Abstract
Aim
Habitual aerobic exercise results in a significant increase in central arterial compliance. Endothelin-1 (ET-1) is a potent endothelium-derived vasoconstrictor peptide and could play a role in mediating the habitual aerobic exercise-induced increase in central arterial compliance. The aim of the present study was to examine whether ET-1 involves in the mechanisms underlying the increase in central arterial compliance to aerobic exercise training.
Methods
Seven apparently healthy middle-aged and older (60±3 yr) adults underwent systemic endothelin-A/B (ETA/B)-receptor blockade (500 mg of Tracleer) before and after 12 weeks of aerobic exercise training (70±1% of maximal heart rate, 44±2 min/day, 4.4±0.1 days/week).
Results
Basal carotid arterial compliance (via simultaneous B-mode ultrasound and arterial applanation tonometry on the common carotid artery) increased significantly after exercise training. Resting plasma ET-1 concentration decreased significantly after the exercise training. Before the exercise intervention, carotid arterial compliance increased significantly with the administration of ETA/B-receptor blockade. After the training, however, increases in carotid arterial compliance previously observed with the ETA/B-receptor blockade before the training were abolished.
Conclusions
Regular aerobic exercise training enhances central arterial compliance in middle-aged and older humans. The increase in arterial compliance was associated with the corresponding reduction in plasma ET-1 concentration as well as the elimination of ET-1 mediated vascular tone. These results suggest that reductions in ET-1 may be an important mechanism underlying the beneficial effect of exercise training on central artery compliance.
Keywords: endothelium, exercise training, elasticity
INTRODUCTION
As illustrated by the William Osler's axiom that “man is as old as his arteries” (Osler 1892), a hallmark feature of cardiovascular aging is the stiffening of large elastic arteries (Lakatta & Levy 2003). Arterial stiffening impairs the artery's ability to buffer and cushion the pulsation of blood pressure and flow and contributes to a variety of diseases (hypertension, coronary ischemia, etc.) that are common to the elderly (Blacher et al. 1999, Laurent et al. 2001). Aerobic exercise training has emerged as one of the most effective means to improve the elastic property of arteries (Cameron & Dart 1994, Tanaka et al. 2000, Kakiyama et al. 2005). However, the physiological mechanisms underlying the destiffening effects of aerobic exercise training remain unclear.
Chronic increases in basal vascular tone have been implicated in the pathogenesis of a age-associated vascular disease, including arterial stiffening (Tanaka & Safar 2005). The age-related elevation in vascular tone is mediated not only by chronically elevated sympathetic vasoconstrictor tone but also by the endothelial function (Dobrin & Rovick 1969). In addition to relaxing factors such as NO, the vascular endothlium also synthesizes and releases vasoconstricting factors, including endothelin-1 (ET-1) (Yanagisawa et al. 1988). ET-1 is the most potent vasoconstrictor peptide secreted by vascular endothelial cells (Yanagisawa et al. 1988). Aging is associated with the increases in both plasma ET-1 concentrations (Maeda et al. 2003) and ET-1 mediated vasoconstrictor (Van Guilder et al. 2007) even in healthy subjects. Moreover, synthesis and release of ET-1 peptide is elevated in cultured endothelial cells extracted from the aorta of older adults compared with cells from younger adults (Kumazaki et al. 1994).
Considering our previous findings that plasma ET-1 concentration decreases with aerobic exercise training (Maeda et al. 2001, 2003), it is reasonable to hypothesize that endogeneous ET-1 may be involved in the mechanisms underlying the increase in central arterial compliance to aerobic exercise training. However, the direct evidence to support such hypothesis was lacking. Accordingly, the present investigation was undertaken to determine the effects of acute endothelin-A/B (ETA/B)-receptor blockade on arterial compliance before and after aerobic exercise training in healthy middle-aged and older humans.
METHODS
Subjects
Seven sedentary but apparently healthy middle-aged and older adults (60±3 yr, 2 men and 5 postmenopausal women) were studied. Candidates who were current smokers or smoked in the past 2 yr, who took medications (including hormone replacement therapy), or who had significant intima-media thickening (>1.0 mm), plaque formation, and/or other characteristics of atherosclerosis (e.g., ankle-brachial index <0.9) were excluded. None of the subjects had engaged in regular physical activity (>2/week) in the past 1 yr, which was verified through the questionnaire. All subjects had no apparent cardiovascular disease as assessed by medical history and physical examination. This study was reviewed and approved by the Institutional Review Board at the University of Tsukuba. All potential risks and procedures of the study were explained to the subjects, and they gave their written informed consent to participate in the study.
Experimental Design
All measurements were performed after an abstinence of caffeine and an overnight fast. Subjects were studied under supine resting conditions in a quiet, temperature-controlled room (24-26°C). All the post-training measurements were performed at least 48 h after the last exercise bout to avoid the acute (i.e., residual) effect of exercise on major dependent variables. After the subject had 15 min of supine rest, baseline measurements were made. After this, a bolus (500 mg) of ETA/B-receptor blockade (Tracleer, Actelion Japan, Tokyo, Japan) was administered orally. The measurements were performed 60 min and 120 min after the ETA/B-receptor blockade. The particular dose of 500 mg of Tracleer was chosen based on the comprehensive pharmacological analyses conducted by Weber et al. (1996) who examined the dose-response issue of an acute administration of ETA/B-receptor antagonist in healthy human subject. Although plasma concentrations of the antagonist increased as oral dose was increased from 3 mg to 2,400 mg, there were no significant differences in the magnitude of blood pressure reduction induced by different doses of Bosentan (approximately 5 mmHg). Additionally, in a study involving chronic treatment of bosentan, i.e., Tracleer, a significant reduction in blood pressure was achieved with a daily dose of ≥500 mg in patients with mild-to-moderate essential hypertension (Krum et al. 1998).
Carotid Artery Compliance
The combination of ultrasound imaging of the common carotid artery with simultaneous applanation tonometry of the contralateral carotid artery was performed to obtain carotid artery compliance as previously described (Tanaka et al. 2000, Miyachi et al. 2004). A longitudinal image of the carotid artery was measured with a duplex ultrasound machine (180II, SonoSite, Bothell, WA) equipped with a high-resolution multi-frequency linear-array transducer (axial resolution of 0.06 mm). These images were recorded on a digital videotape for later off-line analysis. Arterial diameter was determined by a perpendicular measurement from the media/advantitia interface of the near wall to the lumen/intima interface of the far wall of the vessel. Three measurements of arterial lumen diameter were taken per frame and averaged. Data reported are the time averages of ≥10 measurements for all variables. Carotid arterial pressure waveforms were obtained with arterial applanation tonometory incorporating an array of 15 micropiezoresistive transducers (formPWV/ABI, Colin Medical Technology, Komaki, Japan) (Cortez-Cooper et al. 2003) and calibrated by equating the carotid mean arterial and diastolic blood pressure to the brachial values (Armentano et al. 1995). Arterial compliance was calculated using the equations: [(D1-D0)/D0]/[2*(P1-P0)*π*D0] where D1 and D0 are the maximal and minimum arterial diameters and P1 and P0 are the highest and lowest blood pressures (Tanaka et al. 2000, Miyachi et al. 2004). At each time point, averages of 10 cardiac cycles were analyzed. In order to exclude inter-investigator variability, one investigator, who was blinded to the time points, analyzed all ultrasound images and blood pressure waveform. The day-to-day CVs for carotid artery diameter, pulse pressure, and arterial compliance were 2±1, 7±3, and 5±2%, respectively.
Arterial Blood Pressure and Ankle-Brachial Index
Brachial and ankle arterial blood pressure and ankle-brachial index were also measured with the automated device (formPWV/ABI, Colin Medical Technology) (Cortez-Cooper et al. 2003). An automatic oscillometric blood pressure device was used to eliminate potential investigator bias associated with the auscultation.
Body Fatness
Percent body fat was determined by bioelectric impedance (HBF-357, Omron Healthcare, Tokyo, Japan) as previously described (Miyachi et al. 2004).
Blood Samples
A blood sample was collected from the antecubital vein using venipuncture after an overnight fast. Plasma concentrations of glucose, lipids, and lipoproteins were determined by use of the standard enzymatic technique as previously described (Tanaka et al. 1998). Plasma ET-1 concentration was determined by using a sandwich-EIA Kit (Immuno-Biological Laboratories, Fujioka, Japan) as previously described (Maeda et al. 2001, 2003). Plasma samples from each subject before and after the training intervention were analyzed in the same assay run.
Aerobic Capacity
All subjects underwent an incremental cycle exercise test (after 2 min at 20 W, with 15 W increases every 1 min) until they reached 85% of the age-predicted maximal heart rate (208-age*0.7) (Tanaka et al. 2001). Heart rate (via ECG) was measured throughout the protocol. Work rate corresponding to 85% of the age-predicted maximal heart rate (PWC85%) was used as a measure of aerobic capacity. PWC85% has been shown to correlate well with directly measured maximal oxygen consumption (Miyashita et al. 1985).
Aerobic Exercise Training Intervention
Subjects underwent aerobic exercise training 4-5 days per week (walk/jog, 2 supervised and additional home-based trainings) for 12 wk. The prescribed duration and intensity of the training were 30-45 min/day and 65-75% of their individual maximum heart rate. Adherence to the exercise prescription was documented through the use of Polar heart rate monitors and physical activity logs. The actual mean heart rate during the exercise training was 70±1% of their individual maximal heart rate, and the actual frequency and duration of the exercise training were 4.4±0.1 days/wk and 44±2 min/day, respectively.
Statistical Analyses
Student's t-test for paired values was used to compare dependent variables before and after exercise training. ANOVA and MANOVA with repeated measures were used to determine the effects of exercise training intervention and systemic ETA/B-receptor blockade on carotid arterial compliance and other hemodynamic variables. ANCOVA was performed to compare responses of arterial compliance to systemic ETA/B-receptor blockade before and after exercise intervention after taking account into the influence of corresponding changes in mean arterial pressure as a covariate. All data are reported as mean±SEM. Statistical significance was set a priori at P<0.05.
RESULTS
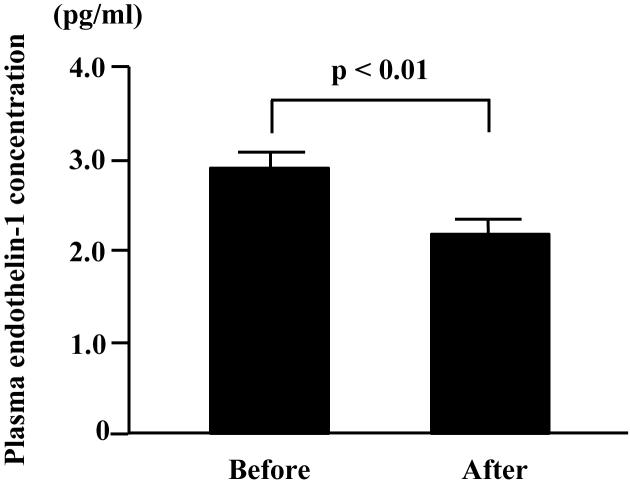
There were no significant changes in body mass, adiposity, and plasma concentrations of cholesterol and glucose with the exercise training intervention (Table 1). PWC85%, a measure of aerobic capacity, increased significantly after the exercise training intervention. Carotid diastolic diameter at rest was not affected by the exercise training intervention. Brachial and carotid systolic blood pressure and carotid pulse pressure at rest decreased significantly after exercise training, whereas brachial diastolic blood pressure, and heart rate at rest did not change (Table 2). The resting plasma concentration of ET-1 significantly decreased after exercise training (Fig. 1).
TABLE 1.
Selected Subject Characteristics
| Before Training | After Training | |
|---|---|---|
| Age, years | 60 ± 3 | … |
| Height, cm | 161 ± 2 | … |
| Body mass, kg | 60 ± 4 | 60 ± 4 |
| Body mass index, kg/m2 | 22.9 ± 1.2 | 23.1 ± 1.2 |
| Body fat, % | 28 ± 2 | 28 ± 2 |
| Total cholesterol, mmol/L | 5.8 ± 0.3 | 5.4 ± 0.2 |
| HDL-cholesterol, mmol/L | 1.6 ± 0.1 | 1.6 ± 0.2 |
| LDL-cholesterol, mmol/L | 3.6 ± 0.2 | 3.2 ± 0.2 |
| Triglyceride, mmol/L | 1.3 ± 0.3 | 1.3 ± 0.3 |
| Blood glucose, mmol/L | 5.2 ± 0.1 | 5.4 ± 0.2 |
| Carotid diastolic diameter, mm | 5.9 ± 0.2 | 6.0 ± 0.3 |
| Ankle-brachial index | 1.18 ± 0.03 | 1.18 ± 0.03 |
| PWC85%, watt | 104 ± 11 | 114 ± 11 * |
Data are mean±SEM.
P<0.05 vs. before training.
PWC85%=Physical work capacity corresponding to 85% of the age-predicted maximal heart rate.
TABLE 2.
Hemodynamic changes in response to endothelin receptor blockade
| Endothelin-Receptor Blockade |
||||
|---|---|---|---|---|
| Baseline | 60 min | 120 min | ||
| Heart rate, beat/min | Before | 61 ± 4 | 60 ± 4 | 61 ± 4 |
| After | 59 ± 5 | 58 ± 4 | 59 ± 5 | |
| Brachial SBP, mmHg | Before | 130 ± 8 | 119 ± 8 † | 118 ± 6 † |
| After | 124 ± 6 * | 121 ± 5 | 115 ± 6 † | |
| Brachial DBP, mmHg | Before | 77 ± 4 | 71 ± 4 † | 69 ± 4 † |
| After | 74 ± 4 | 71 ± 4 † | 67 ± 4 † | |
| Brachial MAP, mmHg | Before | 98 ± 6 | 88 ± 5 † | 87 ± 5 † |
| After | 94 ± 5 | 90 ± 4 | 89 ± 5 | |
| Brachial PP, mmHg | Before | 54 ± 5 | 48 ± 4 | 48 ± 4 |
| After | 50 ± 4 | 50 ± 2 | 48 ± 3 | |
| Carotid SBP, mmHg | Before | 119 ± 8 | 108 ± 7 † | 108 ± 6 † |
| After | 114 ± 6 * | 110 ± 5 | 105 ± 6 † | |
| Carotid PP, mmHg | Before | 43 ± 5 | 37 ± 4 † | 38 ± 4 † |
| After | 39 ± 4 * | 39 ± 3 | 38 ± 4 † | |
| Carotid arterial compliance, mm2/mmHg |
Before | 0.072 ± 0.006 | 0.089 ± 0.008 † | 0.084 ± 0.007 † |
| After | 0.080 ± 0.008 * | 0.080 ± 0.009 | 0.077 ± 0.006 | |
Data are mean±SEM. SBP, systolib blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure.
P<0.05 vs before training.
P<0.05 vs. baseline.
Figure 1.
Venous plasma concentration of endothelin-1 before and after 3 mo of regular aerobic exercise training in healthy middle-aged and older adults. Data are mean±SEM.
There were no significant changes in heart rate and brachial pulse pressure with ETA/B-receptor blockade before and after the exercise training (Table 2). Brachial systolic and diastolic blood pressures, carotid systolic blood pressure, and carotid pulse pressure significantly decreased with the administration of ETA/B-receptor antagonist both before and after the exercise training, but the magnitude of these changes were not different before and after the exercise training.
Basal (resting) carotid arterial compliance increased significantly after exercise training (Table 2). Before the exercise intervention, carotid arterial compliance increased significantly with ETA/B-receptor blockade (+23.6±3.8% and +17.3±6.2%; 60 min and 120 min after the ETA/B-receptor blockade, respectively; Fig. 2). After the exercise intervention, however, carotid arterial compliance did not change significantly after the administration of ETA/B-receptor antagonist (+0.4±5.9% and −2.5±3.6%; 60 min and 120 min after the ETA/B-receptor blockade, respectively; Fig. 2). Responses of carotid arterial compliance to the ETA/B-receptor blockade were significantly greater before than after the exercise intervention. These differential responses in arterial compliance before and after exercise training remained statistically significant (P<0.05) even after the influence of corresponding changes in mean arterial pressure was accounted for using ANCOVA. Additionally, changes in arterial compliance were not significantly related to the corresponding changes in mean arterial blood pressure.
Figure 2.
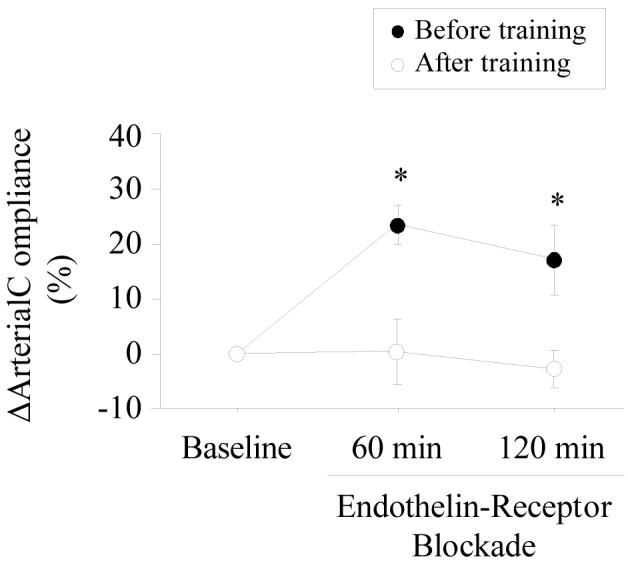
Changes in carotid artery compliance before and after ETA/B-receptor blockade. Data are mean±SEM. *P<0.05 vs. after exercise training.
DISCUSSION
Using the pharmacological approach, we determined whether ET-1 was involved in the physiological mechanism underlying the influence of regular aerobic exercise on central arterial compliance. We found that regular aerobic exercise training increased central arterial compliance in middle-aged and older adults. The improvement in arterial compliance was associated with decreased plasma ET-1 concentration. Additionally, increases in central artery compliance observed with the ETA/B-receptor blockade before the exercise intervention were abolished after the exercise training. These results suggest that endogenous ET-1 plays a role, at least in part, in the mechanism underlying the habitual exercise-induced increase in central arterial compliance.
It is well established that increased vascular tone of vascular smooth muscle cells decrease arterial compliance. Numerous local and neurohumoral factors could influence the vasoconstrictor tone exerted by its smooth muscle cells. Among them, as the most potent vasoconstrictor peptide released by the endothelial cells (Yanagisawa et al. 1988, Rubanyi & Polokoff 1994), ET-1 has been implicated in the pathogenesis of arterial stiffening (McEniery et al. 2003, Vuurmans et al. 2003). McEniery et al. (2003) reported that the administration of ET-1 increases arterial pulse wave velocity (PWV), a traditional index of regional arterial stiffness, and treatment with ET receptor antagonist reduces PWV. In the present study, we demonstrated that directly-measured central arterial compliance increased with ETA/B-receptor blockade before the exercise training intervention. Taken together, these results indicate that endogenously-generated ET-1 contributes to the regulation of arterial stiffness and arterial compliance in humans.
After 3 months of regular aerobic exercise, we observed a significant decline in plasma ET-1 concentration in middle-aged and older humans. This is consistent with our previous exercise training studies in young adults (Maeda et al. 2001). However, plasma ET-1 concentration may not provide a good measure of ET-1 activity in the tissue. For example, in deoxycorticosterone acetate-salt hypertensive rats, the tissue ET-1 concentration in the blood vessels is higher than that in normotensive rats without a change in plasma ET-1 levels (Lariviere et al. 1993). Moreover, in rats with cardiac hypertrophy induced by aortic banding, tissue ET-1 concentration in the heart is higher than that in control sham-operated rats without a change in plasma ET-1 levels (Yorikane et al. 1993). Therefore, the present investigation using the pharmacological blockade of ET receptors was needed to determine the involvement of ET-1 in modifying central arterial compliance.
We found that before the exercise training intervention, arterial compliance increased significantly with the administration of ETA/B-receptor blockade. After the exercise training, however, such increases in arterial compliance were abolished as there was no statistically significant increase in carotid artery compliance with the ET receptor blockade. These results indicate that chronic restraint placed on large elastic arteries by ET-1 was reduced with aerobic exercise training, resulting in improved arterial compliance.
There are several limitations of this study that should be emphasized. First, no test was conducted to confirm completeness of ETA/B-receptor blockade. Second, in order to target the compliance of “central (cardiothoracic)” arteries, where cardiac ejection is effectively buffered, systemic, rather than local, administration of drug was used in this study. Third, the present study only deals with the effects of ET-1. It is likely that other vasodilators could produce a reduction in arterial compliance after exercise training. Indeed, using the gene microarray analyses of rat aorta, we demonstrated that prostaglandin EP2 receptor (PGE-EP2R), prostaglandin EP4 receptor (PGE-EP4R), C-type natriuretic peptide (CNP), and endothelial nitric oxide synthase (eNOS) genes were differentially expressed after a period of aerobic exercise training (Maeda et al. 2005). Fourth, a lack of appropriate control condition (e.g., sedentary control group and control infusion) and a small sample size are clearly limitations of this study.
In conclusion, the present study has demonstrated that regular aerobic exercise increased central arterial compliance in middle-aged and older adults. The improvements in arterial compliance were associated with a reduction in plasma ET-1 concentration. Moreover, the increase in central arterial compliance observed with the ET-receptor blockade before the exercise intervention was eliminated after the exercise training intervention. These results indicate that endogenous ET-1 participates in the mechanism underlying the beneficial influence of regular aerobic exercise on central arterial compliance.
ACKNOWLEDGMENTS
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (18300215, 18650186) and NIH grant AG20966.
Footnotes
CONFLICT OF INTEREST
We have no financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
REFERENCES
- Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension. 1995;26:48–54. doi: 10.1161/01.hyp.26.1.48. [DOI] [PubMed] [Google Scholar]
- Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol Heart Circ Physiol. 1994;266:H693–H701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol. 2003;91:1519–1522. doi: 10.1016/s0002-9149(03)00416-8. [DOI] [PubMed] [Google Scholar]
- Dobrin PB, Rovick AA. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969;217:1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- Kakiyama T, Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M. Effects of short-term endurance training on aortic distensibility in young males. Med Sci Sports Exerc. 2005;37:267–271. doi: 10.1249/01.mss.0000152733.12578.5a. [DOI] [PubMed] [Google Scholar]
- Kumazaki T, Fujii T, Kobayashi M, Mitsui Y. Aging- and growth-dependent modulation of endothelin-1 gene expression in human vascular endothelial cells. Exp Cell Res. 1994;211:6–11. doi: 10.1006/excr.1994.1051. [DOI] [PubMed] [Google Scholar]
- Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lariviere R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993;21:294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Maeda S, Iemitsu M, Miyauchi T, Kuno K, Matsuda M, Tanaka H. Aortic stiffness and aerobic exercise: mechanistic insight from microarray analyses. Med Sci Sports Exerc. 2005;37:1710–1716. doi: 10.1249/01.mss.0000175052.37087.f8. [DOI] [PubMed] [Google Scholar]
- Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001;69:1005–1016. doi: 10.1016/s0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol. 2003;95:336–341. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. doi: 10.1016/j.jacc.2003.06.016. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation. 2004;110:2858–2863. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Mutoh Y, Yoshioka N, Sadamoto T. PWC75%HRmax: a measure of aerobic work capacity. Sports Med. 1985;2:159–164. doi: 10.2165/00007256-198502030-00001. [DOI] [PubMed] [Google Scholar]
- Osler W. The Principles and Practice of Medicine. D. Appleton and Company; New York: 1892. [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- Tanaka H, Clevenger CM, Jones PP, Seals DR, DeSouza CA. Influence of body fatness on the coronary risk profile of physically active postmenopausal women. Metabolism. 1998;47:1112–1120. doi: 10.1016/s0026-0495(98)90286-4. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005;18:137–144. doi: 10.1016/j.amjhyper.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension. 2007;50:403–409. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- Vuurmans TJ, Boer P, Koomans HA. Effects of endothelin-1 and endothelin-1 receptor blockade on cardiac output, aortic pressure, and pulse wave velocity in humans. Hypertension. 2003;41:1253–1258. doi: 10.1161/01.HYP.0000072982.70666.E8. [DOI] [PubMed] [Google Scholar]
- Weber C, Schmitt R, Birnboeck H, Hopfgartner G, van Marle SP, Peeters PA, Jonkman JH, Jones CR. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yorikane R, Sakai S, Miyauchi T, Sakurai T, Sugishita Y, Goto K. Increased production of endothelin-1 in the hypertrophied rat heart due to pressure overload. FEBS Lett. 1993;332:31–34. doi: 10.1016/0014-5793(93)80476-b. [DOI] [PubMed] [Google Scholar]