Abstract
Glial cells, which outnumber neurones in the central nervous system, have traditionally been considered to be electrically inexcitable and to play only a passive role in the electrical activity of the brain1. Recent reports have demonstrated, however, that certain glial cells, when maintained in primary culture, possess voltage-dependent ion channels2-4. It remains to be demonstrated whether these channels are also present in glial cells in vivo. I show here that Müller cells, the principal glial cells of the vertebrate retina, can generate ‘Ca2+ spikes’ in freshly excised slices of retinal tissue. In addition, voltage-clamp studies of enzymatically dissociated Müller cells demonstrate the presence of four types of voltage-dependent ion channels: a Ca2+ channel, a Ca2+-activated K+ channel, a fast-inactivating (type A) K+ channel and an inward-rectifying K+ channel. Currents generated by these voltage-dependent channels may enhance the ability of Müller cells to regulate extracellular K+ levels in the retina and may be involved in the generation of the electroretinogram.
Recordings were made from Müller cells of the salamander Ambystoma tigrinum (aquatic stage) as described previously5,6. Preparations were maintained at ∼15 °C in perfusate containing (in mM): NaCl, 110; KCl, 2.5; CaCl2, 2; MgCl2, 1; HEPES, 5; dextrose, 5; equilibrated with 100% O2 (pH 7.4). KCl was substituted for NaCl in high-[K+] perfusate6. Recordings were made from preparations within 2 h of isolation.
Müller cells have a high resting membrane conductance to K+ (ref. 6) which would shunt out other ionic currents, were they present. To unmask possible voltage-dependent currents, the K+ conductance of Müller cells in retinal slices was blocked by pressure-injecting a saturated solutions of Cs+ and tetraethyl-ammonium (TEA), both K+-channel blockers, from recording micropipettes. (External application of Ba2+, TEA or 4-aminopyridine (4-AP) did not block the K+ conductance of Müller cells in the slice preparation.)
Müller cells in retinal slices were penetrated in their endfeet (in the nerve fibre layer) and stimulated by passing current pulses through the recording electrode. The cells, which are normally coupled by gap junctions7, were uncoupled by lowering the perfusate pH to 6.8 (by addition of 5% CO2). (Application of low pH perfusate was accompanied by an increase in cell input resistance, indicating that the cells were successfully uncoupled.)
Depolarizing current pulses evoked a regenerative response in Cs+, TEA-injected Müller cells bathed in pH 6.8 perfusate (Fig. 1a). This response was significantly increased when perfusate [Ca2+] was raised from 2 to 20 mM (n = 10; Fig, 1b). The regenerative response was caused by an influx of Ca2+ triggered by cell depolarization: the Ca2+ spikes were not diminished by addition of 1 μM tetrodotoxin (TTX), a Na+-channel blocker, but were reduced more than 70% by 60 μM verapamil, a selective Ca2+-channel blocker8.
Fig. 1.
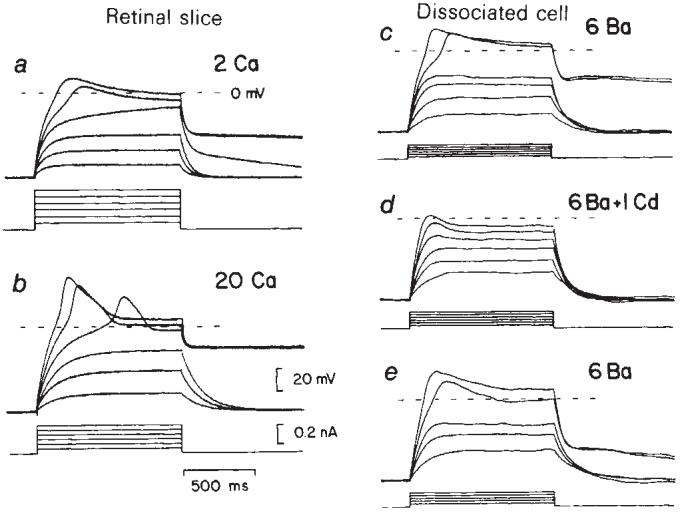
Regenerative ‘Ca2+ spikes’ evoked by depolarizing current pulses in Müller cells of the salamander. a, b, Voltage recordings from a cell in a retinal slice. The regenerative response in control perfusate (a) increased significantly when [Ca2+] was raised from 2 to 20 mM (b). The plateau of the action potential lasted for several seconds following stimulation. The cell was pressure-injected with Cs+ and TEA to reduce the resting K+ conductance and bathed in pH 6.8 perfusate to de-couple the cell from other Müller cells. c-e, Recordings from a dissociated Müller cell in 6 mM Ba2+. Current pulses evoked a large sustained Ba2+ spike (c) which was substantially reduced by addition of 1 mM Cd2+ (d). The regenerative response returned when the Cd2+ was washed out (e). In both cells the resting potential was maintained at -70 mV by injection of small continuous currents. Current pulse amplitudes are indicated below each series of traces.
To investigate this voltage-dependent current in more detail, additional recordings were made from enzymatically dissociated Müller cells of salamanders6. Recordings were made with patch-clamp electrodes (filled with 125 mM KCl) used in the ‘whole cell’ recording mode9.
When dissociated Müller cells were bathed in 6 mM Ba2+, which effectively blocked the resting K+ conductance, large regenerative spikes were evoked by depolarizing current pulses (n = 25; Fig. 1c). This response was substantially reduced by addition of 1mM Cd2+, a potent Ca2+-channel blocker10 (Fig. 1d) and recovered following removal of the Cd2+ (Fig. 1e). The regenerative response was also blocked by 50 μM verapamil, but was unaffected by 1 μM TTX.
The voltage-dependent currents present in Müller cells were further characterized with voltage-clamp recordings from dissociated cells. Single electrode clamps were made with patch electrodes in the ‘whole cell’ recording configuration9.
When cells were bathed in Ba2+ and TEA (both K+ channel blockers), a long lasting voltage-dependent inward current was recorded (n = 21). This current increased significantly when external Ba2+ (which passes freely through Ca2+ channels10,11) was raised from 2 to 20 mM (Fig. 2a). The inward current was blocked by 2 mM Cd2+ (Fig. 2a) and 50 μM verapamil. The current-voltage (I-V) relation of the inward current, recorded in 2 mM Ba2+ (Fig. 3a, Ca) peaked at +5 mV and had a (chord) conductance of 2.6 ± 1.7 nS (mean ±s.d.) at 0 mV (n = 3). These characteristics are typical of current generated by voltage-dependent Ca2+ channels10,11.
Fig. 2.
Voltage-clamp records of voltage-dependent currents in dissociated salamander Müller cells. a, Inward Ca2+ current. The cell potential was stepped to the value which evoked maximal inward current (+15 mV in 20 mM Ba2+ and +5 mV in mM Ba2+). The inward current increased when [Ba2+] was raied from 2 to 20 mM and was abolished by addition of 2 mM Cd2+. The perfusate contained 5 mM TEA to reduce Ca2+-activated K+ currents. Recordings made from a cell with endfoot intact. b, Outward Ca2+-activated K+ current. In 10 mM Ca2+ perfusate (10 Ca), a slowly developing outward current was superimposed on the inward Ca2+ current. 1 mM TEA reduced the outward current while 2 mM Cd2+ eliminated both the inward and outward currents. Recordings from a cell lacking its endfoot process. c, Transient outward (type A) K+ current. The transient current was eliminated when the voltage step was preceded by a 2-s prepulse to -40 mV. d, Transient current shown in isolation by subtracting records preceded by a prepulse from records without a prepulse. The ‘control’ is the difference of the two records in c. The transient current in control perfusate was unaltered by 5 mM TEA but abolished by 5 mM 4-AP. Records from c and d were from the same endfoot-shorn Müller cell. e, Inward-rectifying K+ current evoked by depolarizing and hyperpolarizing voltage steps in 80 mM K+ perfusate. Holding potential (Eh), -4 mV, the membrane potential of the cell in 80 mM K+. Recordings from an endfoot-shorn cell. In a-e, inward currents are shown as downward deflections. Capacitive transients were reduced using transient cancellation circuitry. In a and b, linear contributions of the ‘leakage current’ were removed by subtracting a scaled version of records evoked by depolarizations to ∼ -60 mV.
Fig. 3.
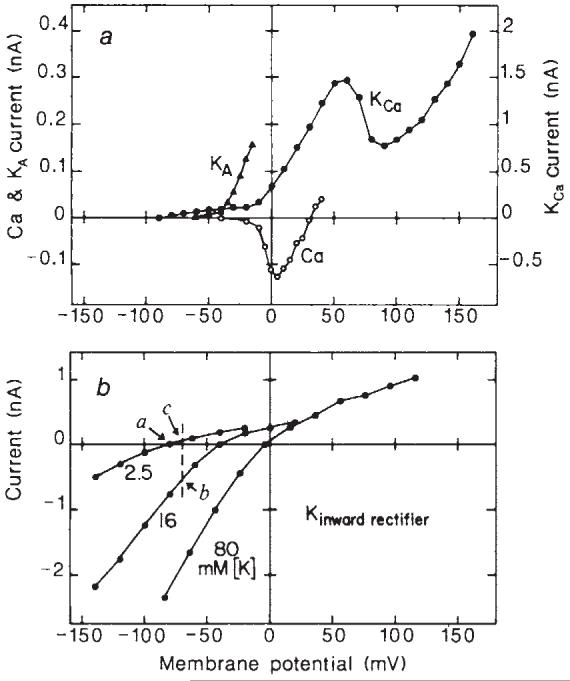
Current-voltage plots of the voltage-dependent ionic currents illustrated in Fig. 2. a, The Ca2+ current relation (Ca) was obtained from an intact Müller cell bathed in perfusate containing 2 mM Ba2+ and 5 mM TEA. It represents peak inward current, corrected for an extrapolated linear leakage current. Eh, -80 mV. The Ca2+-activated K+ current relation (KCa) was obtained from an endfoot-shorn cell in control perfusate and represents the steady-state current attained during a 3.5-s voltage pulse. The small outward current on the KCa curve between -80 and -30 mV is inward rectifier current. Eh, -90 mV. The transient (type A) K+ current relation (KA) was obtained from an endfoot-shorn Müller cell in control perfusate. Current amplitude was measured between the peak of the response and the value at the end of an 800 ms pulse. Eh, -90 mV. b, The inward rectifier K+ current relation was recorded from an endfoot-shorn cell bathed sequentially in 2.5, 16 and 80 mM K+ perfusate. Current amplitudes represent the maximal values attained during an 800-ms voltage step. Eh, -80, -40 and -4 mV, the cell membrane potential in the three perfusates. The dashed line and points a-c in b are used to illustrate changes in membrane conductance caused by a localized [K+]o increase. See text for details. Note differences in the vertical scales for the four currents.
In perfusate containing either 2 or 10 mM Ca2+, depolarizing voltage steps to ∼+15 mV evoked a transient inward current followed by a slowly developing outward current (n = 14; Fig. 2b, ‘10 Ca’). The outward current was partially blocked by 1 mM TEA (known to block Ca2+-activated K+ channels) while 2 mM Cd2+ blocked both the inward Ca2+ current and the outward current (Fig. 2b). The I-V relation of the sustained outward current (Fig. 3a, KCa) had a prominent N-shape, characteristic of current generated by Ca2+-activated K+ channels12. Conductance was 2.7±1.0 nS at 0 mV and 6.3 ± 3.2 nS at +50 mV (n = 3).
A second outward current, which peaked at ∼40 mS and decayed within 1 s, was evoked by depolarizing voltage steps to ∼-5 mV (n = 5). This outward current could be isolated from inward Ca2+ current by recording from cells for more than 20 min, at which time the Ca2+ current was diminished. The transient outward current was completely inactivated by a 2-s prepulse to -40 mV (Fig. 2c). It was abolished by 5 mM 4-AP (known to block fast-inactivating K+ channels) but was unaffected by 5 mM TEA (Fig. 2d). The I-V relation of the transient current (Fig. 3a, KA) shows that it turns on at between -60 and -40 mV. Conductance was 1.8±0.7 nS at 0 mV (n = 5). These properties demonstrate that the current is generated by fast-inactivating (type A) K+ channels13,14.
A third type of K+ current was recorded from Müller cells when the K+ concentration of the perfusate was raised above control level (n = 6). A series of voltage-clamp records in 80 mM K+ (Fig. 2e) shows that evoked current was much larger when the cell was hyperpolarized then when it was depolarized. This inward rectification is shown clearly in the I-V relation of a cell (Fig. 3b) which was bathed sequentially in perfusate containing 2.5, 16 and 80 mM K+. The I-V curves are typical of current arising from inward-rectifying K+ channels15. Conductance was 7.1±2.4 nS in 2.5 mM K+ at -120 mV (n = 4) and 31±5.6 nS in 80 mM K+ at -100 mV (n = 3).
The magnitudes of the four voltage-dependent currents were approximately the same whether they were recorded from cells having intact endfeet or from cells whose endfeet had been shorn off during the dissociation procedure. Thus, the channels producing these four currents were not localized principally in the endfoot process of the cell. Except where indicated, all recordings reported here were made from cells shorn of their endfeet in order to eliminate the large, voltage-independent K+ conductance which is localized in the endfoot process5,6. (The conductance of Müller cells at resting potential is 127 nS in intact cells and 6.6 nS cells shorn of their endfeet6.)
The findings described here demonstrate that four types of voltage-dependent ion channels are present in freshly dissociated Müller cells of salamanders: Ca2+ channels, Ca2+-activated K+ channels, type A K+ channels and inward-rectifying K+ channels. At least one of these, the Ca2+ channel, is also present in Müller cells in retinal slices and thus probably occurs in vivo as well. The results confirm findings from cultured astrocytes, where Ca2+ channels3 and Ca2+-activated K+ channels16 have recently been reported. Inward-rectification has also been noted in turtle Müller cells7. Axonal-type Na+ channels and delayed rectifier K+ channels, reported in primary cultures of Schwann cells2 and astrocytes4,17, were not observed in the present study.
Of the four voltage-dependent ion channels found in Müller cells, the inward-rectifying K+ channel may be the most important functionally, as it is the only one that appears to be open in normal physiological conditions. This channel may be involved in regulating extracellular K+ levels, [K+]o, in the retina: K+ is thought to flow into Müller cells in regions of light-evoked [K+]o increase and to flow out through the Müller cell endfeet, which contain 95% of the total cell membrane conductance6. This process, which has been termed K+ siphoning18, functions to transfer excess K+ directly from the retina to the vitreous humor. The effectiveness of K+ siphoning is limited by the low resting conductance of the cell body membrane of Müller cells (137 μS cm-2, ref. 6). However, the voltage and K+-dependence of the inward-rectifying K+ channel may lead to an increase in this membrane conductance when [K+]o rises.
The effect of inward-rectifying K+ channels on K+ siphoning by Müller cells can be appreciated by considering the I-V relation of the channel. If, for example, [K+]o is raised from 2.5 to 16 mM in a localized region of the cell, the I-V relation of those channels exposed to the K+ increase will be shifted to the right (Fig. 3b, curves 2.5 and 16). The membrane potential of the cell (dashed vertical line in Fig. 3b) will be depolarized by the [K+]o increase, but not to the degree predicted by the new K+ equilibrium potential; the high conductance of the Müller cell endfoot has the effect of clamping the membrane potential at near the resting level (see ref. 6). The conductance of the inward-rectifying channels exposed to 16 mM K+ (given by the slope of the I-V curve at the point where it intersects the vertical line, Fig. 3b, point b) is larger than at rest (Fig. 3b, point a). In this example, the conductance is 4.2 times the resting value. At the same time, the I-V relation of those channels not exposed to the [K+]o increase remains unshifted (Fig. 3b, curve 2.5). Because the cell is partially depolarized by the [K+]o increase, however, the conductance of these channels will be smaller (by 20%) than at rest (as indicated by the slope of the I-V curve at point c compared with the slope at point a, Fig. 3b). Note that, in physiological conditions, the changes in inward rectifier conductance will probably be less than those illustrated here, as light-evoked increases in retinal [K+]o are not thought to reach 16 mM (ref. 19).
Thus, the properties of inward-rectifying K+ channels enhance the process of K+ siphoning by increasing membrane conductance in regions of [K+]o increase, leading to a greater influx of K+ into Müller cells, and by reducing membrane conductance in other regions of the cell, reducing the amount of K+ deposited in adjacent retinal tissue. (Efflux of K+ from the endfoot process will not be reduced by cell depolarization as it occurs through K+ channels which are largely voltage-independent).
The functions of the other three types of voltage-dependent channels reported here are less clear, as they normally remain closed. However, large increases in [K+]o, such as encountered during spreading depression episodes20, might depolarize Müller cells sufficiently to open these channels. The resulting increase in membrane K+ conductance would aid in clearing K+ from the retina by augmenting siphoning currents.
Acknowledgments
I thank Gary R. Strichartz, Jerome Y. Lettvin, Peter H. Hartline, Janice I. Gepner and David M. Berson for their valuable comments on the manuscript. This work was supported by NIH grant EY 04077.
References
- 1.Kuffler SW. Proc. R. Soc. 1967;B168:1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- 2.Chiu SY, Schrager P, Ritchie JM. Nature. 1984;311:156–157. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- 3.MacVicar BA. Science. 1984;226:1345–1347. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- 4.Bevan S, Raff M. Nature. 1985;315:229–232. doi: 10.1038/315229a0. [DOI] [PubMed] [Google Scholar]
- 5.Newman EA. Nature. 1984;309:155–157. doi: 10.1038/309155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman EA. J. Neurosci. 1985;5:2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conner JD, Detwiler PB, Sarthy PV. J. Physiol., Lond. 1985;362:79–92. doi: 10.1113/jphysiol.1985.sp015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleckenstein A. A. Rev. Pharmac. Tox. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- 9.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Plügers Arch. ges. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 10.Byerly L, Chase PB, Stimers JR. J. gen. Physiol. 1985;85:491–518. doi: 10.1085/jgp.85.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagiwara S, Ohmori H. J. Physiol., Lond. 1982;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meech RW. A. Rev. Biophys. Bioengng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- 13.Connor JA, Stevens CF. J. Physiol., Lond. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson SH. J. Physiol., Lond. 1971;265:465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagiwara S, Miyazaki S, Rosenthal NP. J. gen. Physiol. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quandt FN, MacVicar BA. Soc. Neurosci. Abstr. 1984;10:939. [Google Scholar]
- 17.Bevan S, Chiu SY, Gray PTA, Ritchie JM. J. Physiol., Lond. 1985;361:18P. [Google Scholar]
- 18.Newman EA, Frambach DA, Odette LL. Science. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kline RP, Ripps H, Dowling JE. Proc. natn. Acad. Sci. U.S.A. 1978;75:5727–5731. doi: 10.1073/pnas.75.11.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori S, Miller WH, Tomita T. Proc. natn. Acad. Sci. U.S.A. 1976;73:1351–1354. doi: 10.1073/pnas.73.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]



