria Quan Fellowship Program at Harvard Medical School for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
•• of outstanding interest
- 1.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 2.Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya A, Watson FL, Bradlee TA, Pomeroy SL, Stiles CD, Segal RA. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 5.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- 6.Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV. Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci. 2001;21:RC125. doi: 10.1523/JNEUROSCI.21-03-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Curr Opin Neurobiol. 2005;15:40–48. doi: 10.1016/j.conb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 10.Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999;19:8207–8218. doi: 10.1523/JNEUROSCI.19-19-08207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson FL, Heerssen HM, Moheban DB, Lin MZ, Sauvageot CM, Bhattacharyya A, Pomeroy SL, Segal RA. Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J Neurosci. 1999;19:7889–7900. doi: 10.1523/JNEUROSCI.19-18-07889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 13.Grimes ML, Beattie E, Mobley WC. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci U S A. 1997;94:9909–9914. doi: 10.1073/pnas.94.18.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdez G, Philippidou P, Rosenbaum J, Akmentin W, Shao Y, Halegoua S. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc Natl Acad Sci U S A. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdez G, Akmentin W, Philippidou P, Kuruvilla R, Ginty DD, Halegoua S. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci. 2005;25:5236–5247. doi: 10.1523/JNEUROSCI.5104-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci U S A. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. Using quantum dot labeled-NGF (QD-NGF) the authors show a novel way to visualize real-time transport of NGF-containing signaling endosomes. QD-NGF transport proceeded in a retrograde direction, with frequent pauses, and was associated with signaling components including Trk, Rab5B, and P-Erk1/2. Interestingly, quantitative analysis showed that single NGF dimers were sufficient to maintain signaling capabilities. The new QD-NGF technology will allow more precise study of signaling endosome composition and trafficking.
- 20.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 21.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 22.Weible MW, 2nd, Hendry IA. What is the importance of multivesicular bodies in retrograde axonal transport in vivo? J Neurobiol. 2004;58:230–243. doi: 10.1002/neu.10318. [DOI] [PubMed] [Google Scholar]
- 23. Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. In this study, the authors show CREB mRNA is localized to the axons of sensory neurons and selectively translated in response to NGF. Newly synthesized CREB is retrogradely transported to the nucleus in association with activated components of the signaling endosome. In compartmentalized Boyden chambers, specific knockdown of CREB in axonal compartments demonstrated a requirement for axonal CREB to mediate induction of CRE-dependent transcription for NGF-induced retrograde survival.
- 24.Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Ramirez A, Cui B, Ding J, Delcroix JD, Valletta JS, Liu JJ, Yang Y, Chu S, Mobley WC. A functional dynein-microtubule network is required for NGF signaling through the Rap1/MAPK pathway. Traffic. 2007;8:1503–1520. doi: 10.1111/j.1600-0854.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 27. Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. The authors investigate the functions of dynein intermediate chains (ICs) isoforms by live-cell imaging of fluorescently-tagged dynein-ICs in PC12 cells and primary neurons. |The neuron specific IC-1B isoform was found to bind selectively to TrkB, whilst the ubiquitously expressed IC-2C isoform did not. This provides a mechanism whereby IC isoforms are involved in binding specific cargoes to the dynein complex.
- 28.Pfister KK, Salata MW, Dillman JF, 3rd, Vaughan KT, Vallee RB, Torre E, Lye RJ. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- 29.Pfister KK, Salata MW, Dillman JF, 3rd, Torre E, Lye RJ. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Mol Biol Cell. 1996;7:331–343. doi: 10.1091/mbc.7.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers KR, Lo KW, Lye RJ, Kogoy JM, Soura V, Hafezparast M, Pfister KK. Intermediate chain subunit as a probe for cytoplasmic dynein function: biochemical analyses and live cell imaging in PC12 cells. J Neurosci Res. 2007;85:2640–2647. doi: 10.1002/jnr.21213. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan KT, Holzbaur EL, Vallee RB. Subcellular targeting of the retrograde motor cytoplasmic dynein. Biochem Soc Trans. 1995;23:50–54. doi: 10.1042/bst0230050. [DOI] [PubMed] [Google Scholar]
- 32. van Niekerk EA, Willis DE, Chang JH, Reumann K, Heise T, Twiss JL. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc Natl Acad Sci U S A. 2007;104:12913–12918. doi: 10.1073/pnas.0611562104. The RNA binding protein La is post-translationally modified by sumoylation to confer transport directionality in sensory neurons. While La binds kinesin-1 in the absence of sumoylation to be anterogradely transported, addition of SUMO is required for direct binding of La to dynein and retrograde transport. SUMO could potentially play a role in targeting other signalling endosome components to bind dynein for retrograde transport.
- 33.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 34.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 35. Wickramasinghe SR, Alvania RS, Ramanan N, Wood JN, Mandai K, Ginty DD. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532–545. doi: 10.1016/j.neuron.2008.03.006. Using SRF conditional mutant mice, the authors demonstrate that although SRF is not required for survival or differentiation of dorsal root ganglia neurons, SRF is necessary for NGF-dependent axonal branching and extension both in vivo and in vitro. It was further established that SRF-dependent gene expression relies on MEK/ERK and MAL signaling pathways to mediate NGF-dependent axonal growth target innervation.
- 36.Holzbaur EL. Motor neurons rely on motor proteins. Trends Cell Biol. 2004;14:233–240. doi: 10.1016/j.tcb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 38.Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 40.Chevalier-Larsen ES, Wallace KE, Pennise CR, Holzbaur EL. Lysosomal proliferation and distal degeneration in motor neurons expressing the G59S mutation in the p150Glued subunit of dynactin. Hum Mol Genet. 2008;17:1946–1955. doi: 10.1093/hmg/ddn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28:1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebbing B, Mann K, Starosta A, Jaud J, Schols L, Schule R, Woehlke G. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum Mol Genet. 2008;17:1245–1252. doi: 10.1093/hmg/ddn014. [DOI] [PubMed] [Google Scholar]
- 44.Ferreirinha F, Quattrini A, Pirozzi M, Valsecchi V, Dina G, Broccoli V, Auricchio A, Piemonte F, Tozzi G, Gaeta L, et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J Clin Invest. 2004;113:231–242. doi: 10.1172/JCI20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, Helm JR, Bissada N, Cruz-Aguado R, Davidson TL, et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci U S A. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadano S, Benn SC, Kakuta S, Otomo A, Sudo K, Kunita R, Suzuki-Utsunomiya K, Mizumura H, Shefner JM, Cox GA, et al. Mice deficient in the Rab5 guanine nucleotide exchange factor ALS2/alsin exhibit age-dependent neurological deficits and altered endosome trafficking. Hum Mol Genet. 2006;15:233–250. doi: 10.1093/hmg/ddi440. [DOI] [PubMed] [Google Scholar]
- 48.Deng HX, Zhai H, Fu R, Shi Y, Gorrie GH, Yang Y, Liu E, Dal Canto MC, Mugnaini E, Siddique T. Distal axonopathy in an alsin-deficient mouse model. Hum Mol Genet. 2007;16:2911–2920. doi: 10.1093/hmg/ddm251. [DOI] [PubMed] [Google Scholar]
- 49.Yamanaka K, Miller TM, McAlonis-Downes M, Chun SJ, Cleveland DW. Progressive spinal axonal degeneration and slowness in ALS2-deficient mice. Ann Neurol. 2006;60:95–104. doi: 10.1002/ana.20888. [DOI] [PubMed] [Google Scholar]