Abstract
This article explores initial findings and the implications of neuroscientific research on meditation. Meditation is conceptualized here as a family of complex emotional and attentional regulatory training regimes developed for various ends, including the cultivation of well-being and emotional balance. The review focuses on the mental processes and the underlying neural circuitry that are critically involved in two styles of meditation. One style, Focused Attention (FA) meditation, entails the voluntary focusing of attention on a chosen object. The other style, Open Monitoring (OM) meditation, involves non-reactive monitoring of the content of experience from moment to moment. We discuss the potential regulatory functions of these practices on attention and emotion processes and their putative long-term impact on the brain and behavior.
Meditation as an explanandum
This article provides a review of recent studies examining the effects of meditation training on attention and emotion. Despite a large number of scientific reports and theoretical proposals 4–8, little is known about the neurophysiological processes involved in meditation and the long-term impact of meditation on the brain. The lack of statistical evidence, control populations and rigor of many of the early studies, the heterogeneity of the studied meditative states, and the difficulty in controlling the degree of expertise of practitioners can in part account for the limited contributions made by neuroscience-oriented research on meditation. The absence of a clear operational definition of meditation limits this research. Here we offer a theoretical framework, based on traditional meditation texts and modern neuroscientific conceptions, in which some standard meditations are grouped into two broad categories: focused attention (FA) and open monitoring (OM) meditation (box 1–box 2, table 1). These categories are used to delineate the specific psychological processes implicated in these two practices and to derive neuro-functional predictions. We also present key findings illustrating how meditation may affect mental processing and the brain. The overall purpose of this framework is to produce an operational definition for FA and OM meditative practices that can be adopted in the scientific study of effects of meditation training on the mind and the brain (see also 9–11).
BOX 1: Focused Attention (FA) meditation
A widespread style of Buddhist practice consists in sustaining selective attention moment by moment on a chosen object, such as a subset of localized sensations caused by respiration. To sustain this focus, the meditator must also constantly monitor the quality of attention. At first, the attention wanders away from the chosen object, and the typical instruction is to recognize the wandering and then restore attention to the chosen object. For example, while intending to focus on localized sensations around the nostril caused by breathing, one may notice that the focus has shifted to the pain in one’s knee. One then “releases” this distraction, and returns to the intended object. Thus, while cultivating the acuity and stability of sustained attention on a chosen object, this practice also develops three skills regulative of attention: the first is the monitoring faculty that remains vigilant to distractions without destabilizing the intended focus. The next skill is the ability to disengage from a distracting object without further involvement. The last consists in the ability to redirect focus promptly to the chosen object.
Progress in this form of meditation is measured in part by the degree of effort required to sustain the intended focus. The novice contends with more distractions, and the three regulative skills are frequently exercised. As one advances, the three regulative skills can be developed to the point that, for example, advanced practitioners have an especially acute ability to notice when the mind has wandered. Eventually FA induces a trait change whereby the attention rests more readily and stably on the chosen focus. At the most advanced levels, the regulative skills are invoked less and less frequently, and the ability to sustain focus thus becomes progressively “effortless.”
In advanced practitioners, FA practices create a sense of physical lightness or vigor, and the need for sleep is said to be reduced. Advanced levels of concentration are also thought to correlate with a significant decrease in emotional reactivity. FA practices typically involve a relatively narrow field of focus, and as a result, the ability to identify stimuli outside that field of focus may be reduced.
BOX 2: Open Monitoring (OM) meditation
While varied, OM practices share a number of core features, including especially the initial use of FA training to calm the mind and reduce distractions. As FA advances, the well developed monitoring skill becomes the main point of transition into OM practice. One aims to remain only in the monitoring state, attentive moment by moment to anything that occurs in experience without focusing on any explicit object. To reach this state, the practitioner gradually reduces the focus on an explicit object in FA, and the monitoring faculty is correspondingly emphasized. Usually there is also an increasing emphasis on cultivating a “reflexive” awareness that grants one greater access to the rich features of each experience, such as the degree of phenomenal intensity, the emotional tone, and the active cognitive schema (see Box 3).
Although the enhancement of the monitoring awareness continues until no explicit focus is maintained, the monitoring itself does not create any new explicit focus. Thus, unlike FA, OM involves no strong distinction between selection and deselection. For example, the FA monitoring faculty detects a state’s emotional tone as a background feature of the primary focus, but in OM the emotional tone is detected without it or any other object becoming an explicit or primary focus. It is as if emotional tone and such remain in the background, even though there is no contrasting cognitive foreground. In this way, the “effortful” selection or “grasping” of an object as primary focus is gradually replaced by the “effortless” sustaining of an awareness without explicit selection.
This distinction between the “effortful” and the “effortless” points to the contrast between skills employed during the state and traits developed as practice progresses60. For example, initially the practitioner frequently “grasps” to objects in a way that requires the skill to deliberately disengage that focus, but eventually a trait emerges such that one can sustain the “non-grasping” state, which has no explicit focus.
A central aim of OM practice is to gain a clear reflexive awareness of the usually implicit features of one’s mental life. It is said that awareness of such features enables one to more readily transform cognitive and emotional habits. In particular, OM practice allegedly leads one to a more acute but less emotionally reactive awareness of the autobiographical sense of identity that projects back into the past and forward into the future. Finally, heightened sensitivity to body and environment occurs with a decrease in the forms of reactivity that create mental distress.
Table 1.
Schematic descriptions of FA and OM meditations
| Focus Attention Meditation | - directing and sustaining attention on a selected object (e.g., breath sensation) |
| - detecting mind wandering and distractors (e.g., thoughts) | |
| - disengagement of attention from distractors and shifting of attention back to the selected object | |
| - cognitive reappraisal of distractor (e.g. “just a thought”, “it is okay to be distracted”) | |
| Open Monitoring Meditation | - no explicit focus on objects |
| - non-reactive meta-cognitive monitoring (e.g. for novices, labeling of experience) | |
| - non-reactive awareness of automatic cognitive and emotional interpretations of sensory, perceptual and endogenous stimuli. | |
The term ‘meditation’ refers to a broad variety of practices, ranging from techniques designed to promote relaxation to exercises performed with a more far-reaching goal such as a heightened sense of well-being. It is thus essential to be specific about the type of meditation practice under investigation. Failure to make such distinctions would be akin to the use of the word “sport” to refer to all sports as if they were essentially the same. For example, the overly generic description of meditation as a mere relaxation technique12 becomes extremely problematic when one attends to the details of many practices (see 7 and Box 1 and Box 2)7,13,14. In contrast, here we conceptualize meditation as a family of complex emotional and attentional regulatory strategies developed for various ends, including the cultivation of well-being and emotional balance (see 10). In order to narrow the explanandum to a more tractable scope, this article uses Buddhist contemplative techniques and their clinical secular derivatives as a paradigmatic framework (see e.g., 9,10 or 7,9 for reviews including other types of techniques, such as Yoga and Transcendental Meditation). Among the wide range of practices within the Buddhist tradition, we will further narrow this review to two common styles of meditation, FA and OM (see box 1–box 2), that are often combined, whether in a single session or over the course of practitioner’s training. These styles are found with some variation in several meditation traditions, including Zen, Vipassanā and Tibetan Buddhism (e.g. 7,15,16). Both styles are also implicated in secular interventions that draw on Buddhist practices, such as mindfulness-based stress reduction 17. The first style, FA meditation, entails voluntary focusing attention on a chosen object in a sustained fashion. The second style, OM meditation, involves non-reactively monitoring the content of experience from moment to moment, primarily as a means to recognize the nature of emotional and cognitive patterns A functional characterization of these states is proposed in table 1. Below we propose specific neurofunctional predictions and review key findings of recent studies on meditation.
Neuroscientific study of Focused Attention Meditation
The selective nature of attention and its importance for guiding goal-directed behavior has been one of the most extensively studied areas of western psychology and neuroscience. Notably, there are remarkable parallels between the processes involved in FA meditation as described in many meditation texts (Table 1), and recent cognitive (neuro)science conceptualizations of attention. Both Western scientists and Buddhist scholars recognize that the ability to focus and sustain attention on an intended object requires skills involved in monitoring the focus of attention and detecting distraction, disengaging attention from the source of distraction, and (re)directing and engaging attention to the intended object. These capacities have been associated with dissociable systems in the brain 18–20. A first, straightforward prediction is therefore that the specific neural systems associated with conflict monitoring (e.g., dorsal anterior cingulate cortex and dorsolateral prefrontal cortex 21–23), selective attention (e.g., the temporoparietal junction, ventrolateral prefrontal cortex, frontal eye fields, and intraparietal sulcus 18) and sustaining attention (e.g., right frontal and parietal areas and the thalamus 19,24) are involved in inducing and maintaining the state of FA meditation. In addition, sustained activity may be observed in areas representing the object of attention (e.g., 25). These neurophysiological changes induced by meditation training should be correlated with improvements in behavioral measures of sustained attention, such as performance in continuous performance tasks or binocular rivalry tasks, and of selective attention, such as performance in the Posner cueing task 26(see below). A second prediction concerns the long-term changes in mental and brain function that FA meditation may produce. Buddhist descriptions of the development of this practice indicate that while the processes of voluntary sustaining and orienting initially play an important role in focusing attention and expertise in the deployment of these processes may strengthen as a function of practice, with more practice, the need for these processes is greatly reduced, resulting in a form of ‘effortless concentration’ (Box 1) In expert meditators, one may therefore expect reduced activation in neural systems implicated in regulating attention, which may be associated with optimized performance in sustained and selective attention.
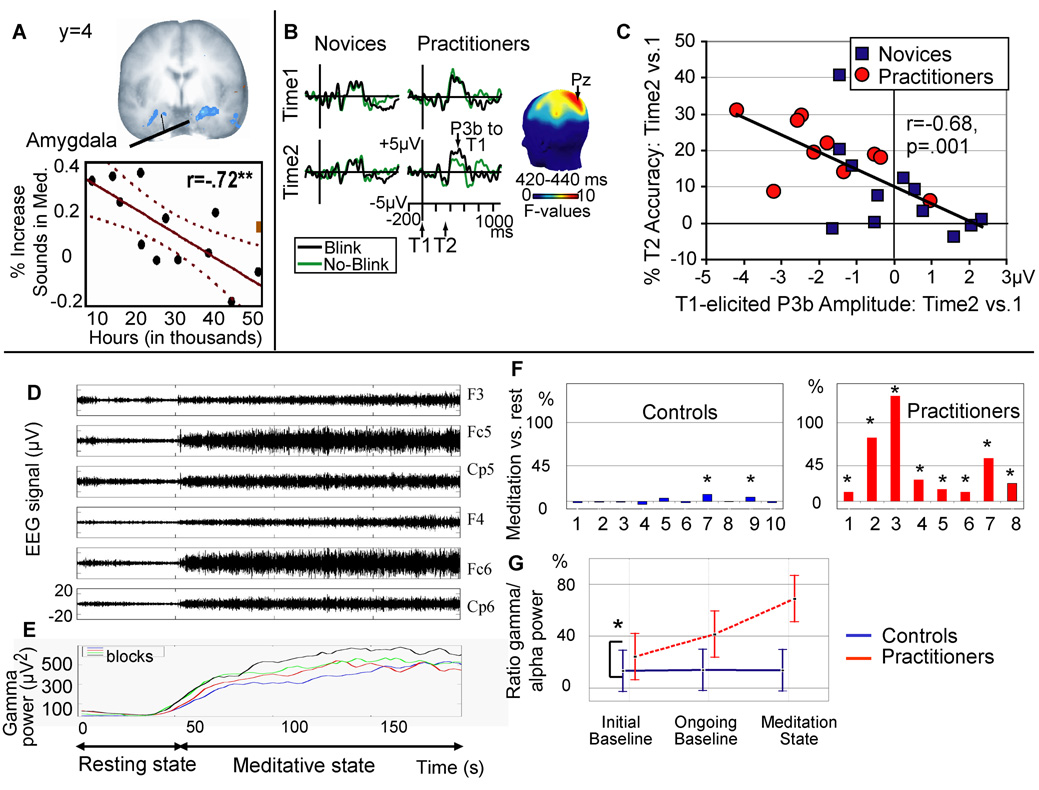
Several recent studies have reported expertise-related changes in attentional processing 21,27 and brain structures 28,29 in those proficient in FA meditation. For instance, Carter et al. 21 found that Tibetan Buddhist monks were able to perceive a stable, superimposed percept of two dissimilar, competing images presented to separate eyes for a longer duration both during and after FA meditation, but not a form of ‘compassion’ meditation. These behavioral findings obtained using a ‘binocular rivalry’ task support the hypothesis that extensive training in FA meditation may improve the practitioner’s ability to sustain attention on a particular object for a prolonged period of time. Another study from our laboratory (27) used functional magnetic resonance imaging (fMRI) to interrogate the neural correlates of FA meditation in experts and novices. In this study, FA meditation on an external visual point compared to a rest condition was associated with activation in multiple brain regions implicated in monitoring (dorsolateral prefrontal cortex), engaging attention (visual cortex), and attentional orienting (e.g., the superior frontal sulcus and intraparietal sulcus). Although this meditation-related activation pattern was generally stronger for long-term-practitioners compared to novices, activity in many brain areas involved in FA meditation showed an inverted u-shaped curve: Whereas expert meditators with an average of 19,000 hours of practice showed stronger activation in these areas than the novices, expert meditators with an average of 44,000 practice hours showed less activation. This inverted u-shaped function resembles the learning curve associated with skill acquisition in other domains of expertise, such as language acquisition 30, and provides support for the notion that after extensive FA meditation training, minimal effort is necessary to sustain attentional focus. A direct comparison between experts in FA and OM meditation will be important here to distinguish effortless concentration from objectless meditation. In addition, expert meditators showed less activation than novices in the amygdala during FA meditation and activation in this affective region correlated negatively with hours of practice in life (Figure 1A). This finding is unlikely due simply to the long-term practitioners ignoring the stimuli more than controls since auditory processing regions (e.g., superior temporal gyrus) were more significantly activated to sounds in the practitioners compared with controls (see Table 3 in 27). This finding is also in line with a previous behavioral study showing a reduction in habitual responding on the Stroop task following a 20-minute meditation practice31 and may illustrate the notion that training in FA is associated with a significant decrease in emotionally reactive behaviors that are incompatible with stability of concentration. Such inhibition of automatic responses provides preliminary support for earlier proposals and an earlier FA electroencephalography study32 that concentrative meditation leads to partial “deautomatization” of the mental processes that shape and interpret perceptual stimuli 11,33. Collectively these findings underscore the view that at least several subcomponents of attention are best regarded as the product of trainable skills and that FA meditation represents a family of mental practices that are explicitly designed to train such attentional skills.
Figure 1. Neuroimaging and neurodynamical correlates of FA and OM meditations.
(a).Relationship between degree of meditation training (in years) and hemodynamic response in the amygdala (in blue) to distractor sounds during FA meditation in long-term Buddhist practitioners. Individual responses in the right amygdala are plotted (adapted from 27). (b) illustrates the reduction in P3b amplitude, a brain-potential index of resource allocation, to the first of two target stimuli (T1 and T2) presented in a rapid stream of distracter stimuli after three months of intensive Vipassana meditation (48). Shown are scalp-recorded brain potentials from electrode Pz, time-locked to T1 onset as a function of T2 accuracy (detected (no-blink) vs. not detected (blink)), time (before or after three months), and group (practitioners vs. novices). The scalp map shows electrode sites where this three-way interaction was significant between 420 and 440ms. (c) shows that generally, the greater the reduction in brain-resource allocation to T1 was over time, the better able an individual became at accurately identifying T2 (adapted from 48). (d–e) Example of high-amplitude gamma activity during a form of OM meditation, non-referential compassion meditation, in long-term Buddhist practitioners (56). (d) Raw electroencephalographic signals. At t = 45 s, practitioner S4 started meditating. (e) Time course of gamma (25–42 Hz) activity power over the electrodes displayed in (d) during four blocks computed in a 20-s sliding window every 2 s and then averaged over electrodes. (f) Intra-individual analysis on the ratio of gamma to slow oscillations (4–13 Hz) averaged across all electrodes during compassion meditation. The abscissa represents the subject numbers, the ordinate represents the difference in the mean ratio between the initial state and meditative state, and the black and red stars indicate that this increase is greater than two and three times, respectively, the baseline standard deviation. (g) illustrates the significant interaction between group (practitioner, control) and state (initial baseline, ongoing baseline, and meditation state) for this ratio. The relative gamma increase during meditation was higher in the post-meditation session. In the initial baseline, the relative gamma was already higher for the practitioners than the controls and correlated with the length of the long-term practitioners' meditation training through life (adapted from 56). For all further details regarding methods and analysis of these studies, see papers cited.
The neuroscientific study of OM meditation
Several predictions concerning the mental processes and brain systems involved in OM meditation can be derived from the description of this form of meditation given in Table 1. A first prediction is that since OM meditation involves no explicit attentional focus, it does not rely on brain regions involved in sustaining or engaging attention onto a specific object, but on brain regions implicated in monitoring, vigilance and disengaging attention from stimuli, which distract attention from the ongoing stream of experience (see above). Findings from several recent studies of OM meditation 13,14,35–38 provide preliminary support for this idea. For instance, in an early behavioral study, OM meditators showed superior performance on a sustained attention task in comparison with FA meditators when the stimulus was unexpected, but there was no difference between the two groups of meditators when the stimulus was expected, indicating a more distributed attentional focus in the OM meditators 14. In another study, a group randomly assigned to five days of meditation training substantially based on OM meditation showed greater improvement in conflict monitoring than a similarly chosen control group given relaxation 13. Secondly, as OM meditation involves the cultivation of awareness of the subjective features of a given moment, such as its emotional tone (see Box 2), it is conceivable that it engages processes involved in interoception, or the perception of internal bodily responses. These processes rely on meta-representations in the brain of homeostatic afferent activity (e.g., temperature change, pain; Craig, 39), in particular in the anterior insula, somatosensory cortex (SII) and anterior cingulate cortices 39,40. In line with the idea that OM meditation engages processes involved in monitoring one’s body state, a recent study found greater activity in this neural circuitry during a monitoring state relative to a narrative generation state in participants who attended an 8-week course incorporating OM meditation (Mindfulness-based-Stress-Reduction program17) compared to a group of controls37. A third prediction concerns the potential regulatory influences of OM meditation on emotional processes through prefrontal regulation of limbic responses. Recent neuroimaging studies have shown that simply verbally labeling affective stimuli activates right ventrolateral prefrontal cortex and attenuates responses in the amygdala via activity in ventromedial prefrontal cortex 41,42. This strategy of labeling aspects of experience (e.g., ‘this is distress’) is used in Vipassana traditions to support OM practice and is a central feature of many clinical interventions based on OM meditation, in particular mindfulness-based interventions 17 (here we used the term OM instead of the term mindfulness, which has received multiple meanings; see 10). OM practices that sometimes involve verbal labeling may thus call upon emotion regulation pro0cesses instantiated in ventral prefrontal cortex and disrupt or inhibit automatic affective responses in appraisal systems, diminishing their intensity and duration. Recent experimental 29,43,44 and clinical (e.g., 45–47) studies of mindfulness meditation, and studies of individual differences in self-reported mindfulness traits44 provide preliminary support for this possibility.
Long-term practice of OM meditation is also thought to result in enduring changes in mental and brain function. Specifically, as OM meditation fosters nonreactive awareness of the stream of experience without deliberate selection of a primary object, intensive practice can be expected to reduce the elaborative thinking that would be stimulated by evaluating or interpreting a selected object (Box 2; see also 11). In line with this idea, Slagter et al. recently found that three months of intensive OM meditation reduced elaborative processing of the first of two target stimuli (T1 and T2) presented in a rapid stream of distracters, as indicated by a smaller T1-elicited P3b, a brain potential index of resource allocation (Figure 1B) 48. Moreover, this reduction in resource allocation to T1 was associated with improved detection of T2 (Figure 1C). As participants were not engaged in formal meditation during task performance, these results provide support for the idea that one effect of an intensive training in OM meditation may be reduction in the propensity to “get stuck” on a target as reflected in less elaborate stimulus processing and the development of efficient mechanisms to engage and then disengage from target stimuli in response to task demands. From the description in Box 2 we anticipate a similar improvement in the capacity to disengage from aversive emotional stimuli following OM training, enabling greater emotional flexibility.
Neurodynamical framework
As noted above, traditional Buddhist scholars have emphasized the decreased need for voluntary attentional efforts to attain concentration following expertise in FA meditation. In addition, some variations of OM meditation explicitly advise practitioners to drop any explicit effort to control the occurrence of thoughts or emotions in order to further stabilize their meditation. These descriptions suggest that some meditation states might not be best understood as top-down influence in a classical neuroanatomical sense, but rather as dynamical global states that, in virtue of their dynamical equilibrium, can influence the processing of the brain from moment to moment. Moreover, an important hypothesis that derives from the emphasis on trait-like transformation in these traditions is that with systematic practice, there is an enduring alteration of the “baseline” or “default” mode (see e.g., 52) of brain functioning. In this alternative “dynamicist” view of top-down control, spatio-temporal trajectories of neural activity emerge from complex non-linear neural interactions following rules of dynamical theory 53. These large-scale coherent neuronal ensembles, for instance which emerge during FA on the breath, can influence other local neuronal processes, for instance evoked by an external distractor, by entraining local ensembles (54,55). In this view, the brain goes through a succession of large-scale brain states, with each state becoming the source of top-down influences for the subsequent state. We predict that these large-scale integrative mechanisms participate in the regulatory influence of these meditation states.
The finding of high-amplitude pattern of gamma synchrony in expert meditators during an emotional version of OM meditation 56 supports the idea that the state of OM may be best understood in terms of dynamic global states. Lutz et al. studied a group of long-term Tibetan Buddhist practitioners who underwent mental training for 10,000 to 50,000 hours over time periods ranging from 15 to 40 years. Compared to a group of novices, the practitioners self-induced higher-amplitude sustained electroencephalography (EEG) gamma-band oscillations and long-distance phase synchrony, in particular over lateral fronto-parietal electrodes, while meditating 56 (Figure 1D–G). Importantly, this pattern of gamma oscillations was also significantly more pronounced in the baseline state of the long-term practitioners compared with controls, suggesting a transformation in the default mode of the practitioners. Although the precise mechanisms are not clear, such synchronizations of oscillatory neural discharges may play a crucial role in the constitution of transient networks that integrate distributed neural processes into highly ordered cognitive and affective functions 54,55 and are an important constraint for synaptic plasticity 57. The combination of neuroimaging and neurodynamical information, in particular with first-person report (see Box 3), may thus provide a particularly promising approach to the study of the brain mechanisms underlying meditation.
BOX 3: Neurophenomenology and meditation
The neurophenomenology approach 61,62 draws attention to the need to combine quantitative measures of neural activity with first-person data about the subject’s inner experience to shed full light on the mind. This approach actively involves the participant in generating and describing specific and stable experiential or phenomenal categories. These reports can be useful in identifying variability in the brain’s response from moment to moment, and this unique information may guide the detection and interpretion of neural processes.
An illustration of the neurophenomenological approach is given by a study by Lutz and colleagues (2002), which examined the usefulness of this approach using a visual protocol and naive subjects 63. On the day of the experiment, participants were first trained to become aware of subtle trial-by-trial fluctuations in their cognitive context as defined by their attentive state, spontaneous thought processes, and the strategy used to carry out the task. During a subsequent simple visual task, their electrical brain activity and their own report about their cognitive context were recorded. Trials were clustered according to the acquired first-person data, and separate, dynamical analyses were conducted for each cluster. Characteristic patterns of synchrony at frontal electrodes were found for each cluster, depending in particular on the stability of attention and the degree of task preparation. These data showing correlations between synchrony patterns and ongoing conscious states illustrate how information regarding moment-to-moment fluctuations in one’s inner experience can guide the study of brain dynamics.
The collaboration with long-term practitioners will be particularly relevant for extending this research strategy. As verbal reports about inner experience can easily be biased or represent non-veridical constructions based upon learned folk-theoretic accounts, the deployment of precise and rigorous first-person methods, such as meditation, is critical to the usefulness of the neurophenomenological approach. Long-term meditation practitioners can allegedly generate more stable and reproducible mental states than untrained subjects and can also describe these states more accurately and provide more refined phenomenological reports than naive subjects. In this context, the practitioners’ introspective skills could provide a way for experimenters to better control and identify the subjective aspects of attention and emotion regulatory processes. Examples of first-person features are the subjective feeling of mental stability in FA meditation and the monitoring of automatic emotion patterns in OM meditation. A central hypothesis is that the correlations between phenomenological reports and simultaneously measured brain activity should be systematically stronger for long-term practitioners than for naive observers, since the former are putatively better able to veridically report on the content and process of their mind.
Future directions
The neuroscientific study of meditation is clearly still in its infancy, but the initial findings reviewed above promise both to reveal the mechanisms by which such training may exert its effects and underscore the plasticity of the brain circuits that underlie complex regulatory mental functions. These findings will need to be supplemented with more data, most critically from longitudinal studies examining changes over time within the same individuals randomized either to meditation training or to an active control group. Such longitudinal studies, which are designed to test a selected effect of a given, clearly defined meditation, are necessary to exclude the possibility that observed training effects are due to pre-existing differences between groups (i.e., experts and novices) and will allow for a more precise delineation of the developmental trajectory of the trained abilities. Another important area of future research is to study meditation practices that deliberately invoke an emotional state of empathy, affection, and compassion for others. Such practices are often seen as indispensable supplements to FA or OM practices. Despite the importance of these practices, they were not reviewed here, due to the paucity of available empirical data.
Future work will need to address at least three additional types of questions. First, the impact of mental training on peripheral biological processes that are important for physical health and illness is not yet clear. Although several clinical studies have reported changes in, for example, cortisol or immune function as a function of mindfulness-based therapies (e.g., 43,46,), what is largely absent are data that mechanistically link changes in the brain that might be produced by meditation, and alterations in peripheral processes, for example, in immune function. At present, relatively few studies have examined how changes in peripheral biology might be related to changes in brain function following meditation (43,58,59). Second, meditation is always practiced in a particular context, whether it be the context formed by the practitioner’s body and mind (e.g., typically a seated posture, the spine must be kept straight, while the rest of the body should be neither too tense nor too lax), or the wider context formed by ethics, traditions, culture and the environment (e.g., altruistic motivation to practice, ethics of non-harming others, student/ teacher relationship). Future studies will need to examine how this context may modulate the generation of certain mental states. Third, to what extent and how meditation training affects behavior outside of the laboratory and transforms basic mental functions such as emotion and attention in everyday life is another critical question that needs more study. It is our fervent hope that this review will stimulate additional research in these and other directions.
BOX 4: Outstanding Questions
What is the developmental impact of meditation training in children and on the aging process? What is the optimal dosing of particular types of meditation at different ages? More generally, how does meditation training fit in the framework of lifespan development?
Which meditation practices are best used for cultivating different types of cognitive and emotional skills? And how can we best match individuals’ unique cognitive and affective style to specific forms of meditation?
What are the peripheral biological consequences of different forms of meditation and how are these related to the unique patterns of brain function engaged by each? Can we use meditation to examine fundamental mechanisms of mind-brain-body interaction?
Acknowledgements
Support for the work described in this article was provided by NCCAM U01AT002114-01A1, Fyssen foundation to AL and NIMH P50-MH069315 to RJD, and by gifts from Adrianne and Edwin Cook-Ryder, Bryant Wangard, Keith and Arlene Bronstein, and the John W. Kluge Foundation.
References
- 1.Pascual-Leone A, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 2.Maguire EA, et al. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13(2):250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- 3.Munte TF, et al. The musician's brain as a model of neuroplasticity. Nat Rev Neurosci. 2002;3(6):473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- 4.West MA, editor. The psychology of meditation. Clarendon Press; 1987. [Google Scholar]
- 5.Shapiro DH, Walsh RN, editors. Meditation: Classical and contemporary perspectives. Aldine; 1984. [Google Scholar]
- 6.Varela FJ, et al. The Embodied Mind. MIT Press; 1991. [Google Scholar]
- 7.Austin JH. Zen and the brain : toward an understanding of meditation and consciousness. MIT Press; 1999. [Google Scholar]
- 8.Wallace AB, editor. Buddhism and Science. Columbia University Press; 2003. [Google Scholar]
- 9.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 10.Lutz A, et al. Meditation and the Neuroscience of Consciousness: An Introduction. In: Zelazo PD, Thompson E, editors. The Cambridge Handbook of Consciousness. Cambridge University Press; 2006. [Google Scholar]
- 11.Bishop SR, et al. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice. 2004;11(3):230. [Google Scholar]
- 12.Fischer R. A cartography of the ecstatic and meditative states. Science. 1971;174(12):897–904. doi: 10.1126/science.174.4012.897. [DOI] [PubMed] [Google Scholar]
- 13.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentine ER, Sweet PLG. Meditation and attention: a comparison of the effects of concentrative and mindfulness meditation on sustained attention. Mental Health, Religion and Culture. 1999;2(1) [Google Scholar]
- 15.Gunaratana H. Mindfulness in plain English. Wisdom Publications; 2002. [Google Scholar]
- 16.Tenzin Gyatso tFDL, Jinpa T. The world of Tibetan Buddhism : an overview of its philosophy and practice. Wisdom Publications; 1995. [Google Scholar]
- 17.Kabat-Zinn J, et al. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 18.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 19.Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- 20.Weissman DH, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 21.Carter OL, et al. Meditation alters perceptual rivalry in Tibetan Buddhist monks. Curr Biol. 2005;15(11):R412–R413. doi: 10.1016/j.cub.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Ridderinkhof KR, et al. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Carter CS, et al. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 24.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(4):343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 25.Kastner S, et al. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 26.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 27.Brefczynski-Lewis JA, et al. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci U S A. 2007;104(27):11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol Aging. 2007;28(10):1623–1627. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Lazar SW, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai KL. Language acquisition and brain development. Science. 2005;310(5749):815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- 31.Wenk-Sormaz H. Meditation can reduce habitual responding. Altern Ther Health Med. 2005;11(2):42–58. [PubMed] [Google Scholar]
- 32.Kasamatsu A, Hirai T. An electroencephalographic study of Zen meditation (Zazen) Folia Psychiatrica et Neurologica Japonica. 1966;20:315–336. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 33.Deikman AJ. Experimental meditation. J Nerv Ment Dis. 1963;136:329–343. doi: 10.1097/00005053-196304000-00002. [DOI] [PubMed] [Google Scholar]
- 34.James W. Principles of Psychology. H. Holt and company; 1890. [Google Scholar]
- 35.Holzel BK, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 36.Jha AP, et al. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 37.Farb NAS, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007:1–10. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain S, et al. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 39.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 40.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; 2000. [Google Scholar]
- 41.Hariri AR, et al. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman MD, et al. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 43.Davidson RJ, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 44.Creswell JD, et al. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 45.Teasdale JD, et al. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J Consult Clin Psychol. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- 46.Carlson LE, et al. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007 doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Teasdale JD, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 48.Slagter HA, et al. Mental Training Affects Distribution of Limited Brain Resources. PLoS Biol. 2007;5(6):e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward R, et al. The Slow Time-Course of Visual Attention. Cognit Psychol. 1996;30(1):79–109. doi: 10.1006/cogp.1996.0003. [DOI] [PubMed] [Google Scholar]
- 50.Raymond JE, et al. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- 51.Shapiro KL, et al. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. J Exp Psychol Hum Percept Perform. 1994;20(2):357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- 52.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman WJ. Tutorial on neurobiology: from single neurons to brain chaos. International Journal of Bifurcation and Chaos. 1992;3(2):451. [Google Scholar]
- 54.Varela F, et al. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 55.Engel AK, et al. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 56.Lutz A, et al. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci U S A. 2004;101(46):16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bibbig A, et al. Long-range synchronization of gamma and beta oscillations and the plasticity of excitatory and inhibitory synapses: a network model. J Neurophysiol. 2002;88(4):1634–1654. doi: 10.1152/jn.2002.88.4.1634. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi T, et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol. 2005;55(2):199–207. doi: 10.1016/j.ijpsycho.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Lehrer P, et al. Zazen and cardiac variability. Psychosom Med. 1999;61(6):812–821. doi: 10.1097/00006842-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Saling LL, Phillips JG. Automatic behaviour: efficient not mindless. Brain Res Bull. 2007;73(1–3):1–20. doi: 10.1016/j.brainresbull.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Varela F. Neurophenomenology: a methodological remedy to the hard problem. Journal of Consciousness Studies. 1996;3:330–350. [Google Scholar]
- 62.Lutz A, Thompson E. Neurophenomenology: Integrating Subjective Experience and Brain Dynamics in the Neuroscience of Consciousness. Journal of Consciousness Studies. 2003;10(9–10):31–52. [Google Scholar]
- 63.Lutz A, et al. Guiding the study of brain dynamics by using first-person data: synchrony patterns correlate with ongoing conscious states during a simple visual task. Proc Natl Acad Sci U S A. 2002;99(3):1586–1591. doi: 10.1073/pnas.032658199. [DOI] [PMC free article] [PubMed] [Google Scholar]