Abstract
Large epidemiological samples, including the National Collaborative Perinatal Project (NCPP), in which blood/serum was collected during pregnancy and offspring followed longitudinally, offer the unique opportunity to examine neuroendocrine mechanisms underlying prenatal “programming” of adult health and disease. However, in order to conduct longitudinal analyses, it is critical to determine the validity of maternal prenatal samples stored over long periods. We investigated the validity of cortisol, testosterone, and their binding globulins (corticosteroid binding globulin (CBG) and sex hormone binding globulin (SHBG)) in maternal prenatal serum from the NCPP after over 40 years of storage. Study 1 included 64 maternal serum samples collected on the day of delivery; study 2 involved 1099 third trimester serum samples collected between gestational weeks 31 and 36. Across both studies, cortisol and testosterone concentrations were consistent with values from published studies of fresh samples collected at similar points in gestation. CBG and SHBG were present but showed some differences from published studies. Results support the validity of cortisol and testosterone values following 40+ years of storage. Results also provide validation for future longitudinal tests of prenatal “programming” hypotheses within the NCPP. Stability of steroid hormones over decades suggests that stored samples from other longitudinal studies may also allow opportunities to investigate links between prenatal steroids and long-term offspring outcomes.
Keywords: maternal, pregnancy, serum, cortisol, testosterone, validity, storage, prenatal, programming, glucocorticoid
Introduction
Exposure to excessive maternal steroid hormones is a prominent proposed mechanism underlying prenatal “programming” of later offspring physiology and disease (Goy and McEwen, 1980; Forest, 1983; Berenbaum and Hines, 1992; Grimshaw et al, 1995; Barbazanges et al, 1996; Lutchmaya et al, 2002; Birch et al, 2003; Seckl, 2004; Steckler et al, 2005; Weinstock, 2005). Large epidemiological samples, including the National Collaborative Perinatal Project (NCPP), in which blood/serum was collected during pregnancy and offspring were followed longitudinally, offer the unique opportunity to examine links between prenatal exposure to steroid hormones and long-term offspring outcomes. However, although steroid hormones are believed to be some of the most stable and resilient molecules to variations in storage duration and conditions (Kley and Rick, 1984; Miki and Sudo, 1998; Garde and Hansen, 2005), the validity of prenatal levels of steroid hormones after decades of storage has not been determined. As cortisol and testosterone have been proposed as key mediators of prenatal “programming,” we examined stability of concentrations of maternal cortisol and testosterone and their binding globulins (cortisol binding globulins (CBG) and sex hormone binding globulin (SHBG)) after more than forty years of storage. Concentrations were examined in serum stored from participants in the New England Cohorts of the NCPP who were followed to adulthood.
Several types of previous studies have examined the stability of serum and plasma cortisol, testosterone, CBG, and SHBG in relation to variations in storage time and conditions. Studies designed to examine the effects of storage time on serum/plasma levels of steroid hormones have compared levels of cortisol and testosterone from the same samples immediately after collection and after years of storage. In these investigations, testosterone and cortisol concentrations were stable at levels consistent with typical coefficients of variation after up to 10–11 years of storage (Wickings and Nieschlag, 1976; Kubasik et al, 1982; Das et al, 1983; Kley and Rick, 1984; Kley et al, 1985; Diver et al, 1994; Bolelli et al, 1995; Bogdan, 2000, Personal Communication). Other studies have examined effects of variation in storage conditions (e.g., repeated freeze-thaw cycles, storage at varied temperatures) on cortisol and testosterone concentrations (Wickings and Nieschlag, 1976; Kubasik et al, 1982; Das et al, 1983; Kley and Rick, 1984; Garde and Hansen, 2005) as well as CBG and SHBG (Kley and Rick, 1984; Sinnecker, 1989; Reyna et al, 2001). These studies have also revealed stability of cortisol, testosterone, CBG, and SHBG to common variations in storage conditions.
Although not designed to examine stability of hormones over time, several large epidemiological studies (e.g., Multiple Risk Factor Intervention Trial (MRFIT), Baltimore Longitudinal Study of Aging) involved assays for testosterone and SHBG in samples from non-pregnant adults stored for up to 34 years (Lapidus et al, 1986; Cauley et al, 1987; Barrett-Connor et al, 1990; Garland et al, 1992; Haffner et al, 1993; Barrett-Connor and Goodman-Gruen, 1995; Haffner et al, 1996; Zmuda et al, 1997; Harman et al, 2001; Moffat et al, 2002). Most described stability of testosterone and SHBG to years of storage. However, many of these studies provided only brief descriptions of how stability of testosterone and SHBG was determined. Cauley et al. (1987), Lapidus et al. (1986), and Harman et al. (2001) provided somewhat more detailed explanations of analyses to determine effects of storage time on testosterone concentrations. Cauley et al. (1987) found no differences between testosterone concentrations from samples stored for 10 years relative to a pooled sample from healthy volunteers or between samples stored for 10 versus 11 years. Lapidus et al. (1986) found no differences in SHBG concentrations after 16 years of storage, but a seven percent decline in CBG compared to pooled fresh samples. Harman et al. (2001) found evidence for a slow, curvilinear increase in testosterone concentrations and decrease in SHBG concentration with increasing years of storage over 34 years. However, we know of no studies that have assayed samples stored over decades for cortisol.
Finally, although levels of steroid hormones increase dramatically over pregnancy, little is known about the stability and validity of stored samples of prenatal steroid hormones. Two studies from the NCPP and one additional longitudinal study assayed prenatal serum samples for testosterone after twenty to forty years of storage (Henderson et al, 1988; Udry et al, 1995; Zhang et al, 2005). However, similar to studies of non-pregnant adults, limited description was provided of how concentrations of maternal prenatal testosterone were validated. In addition, we know of no studies investigating the validity of stored maternal prenatal cortisol or CBG samples.
Thus, although previous studies suggest that steroid hormones and their binding globulins are stable over time and to variations in storage conditions, the stability of cortisol, testosterone, CBG, and SHBG in stored maternal prenatal samples over decades is not clear. In this investigation, we present results from two validation studies from the NCPP. Study 1 is a pilot study of maternal serum samples collected on the day of delivery; Study 2 focuses on maternal serum samples collected during the third trimester of pregnancy. Our aims were twofold: a) to assess whether cortisol, testosterone, CBG, and SHBG were still present in the prenatal serum samples after over forty years of storage and b) to compare serum concentrations from prenatal samples stored for over forty years with concentrations from published studies of fresh prenatal samples.
Method
Overview of NCPP
The NCPP was a 12-site study of perinatal factors affecting birth and child outcomes (Niswander and Gordon, 1972; Broman et al, 1985; Broman et al, 1987). Over fifty thousand pregnant mothers were enrolled between 1959 and 1966 through prenatal clinics. Maternal serum samples were collected at each prenatal visit and on the day of delivery for enrolled participants. As this study was conducted in conjunction with the provision of routine prenatal care, study participants were informed of the study procedures but no formal informed consent was obtained, as was standard research practice at this time.
Participants/Sample Selection
Participants for the current investigation were selected from a subset of the approximately 17,000 offspring enrolled in the Boston and Providence cohorts of the NCPP. As part of an ongoing study of early environmental and genetic risks for nicotine dependence, the current investigative team had followed and interviewed 1636 offspring as adults (mean age 39 years, SD = 1.9 yrs). All of these recent study participants provided written informed consent, following procedures which had been reviewed and approved by Human Subjects Committees at The Miriam Hospital and the Harvard School of Public Health. From this sample, we conducted an initial pilot study (Study 1) that included serum samples from 64 mothers collected on the day of delivery. Serum samples were selected from mothers who gave birth to live, singleton infants. Mean maternal age at pregnancy was 25.4 years (SD = 5.7). The composition of mothers for this pilot was also selected to include 50% African American mothers and 50% Caucasian mothers, and equal numbers of participants of low and high socio-economic status (SES). SES was based on the lowest and highest quartile of a composite variable based on education, occupation, and income levels assessed at the first prenatal visit (Myrianthopoulos and French, 1968). Offspring included 53% males and 47% females. Mean gestational age at birth was 39.9 weeks (SD = 2.2 weeks) after the last menstrual period.
Study 2 included serum samples from 1099 mothers collected during the third trimester of pregnancy. Samples selected for Study 2 were drawn between 31 and 36 weeks following the last menstrual period and at least 14 days prior to the infant’s birth date, given known effects of labor/delivery on steroid hormone levels (Tuimala et al, 1976; Carr et al, 1981; Vogl et al, 2006). If mothers had more than one draw during weeks 31–36, the latest draw within this window was selected. Weeks 31–36 were selected a) because of links in the prior literature between third trimester hormone levels and stress and offspring neurobehavioral outcomes (Murphy, 1982; Huizink et al, 2003; Gutteling et al, 2004; Gutteling et al, 2005; O’Connor et al, 2005; Davis et al, 2006, Unpublished Data), b) because these weeks both provided a relatively tight window within third trimester to examine hormone levels, and c) because these weeks included the greatest number of participants with available serum samples. Mean gestational age at the time samples were collected was 32.9 weeks (SD = 1.5 weeks) after the last menstrual period.
Serum samples in Study 2 were selected from mothers who gave birth to live, singleton infants. Mean maternal age was 25.0 years (SD = 5.8); 87.5% of mothers were Caucasian, 11.7% African-American, and .7 % other races. Mean SES on the composite scale described above was 5.6 (SD = 1.9) on a 10-point scale. Offspring included 59% males and 41% females. Mean gestational age at birth (based on last menstrual period) was 40.1 weeks (SD = 2.1).
Sample Collection, Storage, and Assays
Non-fasting maternal blood was collected at each prenatal visit and on the day of delivery. No record was available for time of day or recency of food intake relative to blood collection. At least 30 ml of maternal blood was drawn, yielding approximately 15 ml of serum per participant. Samples were refrigerated overnight and then centrifuged at 2000 rpm. Serum was removed with volumetric pipette and distributed equally into 4 sterilized glass vials with not more than 3.2 ml per vials. Vials were tightly sealed with rubber-lined screw caps, stored at –15 to –20 degrees Celsius at research sites, then shipped on dry ice within 3 weeks of collection to a central repository in Bethesda, MD for long-term storage at –20 degrees Celsius. Samples were collected between 1959 and 1966. Ninety-four percent of blood samples were collected between 1960 and 1966, and 63% between 1961 and 1965 (Median year of collection was = 1963). Average length of storage was 42.5 years (SD = 1.7). Samples selected for Studies 1 and 2 were shipped on dry ice by express courier directly from the repository to the laboratory of Clemens Kirschbaum, Ph.D., University of Duesseldorf, Germany. For Study 1, samples from 64 participants were thawed and 0.5 ml aliquots separated and shipped. For Study 2, samples from 1150 participants were shipped in their original glass vials. Five vials arrived broken and 28 vials were revealed to be empty, leaving 1099 samples for all Study 2 analyses.
Samples were assayed for total cortisol, total testosterone, CBG, and SHBG. Because stored serum from the NCPP is limited, all assays were conducted using kits designed for use with small amounts of serum (0.5 ml for all four assays) from Immuno-Biological Laboratories (IBL) of Hamburg. Cortisol, testosterone, and SHBG were measured using enzyme-linked immunosorbent assay (ELISA) kits. CBG was measured using radioimmunoassay. Inter and intra-assay coefficients of variability ranged from 4–10%, 4–9%, 3–12%, and 3–8% for cortisol, testosterone, SHBG, and CBG, respectively. Detailed descriptions of assays and procedures followed can be found at www.ibl-hamburg.com.
Literature Review
The scientific literature was reviewed to determine concentrations and ranges for total cortisol and testosterone, CBG, and SHBG from published studies of fresh samples for comparison with values from Studies 1 and 2. Fresh samples were defined as samples that were not reported to be stored for an appreciable length of time in study methods. Two strategies were utilized to obtain a pool of relevant articles: 1) a review of references from major endocrinology textbooks describing concentrations of hormones and binding globulins across pregnancy, and 2) a Medline Search including “cortisol” or “testosterone” or “corticosteroid binding globulin”/“CBG” or “sex hormone binding globulin”/”SHBG”/“testosterone binding globulin” and “pregnancy”. Only studies that measured concentrations of hormones/binding globulins during third trimester of pregnancy or on the day of delivery in ≥ 10 healthy mothers were selected. At least 5 studies were selected per hormone/binding globulin to allow comparison across a range of studies.
Statistical Analyses
Summary statistics including mean, standard deviation, standard error and range were calculated for cortisol, testosterone, CBG and SHBG concentrations in NCPP Studies 1 and 2; Confidence intervals were determined using t distribution tables. Summary statistics (mean, standard error, and confidence intervals based on t statistic) were also derived for cortisol, testosterone, CBG, and/or SHBG for each published study to allow comparison with values from the present studies. For studies designed to examine concentrations of hormones or binding globulins in patient populations (e.g., mothers with hypertension or pre-eclampsia during pregnancy (Potter et al, 1987; Serin et al, 2001; Carlsen et al, 2005)), only values from the control group were examined. For studies in which samples were divided by group (e.g., weeks of pregnancy (Potter et al, 1987), or gender (van de Beek et al, 2004), summary statistics are presented separately for each group. For ease of comparison across studies, units for hormone and binding globulin concentrations were converted to the units utilized in assays for Studies 1 and 2: ng/ml for cortisol and testosterone, μg/ml for CBG, and nmol/l for SHBG. Molecular weights of 362.46 Daltons for cortisol, 288.43 Daltons for testosterone, and 55,000 Daltons for CBG were utilized for conversion between molar and metric (SI) units. (No conversions were necessary for SHBG studies.)
Values from NCPP Studies 1 and 2 were compared to published studies of fresh samples by examining the overlap in t confidence intervals between studies. Although 95% confidence intervals (CI’s) are typically reported for individual study means, the use of such confidence intervals when conducting pairwise study comparisons via the overlap method leads to a higher likelihood of finding no differences between the studies than the nominal 95% rate expected under the null hypothesis of no difference would suggest (Goldstein & Healy, 1995; Schenker & Gentleman, 2001). To properly calibrate these comparisons to a 5% Type I error rate, we have utilized 83% individual CI’s instead, as recommended by Austin and Hux (2002). This corresponds to narrowing the half-length of the t confidence intervals by about 30%, thus making study overlap less likely, and rejection of the null hypothesis more frequent. Two published studies (Carlsen et al, 2005; Troisi et al, 2003) did not include mean values, thus, t intervals were determined using the median and inter-quartile range. One study (Demey-Ponsart et al, 1982) reported mean concentrations but not variation around the mean; thus, CIs could not be determined and this study was not included in Figure 2. Mean values from this study are discussed in the text.
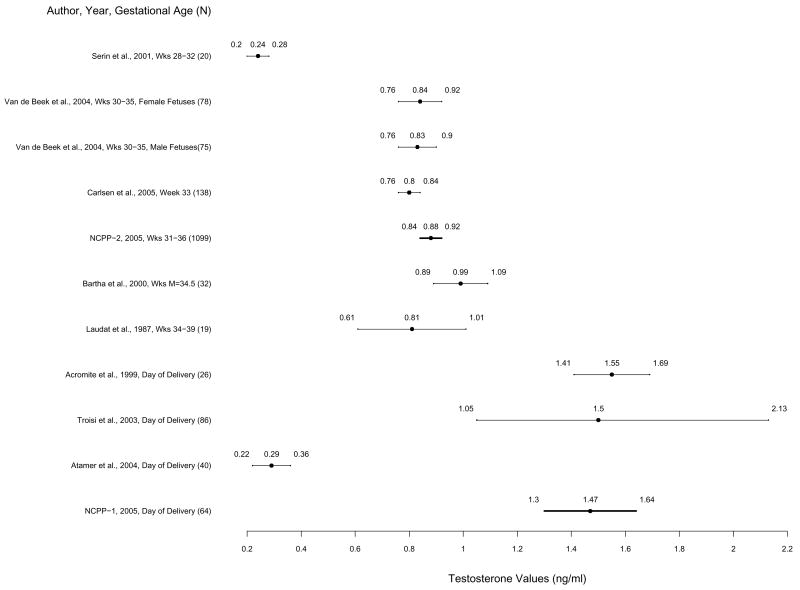
Figure 2.
Mean testosterone levels and 83% confidence intervals based on the t statistic for published studies and NCPP Studies 1 and 2 in order of week of pregnancy. Non-overlapping confidence intervals indicate statistically significant differences between studies by Student’s t test. Means and 83% confidence intervals from Studies 1 and 2 are highlighted in bold.
Results
Cortisol
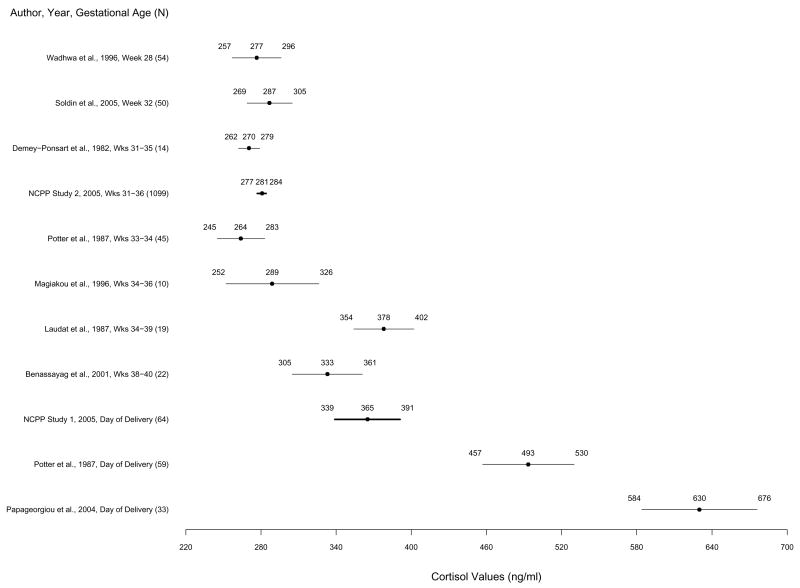
Total cortisol means and confidence intervals (83% CI’s) for Studies 1 and 2 and published studies are presented in Figure 1. In Study 1, mean cortisol concentration was 365 ng/ml (SD = 151). 83% CI was 339 to 391 ng/ml (sample range = 88 – 757 ng/ml). Study 1 cortisol concentrations were significantly higher than concentrations from studies of samples collected earlier in third trimester (28–36 weeks) (Demey-Ponsart et al, 1982; Potter et al, 1987; ; Magiakou et al, 1996; Wadhwa et al, 1996; Soldin et al, 2005) and NCPP Study 2 (31–36 weeks). Study 1 cortisol concentrations did not differ significantly from concentrations measured later in third trimester (34–40 weeks) (Laudat et al, 1987; Benassayag et al, 2001), but were significantly lower than concentrations from both studies of samples collected on the day of delivery (Potter et al, 1987; Papageorgiou et al, 2004).
Figure 1.
Mean cortisol levels and 83% confidence intervals based on the t statistic for published studies and NCPP Studies 1 and 2 in order of week of pregnancy. Non-overlapping confidence intervals indicate statistically significant differences between studies by Student’s t test. Means and 83% confidence intervals from Studies 1 and 2 are highlighted in bold.
In Study 2, mean cortisol concentration was 281 ng/ml (SD=84); 83% CI was 277 to 284 ng/ml (sample range = 3.8 – 795 ng/ml). Study 2 cortisol concentrations did not differ significantly from concentrations in five out of seven published studies of samples from third trimester (weeks 28–36) of pregnancy (Demey-Ponsart et al, 1982; Potter et al, 1987; Magiakou et al, 1996; Wadhwa et al, 1996; Soldin et al, 2005), but were significantly lower than concentrations from two small studies of samples collected late in third trimester (weeks 34–39 and 38–40) (Laudat et al, 1987; Benassayag et al, 2001) and both published studies of samples from the day of delivery (Potter et al, 1987; Papageorgiou et al, 2004).
Testosterone
Total testosterone means and 83% CI’s for Studies 1 and 2 and published studies are presented in Figure 2. In Study 1, mean testosterone concentration was 1.47 ng/ml (SD = .98); 83% CI was 1.30 to 1.64 ng/ml (sample range = .18 to 4.31 ng/ml). Study 1 testosterone concentrations were significantly higher than concentrations from all of samples collected during third trimester of pregnancy (weeks 28–39) (Laudat et al, 1987; Bartha et al, 2000; Serin et al, 2001; van de Beek et al, 2004; Carlsen et al, 2005), concentrations from NCPP Study 2, and one published study of samples collected on the day of delivery (Atamer et al, 2004). Testosterone concentrations in Study 1 overlapped with those measured around the time of delivery in two additional published studies (Acromite et al, 1999; Troisi et al, 2003).
In Study 2, mean testosterone concentration was .88 ng/ml (SD = .52); 83% CI was .84 to .92 ng/ml (range = .01 to 5.79 ng/ml). Concentrations were significantly higher than concentrations from one study of samples collected early in third trimester of pregnancy (weeks 28–32) (Serin et al, 2001) and one study of samples from the day of delivery (Atamer et al, 2004). Concentrations were not significantly different from four of five published studies of samples from third trimester (Weeks 30–39) of pregnancy (Laudat et al, 1987; Bartha et al, 2000; van de Beek et al, 2004; Carlsen et al, 2005), but were lower than the testosterone concentrations from two additional studies from the time of delivery (Acromite et al, 1999; Troisi et al, 2003).
Corticosteroid Binding Globulin (CBG)
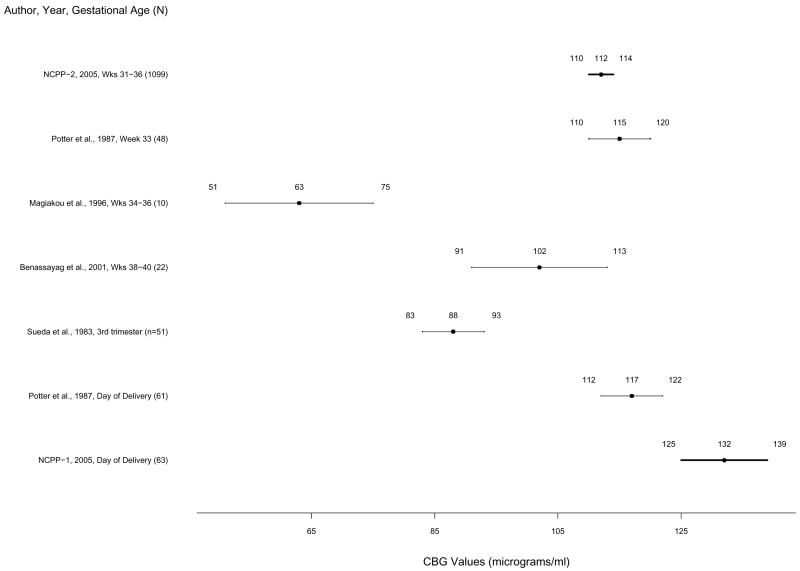
CBG means and 83% CI’s for Studies 1 and 2 and published studies are presented in Figure 3. In Study 1, mean CBG concentration was 143 μg/ml (SD = 90); 83% CI was 126 to 158 μg/ml (sample range = 68 to 788 μg/ml) with the inclusion of one extreme outlier (highest CBG value was 788 μg/ml, but next highest was 231 μg/ml). With the extreme outlier removed, mean CBG concentration was 132 μg/ml (SD = 38); 83% CI was 125 to 139 (sample range 68 to 231 μg/ml). Study 1 CBG concentrations with and without the outlier were significantly higher than CBG concentrations from all 4 published studies of samples collected during third trimester (Weeks 33–40) (Sueda et al, 1983; Potter et al, 1987; Magiakou et al, 1996; Benassayag et al, 2001) and samples from NCPP Study 2. Study 1 CBG concentrations were also significantly higher than those from the one published study of CBG concentrations around the time of delivery (Potter et al, 1987). Study 1 CBG concentrations overlapped with those from one additional published study of third trimester (week 31–35) CBG concentrations (M = 137.5 μg/ml, n=14, no variation around mean reported) (Demey-Ponsart et al, 1982).
Figure 3.
Mean corticosteroid binding globulin levels and 83% confidence intervals based on the t statistic for published studies and NCPP Studies 1 and 2 in order of week of pregnancy. The 83% confidence for NCPP Study 1 is based on exclusion of one extreme outlier. Non-overlapping confidence intervals indicate statistically significant differences between studies by Student’s t test. Means and 83% confidence intervals from Studies 1 and 2 are highlighted in bold.
In Study 2, mean CBG was 112 μg/ml (SD = 47); 83% CI range was 110 to 114 μg/ml (sample range = .25 to 348 μg/ml). Study 2 CBG concentrations were significantly higher than concentrations from two of four published studies of samples collected during third trimester (Sueda et al, 1983; Magiakou et al, 1996), but confidence intervals overlapped with those from two studies from third trimester (weeks 33–38) (Potter et al., 1987; Benassayag et al, 2001) and one study of samples collected on the day of delivery (Potter et al, 1987). Study 2 CBG concentrations were lower than those from the Demey-Ponsart et al. (1982) study described above.
Sex Hormone Binding Globulin (SHBG)
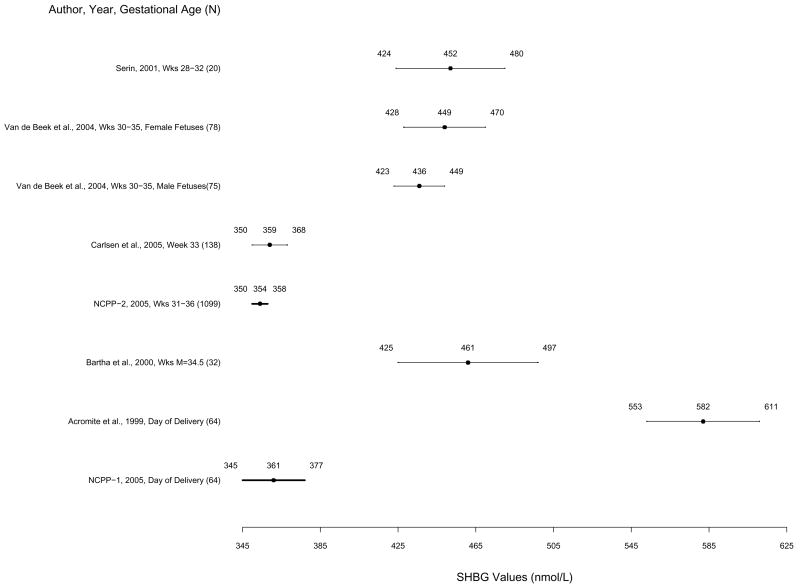
SHBG means and 83% CI’s for Studies 1 and 2 and published studies are presented in Figure 4. In Study 1, mean SHBG concentration was 361 nmol/l (SD = 94); 83% CI was 345 to 377 nmol/l (sample range = 159 to 561 nmol/l). In Study 2, mean SHBG concentration was 355 nmol/l (SD = 102); 83% CI was 350 to 358 nmol/l (sample range = 125 to 1047 nmol/l). SHBG concentrations from Studies 1 and 2 did not differ significantly from those of one of five published studies of samples collected during third trimester (week 33) (Carlsen et al, 2005) or from one another. SHBG concentrations from both studies were significantly lower than four out of five published studies from third trimester (Weeks 28–35) (Bartha et al, 2000; Serin et al, 2001; van de Beek et al, 2004) and one study from the day of delivery (Acromite et al, 1999), suggesting some degradation.
Figure 4.
Mean sex hormone binding globulin levels and 83% confidence intervals based on the t statistic for published studies and NCPP Studies 1 and 2 in order of week of pregnancy. Non-overlapping confidence intervals indicate statistically significant differences between studies by Student’s t test. Means and 83% confidence intervals from Studies 1 and 2 are highlighted in bold.
Discussion
Results from both studies reported here are among the few to examine validity of maternal prenatal steroid hormone and binding globulin concentrations in a large population-based sample after decades of storage. Across two sets of maternal prenatal serum samples from the National Collaborative Perinatal Project (NCPP), cortisol and testosterone concentrations measured after more than forty years of storage were consistent with published values from fresh prenatal samples. Concentrations of corticosteroid and sex hormone binding globulins (CBG and SHBG) after decades of storage were less consistent with published values from fresh samples. In particular, concentrations of SHBG were consistently lower than values from published studies suggesting some degradation.
In examining the validity of samples stored over long periods, there are two primary concerns: a) degradation of the substances of interest resulting in diminished hormone and binding globulin concentrations, and b) evaporation/desiccation of the medium leading to increases in hormone/binding globulin concentrations. With respect to cortisol, samples from third trimester (Study 2) appear to be well within the range of published studies from fresh samples suggesting little degradation or dessication over decades. To our knowledge, the present study is the first to examine the validity of cortisol samples stored over decades. Results extend prior studies showing stability of non-prenatal cortisol samples over years of storage (Kley and Rick, 1984; Kley et al, 1985; Bogdan, 2000, Personal Communication) to prenatal samples stored over decades. Samples from the day of delivery (Study 1), however, revealed lower cortisol concentrations compared to concentrations from two published day-of-delivery studies. Although it is possible that stored delivery samples showed increased degradation compared to third trimester samples, we believe the most likely explanation relates to differences in timing of sample collection relative to delivery. Specifically, in the two published studies, samples were collected simultaneous with delivery—likely the most stressful time of the labor-delivery period, while NCPP samples were collected across a range of times on the day of delivery—presumably including periods somewhat less stressful than delivery itself.
Similar to cortisol, testosterone concentrations from third trimester were within the range of values from most published studies. Day-of-delivery values were similar to values from two of three of published studies, but were higher than values from Atamer et al. (2004). It is notable, however, that testosterone values in the study by Atamer et al. were significantly lower than those from both NCPP studies, the additional two published studies from the time of delivery, and five of six published studies from third trimester of pregnancy, suggesting some idiosyncrasy in these results. In particular, while the majority of published studies of testosterone during pregnancy utilized radioimmunoassay, Atamer et al. utilized an automated immunoassay previously shown to result in lower concentrations relative to other methods and manufacturers (Taieb et al, 2003). Overall, similarity between values from the present study and published studies suggests little degradation or evaporation/desiccation over decades of storage. Results for testosterone are also consistent with several large epidemiological studies of non-pregnant adults in which stored samples were examined after years or decades of storage (Lapidus et al, 1986; Cauley et al, 1987; Barrett-Connor et al, 1990; Garland et al, 1992; Haffner et al, 1993; Barrett-Connor and Goodman-Gruen, 1995; Haffner et al, 1996; Zmuda et al, 1997; Feldman et al, 2002; Moffat et al, 2002), and extend these results to prenatal samples. Results contrast with one previous study of males (Harman et al, 2001), where testosterone concentrations were found to increase over three decades of storage. However, in this study, Harman et al found that SHBG competed with total testosterone for binding with the radioimmunoassay utilized to assess total testosterone concentrations. Because SHBG levels decreased over storage time, Harman et al. suggested that the increase in total testosterone was an artifact of a corresponding decrease in SHBG and decreased competition for binding with their radioimmunoassay. Our use of a different assay type (enzyme-linked immunoassay) to assess total testosterone in the present study may account for differences in effects of storage time on testosterone concentrations between studies.
In contrast to steroid hormones, binding globulin concentrations from stored samples were less consistent with concentrations from published studies of fresh samples. Day-of-delivery (Study 1) CBG concentrations were slightly but significantly higher than those from the one published delivery study (Potter et al, 1987) as well as values from all published third trimester studies. Third trimester (Study 2) CBG concentrations were consistent with values from some published studies from third trimester, higher than values from others, and lower than values from one study. Dessication can be ruled out as an explanation for higher CBG concentrations for third trimester and delivery values compared to some published studies, because dessication would be uniform across analytes (e.g., cortisol, testosterone, and SHBG levels would also be higher than published studies), which was not the case in the present study. We believe them most likely reason for possibly higher CBG values from NCPP compared to published studies from fresh samples is that differences reflect the use of alternative assays (e.g., immunodiffusion and competitive binding assays versus radioimmunoassay) across studies. However, we cannot rule out the possibility that decades of storage may have increased CBG concentrations in NCPP. Results for CBG are not consistent with the one epidemiological study of non-pregnant older women in which serum samples were examined after 16 years of storage (Lapidus et al, 1986). Lapidus et al found a decline in CBG compared to pooled fresh samples; however, description of methods to determine the differences between fresh and stored samples was minimal. CBG values from the present NCPP studies are more consistent with an increase in stored values of CBG relative to those from fresh samples. Thus, the impact of decades of storage on CBG remains equivocal. Because CBG may be utilized to calculate free cortisol indices, evidence for potentially higher CBG values in the present sample suggests that calculations of free cortisol based on both cortisol and CBG in the present sample may lead to lower than expected values.
Third trimester and day-of-delivery SHBG concentrations were lower than those from the majority of published studies, suggesting some degradation over decades of storage. Results are consistent with Harman et al. (2001), who found a slow, curvilinear decline in SHBG concentrations in males determined by radioimmunoassay over 34 years of storage. However, results contrast with those of Lapidus et al (1986), who found no differences in SHBG concentrations relative to pooled fresh samples after 16 years of storage. Again, however, a complete description of methods for comparing stored and fresh samples was not provided. Also potentially impacting both CBG and SHBG levels in NCPP is that nutritional status and insulin levels were not controlled or measured. This is also the case with many of the published studies to which the NCPP studies were compared. Both nutritional status and insulin levels have been shown to potently influence regulation of both CBG and SHBG (Haffner et al., 1989; Fernandez-Real et al, 1999, Tchernof and Despres, 2000; Fernandez-Real et al, 2001; Fernandez-Real et al, 2002; Lewis et al, 2004; Lewis et al, 2006) and should be measured and/or controlled in future studies of binding globulin levels during pregnancy. As SHBG may be utilized to calculate free androgen indices, evidence for degradation of SHBG in the NCPP suggests that higher than expected values of free androgen may emerge in the present sample.
The studies presented here offer a number of significant strengths from both methodological and theoretical perspectives. From a methodological perspective the large sample size for the NCPP third trimester study (Study 2; over 1000 participants) makes it one of the largest studies of stability of hormones and binding globulins in maternal serum stored over decades. Similarly, it is notable that across hormones and binding globulins, confidence intervals from Study 2 were much narrower than those from other published studies, likely due to its much larger sample size. From a theoretical perspective, the stability of steroid hormones from prenatal samples over decades has important implications for investigating mechanisms underlying links between prenatal stress and long-term offspring outcomes. To date, most evidence in support of “programming” mechanisms by steroid hormones has been derived from animal models and a small number of human studies in which offspring have not yet been followed until adulthood (Lutchmaya et al, 2002; de Weerth et al, 2003; Huizink et al, 2003; Gutteling et al, 2004; Gutteling et al, 2005). While it is critical to conduct longitudinal studies designed explicitly to examine steroid programming of later offspring outcomes, evidence for possible links to adult outcomes will not be available for years or decades. The validity of the samples described here and the availability of data on adult outcomes (through third and fourth decades of life) in the New England Cohorts of the NCPP suggests the possibility of novel analyses linking prenatal steroids and long-term behavioral and physical health outcomes. Results from these analyses will extend prior preclinical and human research and offer further empirical evidence regarding prenatal “programming” of long-term outcomes in a large, population-based sample. We hope the present study will also stimulate similar analyses in additional longitudinal studies with access to stored prenatal blood/serum samples.
Although results should spur future longitudinal analyses of stored steroid samples, we acknowledge several limitations in the two validation studies presented here. First, the most optimal strategy to determine stability of stored samples would involve comparison with concentrations of hormones and binding globulins assessed from the same participants at the time of collection (e.g., 1959–1966) and after 40+ years of storage. However, as serum samples were not assayed for cortisol, testosterone, CBG, or SHBG at the time of collection, our best strategy was to compare concentrations from stored samples to published values from fresh samples. Second, because absolute levels of hormones can vary widely between assays, it would have been optimal to compare values from NCPP samples to those from published studies utilizing the same assay types as those in the present study. However, as there was a paucity of studies of fresh samples in pregnant women using identical assays, we selected studies including a number of assay types. Third, similar to concentrations obtained from some published studies, time of day of serum collection was not recorded in NCPP. As both cortisol and testosterone show variation over the course of the day, this represents an important potential confound for future longitudinal studies in this sample. Finally, NCPP samples were collected in non-fasting mothers with no record of recency of food intake/nutritional status prior to serum sampling. Thus, variability in recency of food intake/nutritional status also represents an additional important confound for future longitudinal studies. However, if time of day and nutritional status can be assumed to be random across prenatal visits, variation in hormone levels due to time of day/nutritional status relative to sampling would be included as error variance, and would serve to attenuate rather than strengthen links between prenatal hormones and long-term offspring outcomes.
In conclusion, stored prenatal serum from the National Collaborative Perinatal Project, and other long-term, longitudinal studies beginning in the perinatal period offer the unique opportunity to examine neuroendocrine mechanisms underlying prenatal “programming” of adult health and disease. However, in order to test hypotheses regarding neuroendocrine mechanisms, it is critical to determine the validity and stability of maternal prenatal samples stored over long periods. Results presented here suggest that concentrations of steroid hormones in prenatal serum stored for over four decades are indeed valid compared to concentrations measured in fresh samples at similar points in gestation. Results should lead to future longitudinal studies of neuroendocrine mechanisms underlying links between prenatal factors and offspring behavior and disease within NCPP and other longitudinal studies.
Acknowledgments
Preparation for this manuscript was supported by NIH grants R01 HD043844 and P50 CA84719. We are indebted to John Lewis for his expertise in the biochemistry of binding globulins, and David Stroud for assistance in numerical conversions across published studies. We also thank Clemens Kirschbaum with his assistance in interpreting results, Mark Klebanoff for facilitating retrieval of serum samples from storage, Kathy McGaffigan for statistical programming, and Stephanie Paton for her administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References marked with an asterisk indicate comparison studies utilized to determine validity of stored samples.
- *.Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol. 1999;180:60–63. doi: 10.1016/s0002-9378(99)70150-x. [DOI] [PubMed] [Google Scholar]
- *.Atamer Y, Erden AC, Demir B, Kocyigit Y, Atamer A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstet Gynecol Scand. 2004;83:425–430. doi: 10.1111/j.0001-6349.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Austin PC, Hux JE. A brief note on overlapping confidence intervals. J Vasc Surg. 2002;36:194–195. doi: 10.1067/mva.2002.125015. [DOI] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Garland C, McPhillips JB, Khaw KT, Wingard DL. A prospective, population-based study of androstenedione, estrogens, and prostatic cancer. Cancer Res. 1990;50:169–173. [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. Bmj. 1995;311:1193–1196. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bartha JL, Comino-Delgado R, Romero-Carmona R, Gomez-Jaen MC. Sex hormone-binding globulin in gestational diabetes. Acta Obstet Gynecol Scand. 2000;79:839–845. [PubMed] [Google Scholar]
- *.Benassayag C, Souski I, Mignot TM, Robert B, Hassid J, Duc-Goiran P, et al. Corticosteroid-binding globulin status at the fetomaternal interface during human term pregnancy. Biol Reprod. 2001;64:812–821. doi: 10.1095/biolreprod64.3.812. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychol Sci. 1992;3:203–206. [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Bolelli G, Muti P, Micheli A, Scianjno R, Franceschetti F, Krogh V, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev. 1995;4:509–513. [PubMed] [Google Scholar]
- Broman S, Bien E, Shaughnessy P. Low Achieving Children: The First Seven Years. Hillsdale, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- *.Broman S, Nichols PL, Shaughnessy P, Wallace K. Retardation in Young Children: A Developmental Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. [Google Scholar]
- Carlsen SM, Romundstad P, Jacobsen G. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstet Gynecol Scand. 2005;84:117–121. doi: 10.1111/j.0001-6349.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- Carr BR, Parker CR, Jr, Madden JD, MacDonald PC, Porter JC. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 1981;139:416–422. doi: 10.1016/0002-9378(81)90318-5. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Gutai JP, Kuller LH, Dai WS. Usefulness of sex steroid hormone levels in predicting coronary artery disease in men. Am J Cardiol. 1987;60:771–777. doi: 10.1016/0002-9149(87)91021-6. [DOI] [PubMed] [Google Scholar]
- Das RE, Calam DH, Mitchell FL, Woodford FP, Cawood M, Gaskell SJ, et al. Stability of four steroids in lyophilised human serum. Ann Clin Biochem. 1983;20(Pt 6):364–368. doi: 10.1177/000456328302000607. [DOI] [PubMed] [Google Scholar]
- *.Demey-Ponsart E, Foidart JM, Sulon J, Sodoyez JC. Serum CBG, free and total cortisol and circadian patterns of adrenal function in normal pregnancy. J Steroid Biochem. 1982;16:165–169. doi: 10.1016/0022-4731(82)90163-7. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Hughes JG, Hutton JL, West CR, Hipkin LJ. The long-term stability in whole blood of 14 commonly-requested hormone analytes. Ann Clin Biochem. 1994;31:561–565. doi: 10.1177/000456329403100606. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real J-M, Grasa M, Casamitjana R, Pugeat M, Barret C, Ricart W. Plasma total and glycosylated corticosteroid-binding globulin levels are associated with insulin secretion. J Clin Enocrinol Metab. 1999;84:3192–3196. doi: 10.1210/jcem.84.9.5946. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real J-M, Pugeat M, Emptoz-Bonneton A, Ricart W. Study of the effect of changing glucose, insulin, and insulin-like growth factor-I levels on serum corticosteroid-binding globulin in lean, obese, and obese subjects with glucose intolerance. Metabolism. 2001;50:1248–1252. doi: 10.1053/meta.2001.25647. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real J-M, Pugeat M, Grasa M, Broch MVendrell J, Brun J, et al. Serum corticosteroid-binding globulin concentration and Insulin Resistannce Syndrome: A population study. J Clin Enocrinol Metab. 2002;87:4680–4690. doi: 10.1210/jc.2001-011843. [DOI] [PubMed] [Google Scholar]
- Forest MG. Role of androgens in fetal and pubertal development. Horm Res. 1983;18:69–83. doi: 10.1159/000179780. [DOI] [PubMed] [Google Scholar]
- Garde AH, Hansen AM. Long-term stability of salivary cortisol. Scand J Clin Lab Invest. 2005;65:433–436. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- Garland CF, Friedlander NJ, Barrett-Connor E, Khaw KT. Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. Am J Epidemiol. 1992;135:1220–1230. doi: 10.1093/oxfordjournals.aje.a116228. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual differentiation of the brain. Cambridge, MA: MIT Press; 1980. [Google Scholar]
- Grimshaw GM, Sitarenios G, Finegan JA. Mental rotation at 7 years: relations with prenatal testosterone levels and spatial play experiences. Brain Cogn. 1995;29:85–100. doi: 10.1006/brcg.1995.1269. [DOI] [PubMed] [Google Scholar]
- Goldstein H, Healy MJR. The graphical presentation of a collection of means. J R Stat Soc Ser A. 1995;158:175–177. [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Maternal prenatal stress and 4–6 year old children’s salivary cortisol concentrations pre- and post-vaccination. Stress. 2004;7:257–260. doi: 10.1080/10253890500044521. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Katz MS, Stern MP, Dunn JF. Relationship of sex hormone binding globulin to overall adiposity and body fat distribution in a biethnic population. Int J Obes. 1989;13:1–9. [PubMed] [Google Scholar]
- Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Valdez RA, Morales PA, Hazuda HP, Stern MP. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab. 1993;77:56–60. doi: 10.1210/jcem.77.1.8325960. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. Br J Cancer. 1988;57:216–218. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- Kley HK, Rick W. The effect of storage and temperature on the analysis of steroids in plasma and blood. J Clin Chem Clin Biochem. 1984;22:371–378. [PubMed] [Google Scholar]
- Kley HK, Schlaghecke R, Kruskemper HL. Stability of steroids in plasma over a 10-year period. J Clin Chem Clin Biochem. 1985;23:875–878. [PubMed] [Google Scholar]
- Kubasik NP, Ricotta M, Hunter T, Sine HE. Effect of duration and temperature of storage on serum analyte stability: examination of 14 selected radioimmunoassay procedures. Clin Chem. 1982;28:164–165. [PubMed] [Google Scholar]
- Lapidus L, Lindstedt G, Lundberg PA, Bengtsson C, Gredmark T. Concentrations of sex-hormone binding globulin and corticosteroid binding globulin in serum in relation to cardiovascular risk factors and to 12-year incidence of cardiovascular disease and overall mortality in postmenopausal women. Clin Chem. 1986;32:146–152. [PubMed] [Google Scholar]
- *.Laudat MH, Guilhaume B, Blot P, Fournier C, Giauque JP, Luton JP. The hormonal state of pregnancy: modification of cortisol and testosterone. Ann Endocrinol (Paris) 1987;48:334–338. [PubMed] [Google Scholar]
- Lewis JG, Mopert B, Shand BI, Doogue MP, Soule SG, Frampton CM, et al. Plama variation of corticosteroid-binding globulin and sex hormone-binding globulin. Horm Metab Res. 2006;38:241–245. doi: 10.1055/s-2006-925338. [DOI] [PubMed] [Google Scholar]
- Lewis JG, Shand BI, Elder PA, Scott RS. Plasma sex hormone-binding globulin rather than corticosteroid-binding globulin is a marker of insulin resistance in obese adult males. Diabetes Obes Metab. 2004;6:259–263. doi: 10.1111/j.1462-8902.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and vocabulary size in 18- and 24-month-old infants. Inf Behav Dev. 2002;24:418–424. [Google Scholar]
- *.Magiakou MA, Mastorakos G, Rabin D, Margioris AN, Dubbert B, Calogero AE, et al. The maternal hypothalamic-pituitary-adrenal axis in the third trimester of human pregnancy. Clin Endocrinol (Oxf) 1996;44:419–428. doi: 10.1046/j.1365-2265.1996.683505.x. [DOI] [PubMed] [Google Scholar]
- Miki K, Sudo A. Effect of urine pH, storage time, and temperature on stability of catecholamines, cortisol, and creatinine. Clin Chem. 1998;44:1759–1762. [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Murphy BE. Human fetal serum cortisol levels related to gestational age: evidence of a midgestational fall and a steep late gestational rise, independent of sex or mode of delivery. Am J Obstet Gynecol. 1982;144:276–282. doi: 10.1016/0002-9378(82)90579-8. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2:283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Niswander KR, Gordon M. The Women and Their Pregnancies DHEW Publication NIH-73-379. Washington, D.C.: U.S. Government Printing Office; 1972. [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- *.Papageorgiou I, Messinis I, Milingos S, Boli A, Kolios G, Seferiadis K. Relation between leptin and cortisol values in umbilical vessels at normal vaginal delivery. J Matern Fetal Neonatal Med. 2004;16:303–307. doi: 10.1080/14767050400018130. [DOI] [PubMed] [Google Scholar]
- *.Potter JM, Mueller UW, Hickman PE, Michael CA. Corticosteroid binding globulin in normotensive and hypertensive human pregnancy. Clin Sci (Lond) 1987;72:725–735. doi: 10.1042/cs0720725. [DOI] [PubMed] [Google Scholar]
- Reyna R, Traynor KD, Hines G, Boots LR, Azziz R. Repeated freezing and thawing does not generally alter assay results for several commonly studied reproductive hormones. Fertil Steril. 2001;76:823–825. doi: 10.1016/s0015-0282(01)01986-0. [DOI] [PubMed] [Google Scholar]
- Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat. 2001;55:182–186. [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- *.Serin IS, Kula M, Basbug M, Unluhizarci K, Gucer S, Tayyar M. Androgen levels of preeclamptic patients in the third trimester of pregnancy and six weeks after delivery. Acta Obstet Gynecol Scand. 2001;80:1009–1013. doi: 10.1034/j.1600-0412.2001.801107.x. [DOI] [PubMed] [Google Scholar]
- Sinnecker G. Stability of sex-hormone-binding globulin in serum and plasma. Clin Chem. 1989;35:1253–1254. [PubMed] [Google Scholar]
- *.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701–710. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- *.Sueda K, Ogawa K, Matsui N. Radioimmunoassay of human transcortin. Endocrinol Jpn. 1983;30:737–745. doi: 10.1507/endocrj1954.30.737. [DOI] [PubMed] [Google Scholar]
- Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, et al. Testosterone measured by ten immunoassays and by isotope dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1371–1385. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Despres JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 2000;32:526–536. doi: 10.1055/s-2007-978681. [DOI] [PubMed] [Google Scholar]
- *.Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32:455–460. doi: 10.1093/ije/dyg094. [DOI] [PubMed] [Google Scholar]
- Tuimala R, Kauppila A, Ronnberg L, Jouppila R, Haapalahti J. The effect of labour on ACTH and cortisol levels in amniotic fluid and maternal blood. Br J Obstet Gynaecol. 1976;83:707–710. doi: 10.1111/j.1471-0528.1976.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Udry JR, Morris NM, Kovenock J. Androgen effects on women’s gendered behaviour. J Biosoc Sci. 1995;27:359–368. doi: 10.1017/s0021932000022884. [DOI] [PubMed] [Google Scholar]
- *.van de Beek C, Thijssen JH, Cohen-Kettenis PT, van Goozen SH, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm. Behav. 2004;46:663–669. doi: 10.1016/j.yhbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Vogl SE, Worda Egarter C, Bieglmayer C, Szerkeres T, Huber J, Husslein P. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG. 2006;113:441–445. doi: 10.1111/j.1471-0528.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- *.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wickings EJ, Nieschlag E. Stability of testosterone and androstenedione in blood and plasma samples. Clin Chim Acta. 1976;71:439–443. doi: 10.1016/0009-8981(76)90095-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Graubard BI, Klebanoff MA, Ronckers C, Stanczyk FZ, Longnecker MP, McGlynn KA. Maternal hormone levels among populations at high and low risk of testicular germ cell cancer. Br J Cancer. 2005;92:1787–1793. doi: 10.1038/sj.bjc.6602545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal relation between endogenous testosterone and cardiovascular disease risk factors in middle-aged men. A 13-year follow-up of former Multiple Risk Factor Intervention Trial participants. Am J Epidemiol. 1997;146:609–617. doi: 10.1093/oxfordjournals.aje.a009326. [DOI] [PubMed] [Google Scholar]