Introduction
The programmed elimination of cytoplasmic organelles occurs during terminal differentiation of erythrocytes, keratinocytes and lens fiber cells. In each case, the process is relatively well understood phenomenologically, but the underlying molecular mechanisms have been surprisingly slow to emerge. This brief review considers the particular case of the lens where, in addition to their specialized physiological roles, organelles represent potential sources of light scattering. The article describes how the elimination of organelles from lens cells located on the visual axis contributes to the transparency of lens tissue. Classic anatomical studies of lens organelle degradation are discussed, along with more contemporary work utilizing confocal microscopy and other imaging modalities. Finally, recent data on the biochemistry of organelle degradation are reviewed. Several review articles on lens organelle degradation are available (Wride 1996; Wride 2000; Bassnett 2002; Dahm 2004) and readers are directed to these for a comprehensive discussion of the earlier literature on this topic.
Organelles and light scatter
A substance is transparent if it neither absorbs nor scatters light. In the visible range most biological molecules do not absorb light strongly. The principal reason then, that biological structures are not transparent is that they scatter a significant fraction of the incident light.
Living cells contain varying proportions and concentrations of lipids, proteins and other organic molecules, each with its own characteristic refractive index (n). As a result of variation in refractive index between and within cells, light is invariably scattered (i.e. caused to deviate from its original trajectory) as it passes through living tissue. The size of potential scattering centers in tissues varies enormously, from individual protein molecules to the cells themselves, but light scatter is generally dominated by cells and the organelles they contain (Beauvoit et al. 1994).
The familiar concepts of geometric optics (the laws that govern refraction by lenses and reflection by mirrors) do not adequately describe the interactions of light with sub-cellular particles, such as organelles, that are comparable in size to the wavelength of light. Gustav Mie, studying light scattering by colloidal solutions, provided a complete analytical solution of Maxwell’s equations for the scattering of light by isotropic spheres embedded in a homogeneous medium (Mie 1908). Today, Mie scattering theory is used to explain the appearance of common materials such as milk or latex paint. In biology, Mie theory is used to model light scattering in living tissues (Beauvoit et al. 1994; Mourant et al. 1998; Wilson et al. 2005; Xu et al. 2008) and has been applied to scattering from micrometer-sized multi-lamellar bodies in cataractous lenses (Costello et al. 2007; Gilliland et al. 2008).
Organelles are distributed within a cytoplasm composed mainly of dissolved salts and proteins. The refractive index of cytoplasm is ~1.37 (for pure water n=1.33). The refractive index of organelles is substantially higher (Table 1), in part due to their relatively high lipid content. Consequently, organelles are major scattering centers. The relationship between the scattering cross section of a particle, its size, and the angular distribution of scattered light is complex (Johnsen and Widder 1999). Larger structures (such as the cell nucleus or individual cells in suspension) scatter light predominantly in the forward direction (i.e. in the same general direction as the incident light). In some cases, cell light scattering is adequately described by modeling cells as denser spheres (corresponding to nuclei) embedded in larger, softer spheres (corresponding to cytoplasm) (Brunsting and Mullaney 1974). However, in many cell types, organelles other than nuclei make a significant contribution to light scatter, particularly at higher angles. For example, light scatter from normal and neoplastic tissue has been shown to be proportional to their mitochondrial content (Beauvoit et al. 1995). In liver, light scattering has been attributed almost exclusively to mitochondria, which constitute a particularly large volume fraction in hepatocytes (Beauvoit et al. 1994). Other organelles also contribute significantly to light scatter. Using a photodynamic ablation technique, Wilson and Foster (2007) demonstrated that, due to their high refractive index (n=1.6), lysosomes scatter ~14% of the light from EMT6 cells.
Table 1.
Refractive index of cytoplasmic organelles
| Organelle | Table Refractive Index (n) | Reference |
|---|---|---|
| Extracellular Fluid | 1.35–1.36 | (Beauvoit et al. 1994) |
| Cytoplasm | 1.37 | (Brunsting and Mullaney 1974) |
| Nucleus | 1.39 | (Brunsting and Mullaney 1974) |
| Mitochondria | 1.4 | (Wilson et al. 2007) |
| Lysosome | 1.6 | (Wilson and Foster 2007) |
| Lens membranes | 1.42–1.47 | (Michael et al. 2003) |
The role of the lens is to focus light on the retina. To do so it must be transparent. The degree to which lens organelles and other cellular inclusions scatter light depends largely on the difference in refractive index between the organelle and the surrounding cytoplasm. Organelle light scatter would be eliminated were the refractive index of the cytoplasm to match that of the organelles. The cytoplasmic protein content is much higher in cells located in the center of the lens than in cells located near the periphery. As a result, n varies with depth. In rat lenses, ray tracing experiments suggest that n varies parabolically, ranging from ~1.36 near the surface to ~1.50 in the center (Campbell 1984). Similar gradients, although of lesser magnitude (n=1.37–1.41), are found in the human lens (Kasthurirangan et al. 2008). The gradient index corrects longitudinal spherical aberration and, at least in the human lens, is adjusted over the life span (Augusteyn et al. 2008). The implication for lens organelle light scattering is that there is no stratum of the tissue in which the refractive index of the cytoplasm might permanently match that of the organelles.
Several strategies appear to have been selected for in the lens that minimize potential light scatter from cytoplasmic organelles. The first and most obvious is that organelles are degraded in the course of lens fiber cell differentiation, such that the majority of the fiber cells situated in the light path completely lack organelles. The mechanisms by which this is accomplished are discussed in detail in the following sections. One organelle which is present throughout the lens volume is the plasma membrane. Interestingly, its refractive index closely matches that of the cytosol for cells located on the visual axis (Michael et al. 2003). Those (relatively few) fiber cells in the adult lens that contain cytoplasmic organelles are restricted to the periphery, where they lie in the shadow of the iris (Bassnett and Beebe 1992). Organelle-containing cells located on the visual axis, such as those in the central lens epithelium, constitute a very thin layer. Light scattering is proportional to path length. Consequently, the scattering from organelles in the lens epithelium is negligible. Keratocytes in the corneal stroma are also flattened along their anterior/posterior axis, presumably for the same reason. Even in organelle-containing fiber cells near the lens equator, the density of organelles is unusually low. As discussed earlier, nuclei are important sources of forward scatter, but due to the extremely elongated morphology of lens fiber cells, the volume fraction of nuclei is low. Therefore, their contribution to the overall light scatter is negligible.
The anatomy of organelle breakdown
Due to its unusual growth pattern, in which newly-formed fiber cells are layered onto existing fibers, the lens contains cells at all stages of terminal differentiation. Early anatomical studies of the lens noted the absence of nuclei from the innermost (oldest) fiber cells (Rabl 1899). Fiber cell nuclei appear to pass through distinct morphological stages during terminal differentiation. In the superficial fiber cells, nuclei are ovoid but they assume a more spherical shape prior to their disintegration. The change in nuclear shape may be secondary to dismantling of the nuclear lamina which, according to studies in bovine (Dahm et al. 1998) and chicken (Bassnett and Mataic 1997) lenses, occurs before frank disintegration of the chromatin commences.
Fiber cells are transcriptionally active until quite late in differentiation, as evidenced by their ability to transcribe novel mRNAs, such as those encoding lengsin (Vasiliev et al. 2007; Wyatt et al. 2008) and hop (Vasiliev et al. 2007). Microinjection of expression plasmids into fiber cells located at various depths from the lens surface has confirmed that the majority of nucleated fiber cells are transcriptionally competent (Shestopalov and Bassnett 1999). However, it is likely that transcription is shut down in lens fiber cells sometime prior to remodeling of the nuclear lamina and subsequent nuclear disintegration. Confocal microscopic studies have demonstrated changes in the distribution of fibrillarin and coilin within the nucleus of cells bordering the central, organelle-free zone (OFZ) of the lens (Dahm et al. 1998). Similarly, NuMA, a nuclear matrix component, associates with the Cajal body and nuclear speckle compartments in the fiber cell nuclei prior to their dissolution (Gribbon et al. 2002). These changes have been interpreted as signifying the loss of transcriptional capacity in fiber nuclei shortly before their degradation.
Disintegrating fiber cell nuclei are sometimes described as “pyknotic”, an expression that dates to the end of the 19th century (Arnheim 1890) and denotes chromatin shrinkage and hyperchromatia. In fact, there is considerable species variation in the appearance of disintegrating lens nuclei. In bovine lenses, for example, following reorganization of the lamina, the chromatin condenses into clumps of material within the nucleus. At the nuclear margins, the condensed chromatin associates with nuclear pore clusters (Dahm and Prescott 2002). The clumps of chromatin are readily labeled using the TUNEL technique, which detects free 3′-OH ends in the DNA and is used to monitor the integrity of the DNA. Eventually, the chromatin coalesces into a single condensed, TUNEL-positive remnant, which persists in the cytoplasm for a period before finally disappearing (Dahm et al. 1998; De Maria and Arruti 2004). In chicken lenses, condensed chromatin, stripped of the nuclear membrane, persists for a few days before becoming TUNEL-positive and disappearing (Bassnett and Mataic 1997). Primate lens denucleation appears to involve the disintegration of the nucleus (karyorhexis) and release of particulate, TUNEL-positive material which perdures in the cytoplasm (Bassnett 1997). In contrast, mouse lens denucleation involves karyolysis (nuclear fading), in which a cloud of TUNEL-positive material is released into the cytoplasm without prior condensation or marginalization of the chromatin (Kuwabara and Imaizumi 1974; De Maria and Bassnett 2007). These different morphological presentations probably reflect species differences in the identity and/or role of the nucleases and proteases active during denucleation.
Although organelle breakdown might be ascribed simply to aging (cells in the lens core are among the oldest in the body) it is clear that denucleation is a developmentally programmed event which commences during embryonic development. In the chicken lens, for example, all fiber cells are nucleated until day 12 (E12) of embryonic development, at which time nuclei begin to disappear from the innermost cells (Bassnett and Beebe 1992). In rats (He et al. 1998) and mice (Vrensen et al. 1991), this process begins at E17. In all cases, denucleation occurs simultaneously in a cluster of primary fiber cells and, subsequently, on a cell-by-cell basis, in the secondary fiber cells. It has been suggested that the degradation process differs between primary and secondary fiber cells (Vrensen et al. 1991) but, if a difference exists, its mechanistic basis is obscure.
Once nuclear degradation is complete, the capacity for transcription is lost. In situ hybridization studies in chicken lenses suggest that, following nuclear breakdown, extant RNA decays quite rapidly in the fiber cell cytoplasm (Faulkner-Jones et al. 2003). Ribosomal RNA disappears with an estimated half life of 2.5 days, a decrease paralleled by the loss of morphologically identifiable polysomes and ribosomes from the cytoplasm. Polyadenylated RNA is undetectable within a few cell layers of the last intact nucleus, suggesting the rapid degradation of most or all mRNAs. Quantitative PCR studies have shown that mRNAs encoding housekeeping genes, such as GAPDH, disappear from the cytoplasm of anucleated fibers with a half-life of 3–4 days. In contrast, crystallin mRNAs are detectable several weeks after nuclear disintegration (Treton et al. 1982) but this may reflect the extraordinary initial abundance of those transcripts rather than unusual stability (Faulkner-Jones et al. 2003).
One striking and consistent feature of nuclear breakdown is that it is invariably accompanied by the rapid loss of all other organelles including mitochondria (Bassnett 1992; Bassnett and Beebe 1992; Dahm et al. 1998) and endoplasmic reticulum (Bassnett 1995). The simultaneous disappearance of organelles raises the question of whether the breakdown of one class of organelle is required to trigger the destruction of another. In some instances, fiber cell denucleation is inhibited (Nishimoto et al. 2003) but, in such cases the integrity of other organelles is not similarly preserved. This suggests that organelle breakdown may occurs through multiple independent pathways.
Compared to denucleation, which can be a relatively protracted process, disintegration of mitochondria appears to be quite rapid. In cells bordering the OFZ, mitochondria become swollen and fragmented, losing their ability to accumulate rhodamine 123 (Bassnett and Beebe 1992). Mitochondrial markers such as BAP 37, prohibitin, (Dahm et al. 1998), succinic dehyrogenase, (Bassnett and Beebe 1992) and succinate-ubiquinone oxidoreductase (Zandy and Bassnett 2007) are no longer detectable by immunofluorescence or immunoblot. Some studies indicate that changes in the mitochondria are initiated relatively early in differentiation. In the embryonic chicken lens, for example, depolarized mitochondria are present in newly differentiating fiber cells and cytochrome C, a critical modulator of apoptosis, redistributes from the mitochondria into the cytoplasm in cells located near the periphery (Weber and Menko 2005).
Thus, within the space of a few cell layers, corresponding to a period of as little as 2–4 hours (Bassnett and Beebe 1992), lens fiber cells lose the capacity for de novo protein synthesis, oxidative phosphorylation, intracellular membrane trafficking and the host of other cellular functions normally carried out by cytoplasmic organelles. Fiber cells in the lens OFZ are essentially membrane clad bags of protein with a more than passing resemblance to hemoglobin-filled erythrocytes in the blood or keratin-rich keratinocytes in the stratum corneum of the epidermis, the other cell types that undergo organelle degradation during their terminal differentiation.
Biochemical mechanisms underlying organelle breakdown
Nucleolytic Mechanisms
The failure of the organelle degradation program, as evidenced by the abnormal persistence of nuclei or other organelles in central fiber cells, is a striking feature of many types of cataract in animal models. In rodents, for example, organelle degradation is apparently inhibited in animals lacking hsf4 (Min et al. 2004), AP2 alpha (West-Mays et al. 2002), connexin 50 (Graw et al. 2001), persistently expressing Foxe3 (Landgren et al. 2008), suffering from tryptophan deficiency (Vrensen et al. 2004) or harboring mutations in βA3/A1 crystallin (Sinha et al. 2005; Sinha et al. 2007), γD crystallin (Wang et al. 2007), or γS crystallin (Sinha et al. 2001), to name but a few. Indeed, it is unusual to find mutations affecting the mouse lens that do not affect organelle breakdown to some degree (J. Graw, personal communication). It is possible that some of these mutations impact the organelle breakdown process directly. However, it is also evident that organelle degradation involves a complex series of interdependent steps and that any mutation that disturbs lens homeostasis sufficiently has the potential to disrupt the process.
One knockout mouse model that has provided important insights into organelle breakdown is the DNase IIβ-null mouse. Mammals express two DNases with acidic pH optima: DNase IIα and DNase IIβ (aka DLAD). DNAse IIα is a lysosomal enzyme with a broad tissue expression pattern. In macrophages, where it is expressed particularly strongly, it plays an important role in degrading the DNA of apoptotic cells and DNA contained in nuclei expelled from erythroid precursors (Nagata 2007). DNase IIβ shares 34% sequence homology with DNase IIα, but Northern blot experiments indicate that it is expressed at significant levels only in liver and lens (Shiokawa and Tanuma 1999; Nishimoto et al. 2003). The DNAse IIβ-null mouse develops nuclear cataracts as a result of abnormal retention of undigested DNA in the central fiber cells, suggesting that DNase IIβ plays a critical role in lens denucleation (Nishimoto et al. 2003). Like DNase IIα, DNase IIβ cleaves DNA to produce 3′-phosphoryl/5′-hydroxy ends (Shiokawa and Tanuma 1999). It has been known for many years that 3′-OH ends rather than 5′-OH ends accumulate during fiber cell denucleation (Modak and Bollum 1972) so the realization that DNase IIβ is the major nuclease responsible for lens chromatin degradation was surprising. This apparently paradoxical finding may be explained by the presence of endogenous phosphatases, which rapidly convert 3′-PO4 ends generated by DNAse IIβ digestion into 3′-OH ends (De Maria and Bassnett 2007).
Quantitative PCR measurements have shown that while transcript levels for DNase IIα and DNase IIβ are approximately equivalent in lens epithelial cells, the former is down regulated during fiber differentiation while the latter is upregulated, resulting in a ratio of DNase IIα:DNase IIβ of 1:8000 in cortical fiber cells (De Maria and Bassnett 2007). Microarray analysis of laser dissected lens tissue shows a ~ 3-fold increase in DNase IIβ transcript level in cells bordering the OFZ (Ivanov et al. 2005). Most (De Maria and Bassnett 2007) or all (Nishimoto et al. 2003) of the acid nuclease activity in the fiber cells can be attributed to DNase IIβ. Furthermore, activity assays on fractionated lens lysates suggest that the majority of DNase IIβ activity in the lens is associated with the membrane fraction, specifically the lysosomal fraction (De Maria and Bassnett 2007). Immunofluorescence studies indicate that DNase IIβ co-localizes with lysosomal associated membrane protein-1in the lens cortex (Nakahara et al. 2007). Together with its acidic pH optimum and sequence homology to DNase IIα, these biochemical and immunocytochemical findings strongly suggest that DNase IIβ is a lysosomal enzyme in the lens.
The question arises of how a lysosomal enzyme might gain access to the nuclear compartment during fiber cell differentiation. One possibility is that lysosomes fuse with the nuclear envelope and thereby deliver their enzymatic cargo. This process might be akin to autophagosome formation, although it is important to note that autophagy, per se, is not implicated in organelle breakdown in the lens (Matsui et al. 2006). DNase IIβ-containing lysosomes have been observed in close association with nuclei at the border of the OFZ and ultrastructural studies have identified DNase IIβ within the nuclear compartment in degenerating nuclei (Nakahara et al. 2007).
Although DNase IIβ is clearly critical for denucleation, it may not be the only nuclease involved. Progressive changes in chromatin organization occur in the persistent nuclei found in the center of the DNase IIβ-null lens. These changes, which include chromatin fragmentation and clumping, suggest that the DNA may be subjected to further digestion over time by other nucleases. Several classes of DNase are known to be present in the lens that could perform such an ancillary role (Counis et al. 1998).
Proteolytic Mechanisms
Studies of the proteolytic mechanisms underlying organelle breakdown have been strongly influenced by developments in the field of programmed cell death. The similarities between organelle breakdown and apoptosis have been noted by numerous authors (Dahm 1999; Wride 2000). Fiber cells express most or all components of the cell death apparatus (Wride et al. 1999) and die by apoptosis in response to sufficiently noxious stimuli (Zandy et al. 2005). Several studies have further suggested that elements of the cell death machinery are utilized during organelle breakdown (Sanders and Parker 2003) which, according to this view, constitutes a form of “attenuated” cell death. In support of this notion, retroviral expression of Bcl2, an anti-apoptotic molecule, reduces or delays fiber cell denucleation in embryonic chicken lenses (Sanders and Parker 2003). A similar inhibitory effect on organelle breakdown was noted in studies utilizing mice expressing a Bcl2 transgene in the lens (Fromm and Overbeek 1997).
Authentic apoptotic cell death can be triggered in response to extrinsic or intrinsic signals but, in each case, the cell death pathways converge on a conserved family of cysteine proteases called caspases. Following receipt of an apoptotic signal, caspases are cleaved from inactive precursors to active mature forms. Initiator caspases (caspase-2, -8, and -9) generally act upstream of effector caspases (caspase-3, -6, and -7). The effector caspases in turn cleave critical regulatory or structural proteins such as poly(ADP-ribose) polymerase, lamins, spectrin, and DNA fragmentation factor (all of which are cleaved during organelle breakdown). Western blotting experiments have revealed that several caspases are expressed in the lens (Wride et al. 1999; Zandy et al. 2005) and it has been suggested that at least one of these, caspase-3, is directly involved in organelle breakdown (Ishizaki et al. 1998). Lens caspase activity has been measured using fluorogenic assays. Of the caspase-like activities recovered from rodent lens lysates, VEIDase activity (caspase-6-like activity) is the most prominent (Foley et al. 2004; Zandy et al. 2005). Significantly, VEIDase activity peaks during the developmental period in which organelle degradation commences (Foley et al. 2004), suggesting that caspase-6, or a caspase-6-like enzyme might be implicated in organelle breakdown.
If organelle breakdown represents a form of apoptosis, it has been difficult to explain why the process does not proceed to complete cell death, with all its cytological hallmarks (breakdown of the cytoskeleton, membrane blebbing, formation of apoptotic bodies, etc.). In other systems, cell death can be prevented by αB crystallin, a chaperone molecule with the ability to inhibit the maturation of pro-caspase-3 (Kamradt et al. 2002). Fiber cells contain extraordinarily high levels of α-crystallin, which has both refractive and chaperone functions in the lens. It has been proposed that the presence of α-crystallin serves to blunt the apoptotic cascade in cells undergoing organelle breakdown. In support of this hypothesis, massive apoptotic cell death ensues in the center of lenses lacking αA and αB crystallin (Morozov and Wawrousek 2006).
Despite a preponderance of circumstantial evidence supporting the hypothesis that organelle breakdown in the lens is an apoptosis-like event, recent data have cast doubt on this conclusion. First, there is no direct evidence for caspase activation in cells bordering the OFZ. Specific antibodies against activated caspase-3 do not label cells undergoing organelle breakdown, although the same antibodies label cells in the epithelium undergoing genuine apoptosis (Zandy et al. 2005). Organelle breakdown is not inhibited in mice lacking caspase-3, -6, -7, (the effector caspases shown to be expressed in the lens) or a combination of caspase-3 and -6. Although caspase-like activities can be recovered from lens lysates these are most likely due to the postglutamyl peptide hydrolyzing (PGPH) domain of the proteasome. Using caspase-null mice as controls, no evidence was found for the presence of activated caspases in the healthy lens (Zandy and Bassnett 2007). It is also noteworthy that caspase activated deoxyribonuclease (CAD), the enzyme that promotes DNA cleavage in apoptotic cells (Enari et al. 1998) is not required for organelle breakdown in the lens (Zandy 2006), where chromatin degradation occurs through the action of the unrelated nuclease, DNAse IIβ (Nishimoto et al. 2003).
Although the contribution of caspases to lens organelle degradation is still unresolved, recent data suggest an important role for the ubiquitin proteasome pathway (UPP). The UPP is the major non-lysosomal protein degradation system in cells. Through it, proteins are first tagged for destruction via covalent attachment of a polyubiquitin chain. The chain is added by the coordinated activity of three classes of enzyme: a ubiquitin activating enzyme (E1), a ubiquitin conjugating enzyme (E2) and a ubiquitin ligase (E3). The ubiquitinated substrate is targeted to the 26S proteasome for destruction. Lens fiber cells express a fully function UPP (Pereira et al. 2003) and it has been demonstrated that core components of the UPP redistribute during fiber cell differentiation, many accumulating in the nuclear compartment (Girao et al. 2005) consistent with a role in organelle breakdown.
In the developing chicken lens, mitochondria and other organelles are degraded in the central fiber cells on or about E12 (Bassnett and Beebe 1992). Western blotting experiments have demonstrated that the mitochondrial marker protein, succinate-ubiquinone oxidoreductase (part of complex II of the oxidative phosphorylation pathway and a component of the inner mitochondrial membrane), disappears from the central cells in the period E9–E13. In vivo application of the UPP inhibitor lactacystin prevents the clearance of succinate-ubiquinone oxidoreductase from the central fiber cells during this period (Zandy and Bassnett 2007) providing the first direct evidence that the UPP is involved in the elimination of organelle components from differentiating lens fiber cells. These studies need to be extended to determine whether all organelle components are ultimately cleared from the cell through the UPP.
Future perspectives
Much has been learned about the process of lens organelle degradation in the past five years, but many important questions are unresolved. A striking feature of organelle breakdown is the sharp transition between cells than contain organelles and those that do not but little is known about the spatial cues that trigger organelle degradation in cells located a specific depth from the lens surface. Standing gradients of oxygen are established within the lens (McNulty et al. 2004) and it is possible that these constitute both a spatial cue and a physiological trigger for organelle breakdown (Bassnett and McNulty 2003). It has also been proposed that in both erythrocytes and lens fiber cells, an initial stage in the organelle breakdown process might be the specific permeabilization of organelle membranes through incorporation of the enzyme 15-lipoxgenase (van Leyen et al. 1998). This intriguing hypothesis is supported by studies indicating that inhibitors of 15-lipoxygenase block mitochondrial degradation in reticulocytes (Grullich et al. 2001) and that ectopic expression of 15-lipoxygenase collapses the mitochondrial pH gradient in non-erythroid cells (Vijayvergiya et al. 2004). However, erythrocyte maturation is not impaired in mice deficient in 12-lipoxygenase, the mouse equivalent of human and rabbit 15-lipoxygenase (Sun and Funk 1996), and no ocular defects have been noted, suggesting that if 15-lipoxygenase has a role in organelle breakdown it may not be indispensable.
It seems likely that cell signaling pathways may operate during organelle degradation but the nature and role of such pathways is currently a matter of speculation. Mitochondria and endoplasmic reticulum are major cellular calcium stores and it is expected that with their dissolution calcium will be released into the cytosol. Interestingly, free calcium levels are elevated in the cytoplasm of central, organelle-free cells compared to organelle-containing cells near the periphery (Gao et al. 2004). The gradient is relatively steep (300 – 700 nM) but it is not known whether it arises as a consequence of organelle breakdown. Whatever its origin, calcium-dependent signaling pathways might be activated as cells traverse the calcium gradient during their differentiation. Similarly, calcium dependent proteases (calpains), which have been studied intensively in the lens due to their possible involvement in cataract formation (Biswas et al. 2004), might be differentially activated across the radial calcium gradient and thereby have a role in organelle degradation or other aspects of fiber cell differentiation.
Figure 1.
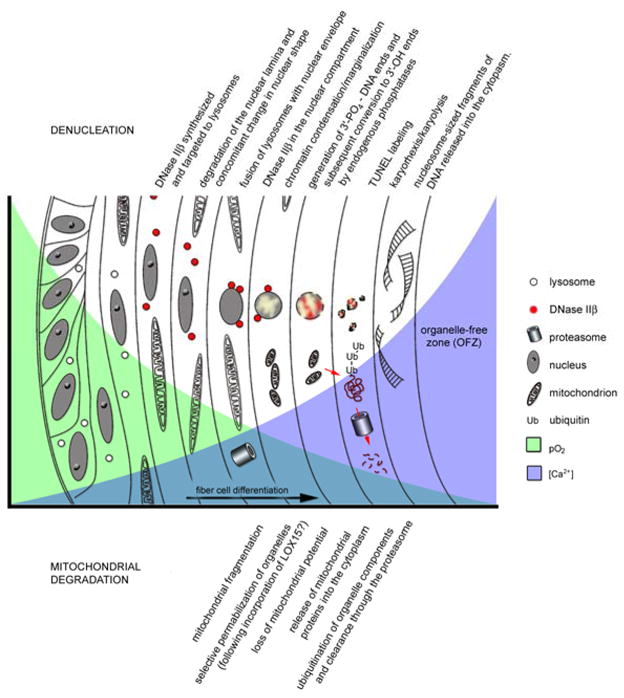
Organelle degradation in differentiating lens fiber cells.
Acknowledgments
This study was supported by NIH grants R01EY09852 and EY02687 (Core grant for vision research), and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness (RPB). The author is the recipient of an RPB Wasserman award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnheim G. Coagulationsnekrose und Kernschwund. Virchows Arch Pathol Anat. 1890;120:367–383. [Google Scholar]
- Augusteyn RC, Jones CE, Pope JM. Age-related development of a refractive index plateau in the human lens: evidence for a distinct nucleus. Clin Exp Optom. 2008;91:296–301. doi: 10.1111/j.1444-0938.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Curr Eye Res. 1992;11:1227–1232. doi: 10.3109/02713689208999548. [DOI] [PubMed] [Google Scholar]
- Bassnett S. The fate of the Golgi apparatus and the endoplasmic reticulum during lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 1995;36:1793–1803. [PubMed] [Google Scholar]
- Bassnett S. Fiber cell denucleation in the primate lens. Invest Ophthalmol Vis Sci. 1997;38:1678–1687. [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev Dyn. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Mataic D. Chromatin degradation in differentiating fiber cells of the eye lens. J Cell Biol. 1997;137:37–49. doi: 10.1083/jcb.137.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S, McNulty R. The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol. 2003;206:4353–4361. doi: 10.1242/jeb.00670. [DOI] [PubMed] [Google Scholar]
- Beauvoit B, Evans SM, Jenkins TW, Miller EE, Chance B. Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal Biochem. 1995;226:167–174. doi: 10.1006/abio.1995.1205. [DOI] [PubMed] [Google Scholar]
- Beauvoit B, Kitai T, Chance B. Contribution of the mitochondrial compartment to the optical properties of the rat liver: a theoretical and practical approach. Biophys J. 1994;67:2501–2510. doi: 10.1016/S0006-3495(94)80740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Harris F, Singh J, Phoenix D. Role of calpains in diabetes mellitus-induced cataractogenesis: a mini review. Mol Cell Biochem. 2004;261:151–159. doi: 10.1023/b:mcbi.0000028750.78760.6f. [DOI] [PubMed] [Google Scholar]
- Brunsting A, Mullaney PF. Differential light scattering from spherical mammalian cells. Biophys J. 1974;14:439–453. doi: 10.1016/S0006-3495(74)85925-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC. Measurement of refractive index in an intact crystalline lens. Vision Res. 1984;24:409–415. doi: 10.1016/0042-6989(84)90039-7. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Johnsen S, Gilliland KO, Freel CD, Fowler WC. Predicted light scattering from particles observed in human age-related nuclear cataracts using mie scattering theory. Invest Ophthalmol Vis Sci. 2007;48:303–312. doi: 10.1167/iovs.06-0480. [DOI] [PubMed] [Google Scholar]
- Counis MF, Chaudun E, Arruti C, Oliver L, Sanwal M, Courtois Y, Torriglia A. Analysis of nuclear degradation during lens cell differentiation. Cell Death Differ. 1998;5:251–261. doi: 10.1038/sj.cdd.4400351. [DOI] [PubMed] [Google Scholar]
- Dahm R. Lens fibre cell differentiation - A link with apoptosis? Ophthalmic Res. 1999;31:163–183. doi: 10.1159/000055530. [DOI] [PubMed] [Google Scholar]
- Dahm R. Dying to see. Sci Am. 2004;291:82–89. doi: 10.1038/scientificamerican1004-82. [DOI] [PubMed] [Google Scholar]
- Dahm R, Gribbon C, Quinlan RA, Prescott AR. Changes in the nucleolar and coiled body compartments precede lamina and chromatin reorganization during fibre cell denucleation in the bovine lens. Eur J Cell Biol. 1998;75:237–246. doi: 10.1016/S0171-9335(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Dahm R, Prescott AR. Morphological changes and nuclear pore clustering during nuclear degradation in differentiating bovine lens fibre cells. Ophthalmic Res. 2002;34:288–294. doi: 10.1159/000065605. [DOI] [PubMed] [Google Scholar]
- De Maria A, Arruti C. DNase I and fragmented chromatin during nuclear degradation in adult bovine lens fibers. Mol Vis. 2004;10:74–82. [PubMed] [Google Scholar]
- De Maria A, Bassnett S. DNase IIbeta distribution and activity in the mouse lens. Invest Ophthalmol Vis Sci. 2007;48:5638–5646. doi: 10.1167/iovs.07-0782. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Faulkner-Jones B, Zandy AJ, Bassnett S. RNA stability in terminally differentiating fibre cells of the ocular lens. Exp Eye Res. 2003;77:463–476. doi: 10.1016/s0014-4835(03)00172-6. [DOI] [PubMed] [Google Scholar]
- Foley JD, Rosenbaum H, Griep AE. Temporal regulation of VEID-7-amino-4-trifluoromethylcoumarin cleavage activity and caspase-6 correlates with organelle loss during lens development. J Biol Chem. 2004;279:32142–32150. doi: 10.1074/jbc.M313683200. [DOI] [PubMed] [Google Scholar]
- Fromm L, Overbeek PA. Inhibition of cell death by lens-specific overexpression of bcl-2 in transgenic mice. Dev Genet. 1997;20:276–287. doi: 10.1002/(SICI)1520-6408(1997)20:3<276::AID-DVG10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Gao J, Sun X, Martinez-Wittinghan FJ, Gong X, White TW, Mathias RT. Connections between connexins, calcium, and cataracts in the lens. J Gen Physiol. 2004;124:289–300. doi: 10.1085/jgp.200409121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland KO, Johnsen S, Metlapally S, Costello MJ, Ramamurthy B, Krishna PV, Balasubramanian D. Mie light scattering calculations for an Indian age-related nuclear cataract with a high density of multilamellar bodies. Mol Vis. 2008;14:572–582. [PMC free article] [PubMed] [Google Scholar]
- Girao H, Pereira P, Taylor A, Shang F. Subcellular redistribution of components of the ubiquitin-proteasome pathway during lens differentiation and maturation. Invest Ophthalmol Vis Sci. 2005;46:1386–1392. doi: 10.1167/iovs.04-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, Hrabe de Angelis M. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res. 2001;73:867–876. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- Gribbon C, Dahm R, Prescott AR, Quinlan RA. Association of the nuclear matrix component NuMA with the Cajal body and nuclear speckle compartments during transitions in transcriptional activity in lens cell differentiation. Eur J Cell Biol. 2002;81:557–566. doi: 10.1078/0171-9335-00275. [DOI] [PubMed] [Google Scholar]
- Grullich C, Duvoisin RM, Wiedmann M, van Leyen K. Inhibition of 15-lipoxygenase leads to delayed organelle degradation in the reticulocyte. FEBS Lett. 2001;489:51–54. doi: 10.1016/s0014-5793(01)02080-4. [DOI] [PubMed] [Google Scholar]
- He HY, Gao C, Vrensen G, Zelenka P. Transient activation of cyclin B/Cdc2 during terminal differentiation of lens fiber cells. Dev Dyn. 1998;211:26–34. doi: 10.1002/(SICI)1097-0177(199801)211:1<26::AID-AJA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Jacobson MD, Raff MC. A role for caspases in lens fiber differentiation. J Cell Biol. 1998;140:153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D, Dvoriantchikova G, Pestova A, Nathanson L, Shestopalov VI. Microarray analysis of fiber cell maturation in the lens. FEBS Lett. 2005;579:1213–1219. doi: 10.1016/j.febslet.2005.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S, Widder EA. The physical basis of transparency in biological tissue: ultrastructure and the minimization of light scattering. J Theor Biol. 1999;199:181–198. doi: 10.1006/jtbi.1999.0948. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008;49:2531–2540. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Imaizumi M. Denucleation process of the lens. Invest Ophthalmol. 1974;13:973–981. [PubMed] [Google Scholar]
- Landgren H, Blixt A, Carlsson P. Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2243. [DOI] [PubMed] [Google Scholar]
- Matsui M, Yamamoto A, Kuma A, Ohsumi Y, Mizushima N. Organelle degradation during the lens and erythroid differentiation is independent of autophagy. Biochem Biophys Res Commun. 2006;339:485–489. doi: 10.1016/j.bbrc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R, van Marle J, Vrensen GF, van den Berg TJ. Changes in the refractive index of lens fibre membranes during maturation--impact on lens transparency. Exp Eye Res. 2003;77:93–99. doi: 10.1016/s0014-4835(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Mie G. Beiträge zur optik trüber medien, speziell kolloidaler metallösungen. Annalen der Physik. 1908;25:377–445. [Google Scholar]
- Min JN, Zhang Y, Moskophidis D, Mivechi NF. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis. 2004;40:205–217. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- Modak SP, Bollum FJ. Detection and measurement of single-strand breaks in nuclear DNA in fixed lens sections. Exp Cell Res. 1972;75:307–313. doi: 10.1016/0014-4827(72)90434-x. [DOI] [PubMed] [Google Scholar]
- Morozov V, Wawrousek EF. Caspase-dependent secondary lens fiber cell disintegration in {alpha}A-/{alpha}B-crystallin double-knockout mice. Development. 2006;133:813–821. doi: 10.1242/dev.02262. [DOI] [PubMed] [Google Scholar]
- Mourant JR, Freyer JP, Hielscher AH, Eick AA, Shen D, Johnson TM. Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics. Appl Opt. 1998;37:3586–3593. doi: 10.1364/ao.37.003586. [DOI] [PubMed] [Google Scholar]
- Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunol Rev. 2007;220:237–250. doi: 10.1111/j.1600-065X.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- Nakahara M, Nagasaka A, Koike M, Uchida K, Kawane K, Uchiyama Y, Nagata S. Degradation of nuclear DNA by DNase II-like acid DNase in cortical fiber cells of mouse eye lens. Febs J. 2007;274:3055–3064. doi: 10.1111/j.1742-4658.2007.05836.x. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Kawane K, Watanabe-Fukunaga R, Fukuyama H, Ohsawa Y, Uchiyama Y, Hashida N, Ohguro N, Tano Y, Morimoto T, Fukuda Y, Nagata S. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–1074. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- Pereira P, Shang F, Hobbs M, Girao H, Taylor A. Lens fibers have a fully functional ubiquitin-proteasome pathway. Exp Eye Res. 2003;76:623–631. doi: 10.1016/s0014-4835(03)00020-4. [DOI] [PubMed] [Google Scholar]
- Rabl C. Uber den Bau und die Entwicklung der Linse. III. Die Linse der Saugetiere:Ruckblick und Schluss. Z Wiss Zool. 1899;67:1–138. [Google Scholar]
- Sanders EJ, Parker E. Retroviral overexpression of bcl-2 in the embryonic chick lens influences denucleation in differentiating lens fiber cells. Differentiation. 2003;71:425–433. doi: 10.1046/j.1432-0436.2003.7107005.x. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Exogenous gene expression and protein targeting in lens fiber cells. Invest Ophthalmol Vis Sci. 1999;40:1435–1443. [PubMed] [Google Scholar]
- Shiokawa D, Tanuma S. DLAD, a novel mammalian divalent cation-independent endonuclease with homology to DNase II. Nucleic Acids Res. 1999;27:4083–4089. doi: 10.1093/nar/27.20.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Hose S, Zhang C, Neal R, Ghosh M, O’Brien TP, Sundin O, Goldberg MF, Robison WG, Jr, Russell P, Lo WK, Samuel Zigler J., Jr A spontaneous mutation affects programmed cell death during development of the rat eye. Exp Eye Res. 2005;80:323–335. doi: 10.1016/j.exer.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-Llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RN, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JS., Jr betaA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2007 doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Wyatt MK, Sarra R, Jaworski C, Slingsby C, Thaung C, Pannell L, Robison WG, Favor J, Lyon M, Wistow G. A temperature-sensitive mutation of Crygs in the murine Opj cataract. J Biol Chem. 2001;276:9308–9315. doi: 10.1074/jbc.M010583200. [DOI] [PubMed] [Google Scholar]
- Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem. 1996;271:24055–24062. [PubMed] [Google Scholar]
- Treton JA, Shinohara T, Piatigorsky J. Degradation of delta-crystallin mRNA in the lens fiber cells of the chicken. Dev Biol. 1982;92:60–65. doi: 10.1016/0012-1606(82)90150-6. [DOI] [PubMed] [Google Scholar]
- van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- Vasiliev O, Rhodes SJ, Beebe DC. Identification and expression of Hop, an atypical homeobox gene expressed late in lens fiber cell terminal differentiation. Mol Vis. 2007;13:114–124. [PMC free article] [PubMed] [Google Scholar]
- Vijayvergiya C, De Angelis D, Walther M, Kuhn H, Duvoisin RM, Smith DH, Wiedmann M. High-level expression of rabbit 15-lipoxygenase induces collapse of the mitochondrial pH gradient in cell culture. Biochemistry. 2004;43:15296–15302. doi: 10.1021/bi048745v. [DOI] [PubMed] [Google Scholar]
- Vrensen GF, Graw J, De Wolf A. Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp Eye Res. 1991;52:647–659. doi: 10.1016/0014-4835(91)90017-9. [DOI] [PubMed] [Google Scholar]
- Vrensen GF, van Marle J, Jonges R, Voorhout W, Breipohl W, Wegener AR. Tryptophan deficiency arrests chromatin breakdown in secondary lens fibers of rats. Exp Eye Res. 2004;78:661–672. doi: 10.1016/j.exer.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Wang K, Cheng C, Li L, Liu H, Huang Q, Xia CH, Yao K, Sun P, Horwitz J, Gong X. GammaD-crystallin associated protein aggregation and lens fiber cell denucleation. Invest Ophthalmol Vis Sci. 2007;48:3719–3728. doi: 10.1167/iovs.06-1487. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem. 2005;280:22135–22145. doi: 10.1074/jbc.M414270200. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Coyle BM, Piatigorsky J, Papagiotas S, Libby D. Ectopic expression of AP-2alpha transcription factor in the lens disrupts fiber cell differentiation. Dev Biol. 2002;245:13–27. doi: 10.1006/dbio.2002.0624. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Bigelow CE, Calkins DJ, Foster TH. Light scattering from intact cells reports oxidative-stress-induced mitochondrial swelling. Biophys J. 2005;88:2929–2938. doi: 10.1529/biophysj.104.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Wride MA. Minireview: apoptosis as seen through a lens. Apoptosis. 2000;5:203–209. doi: 10.1023/a:1009653326511. [DOI] [PubMed] [Google Scholar]
- Wride MA, Parker E, Sanders EJ. Members of the bcl-2 and caspase families regulate nuclear degeneration during chick lens fibre differentiation. Dev Biol. 1999;213:142–156. doi: 10.1006/dbio.1999.9375. [DOI] [PubMed] [Google Scholar]
- Wyatt K, Gao C, Tsai JY, Fariss RN, Ray S, Wistow G. A role for lengsin, a recruited enzyme, in terminal differentiation in the vertebrate lens. J Biol Chem. 2008;283:6607–6615. doi: 10.1074/jbc.M709144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wu TT, Qu JY. Unified Mie and fractal scattering by cells and experimental study on application in optical characterization of cellular and subcellular structures. J Biomed Opt. 2008;13:024015. doi: 10.1117/1.2907790. [DOI] [PubMed] [Google Scholar]
- Zandy AJ. Department of Ophthalmology and Visual Sciences. Washington University; St. Louis: 2006. PhD thesis: Lens Organelle Degradation. [Google Scholar]
- Zandy AJ, Bassnett S. Proteolytic mechanisms underlying mitochondrial degradation in the ocular lens. Invest Ophthalmol Vis Sci. 2007;48:293–302. doi: 10.1167/iovs.06-0656. [DOI] [PubMed] [Google Scholar]
- Zandy AJ, Lakhani S, Zheng T, Flavell RA, Bassnett S. Role of the executioner caspases during lens development. J Biol Chem. 2005;280:30263–30272. doi: 10.1074/jbc.M504007200. [DOI] [PubMed] [Google Scholar]


