Abstract
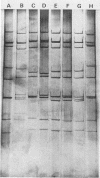
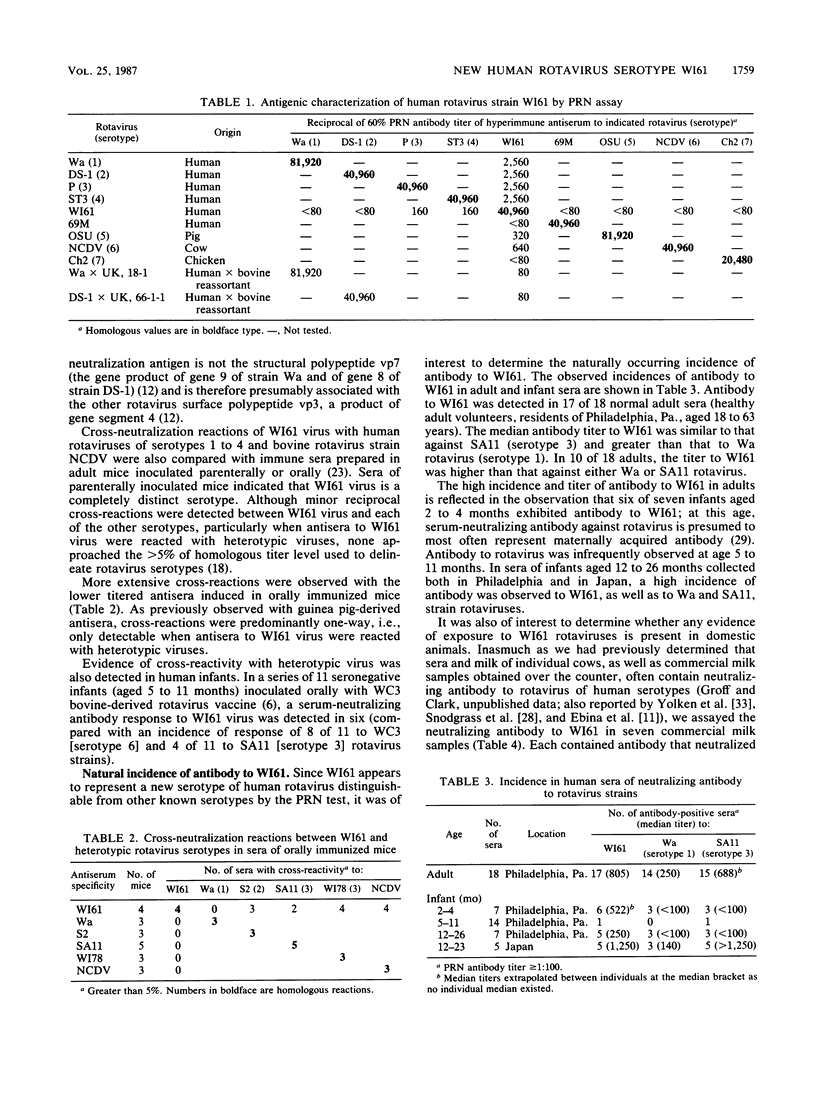
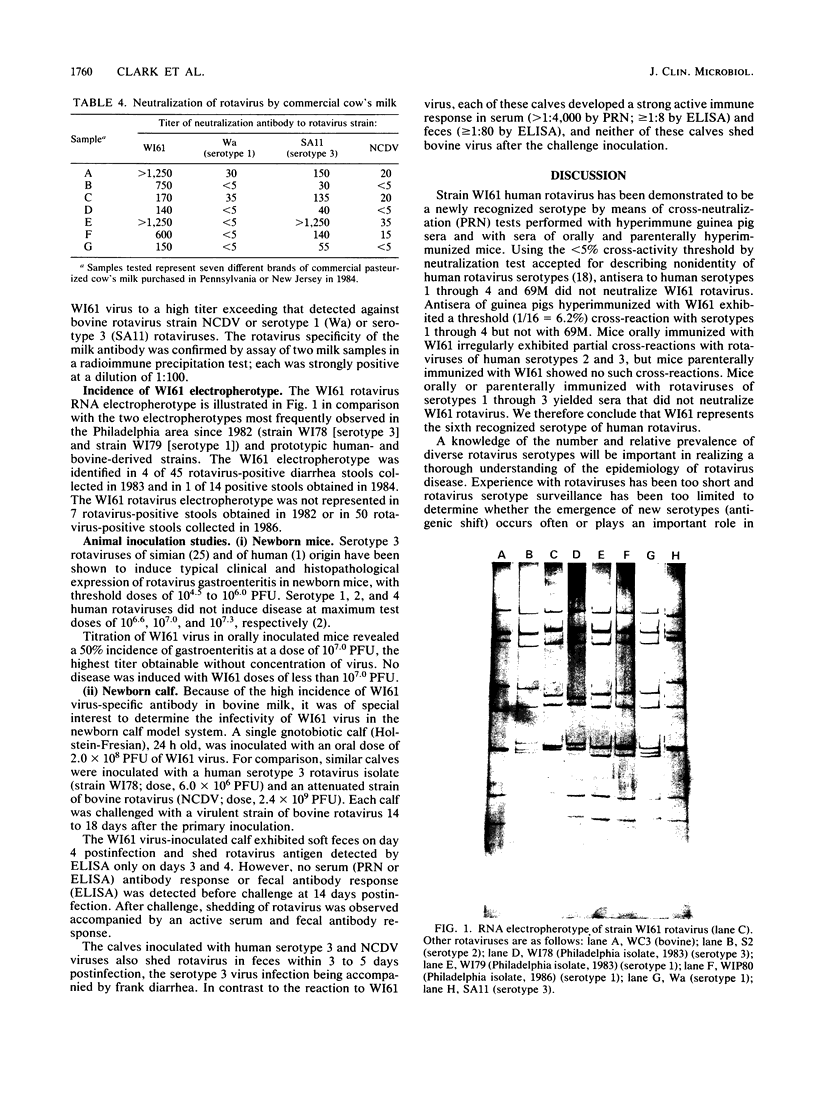
A virus (strain WI61) representing a presumptive new human serotype was isolated from an 18-month-old child with gastroenteritis admitted to Children's Hospital of Philadelphia in February 1983. The WI61 virus was clearly distinguished by cross-neutralization tests from human rotaviruses of serotypes 1, 2, 3, and 4, human 69M, and representative bovine (NCDV), porcine (OSU), and chicken (Ch2) rotaviruses. Antisera generated in guinea pigs hyperimmunized to WI61 virus displayed a partial cross-reactivity with rotaviruses of human serotypes 1, 2, 3, and 4. By means of studies with reassortant rotaviruses, it was presumptively determined that the WI61 virus cross-reactive antigenic determinants are localized on the vp3 surface polypeptide coded by gene segment 4. The characteristic RNA genome electropherotype of WI61 virus was observed in 5 of 59 cases of infant gastroenteritis detected in 1983 and 1984 but has not been observed in a subsequently at Children's Hospital. Serotype WI61-specific neutralizing antibodies were observed in a majority of sera of normal adults and infants of less than 4 or greater than 12 months of age collected in the Philadelphia area. Median antibody titers to WI61 equaled or exceeded those to rotaviruses of serotype 1 or 3. Each of seven samples of commercial cow's milk exhibited neutralizing antibodies to WI61 virus at a titer greater than or equal to that to serotype 1 or 3 or bovine (strain NCDV) rotavirus. However, WI61 rotavirus did not induce disease or a specific serum-neutralizing antibody response when fed to a caesarean-derived colostrum-deprived newborn calf. WI61 rotavirus caused diarrhea in newborn mice with a 50% diarrhea-inducing dose of 10(7.0) PFU.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Bell L. M., Clark H. F., O'Brien E. A., Kornstein M. J., Plotkin S. A., Offit P. A. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc Soc Exp Biol Med. 1987 Jan;184(1):127–132. doi: 10.3181/00379727-184-rc2. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Theil K. W., Saif L. J. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984 Feb;19(2):105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Yokoyama T., Nakata S., Morita Y., Urasawa T., Taniguchi K., Urasawa S., Nakao T. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. Lancet. 1986 Aug 23;2(8504):417–421. doi: 10.1016/s0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Dolan K. T., Horton-Slight P., Palmer J., Plotkin S. A. Diverse serologic response to rotavirus infection of infants in a single epidemic. Pediatr Infect Dis. 1985 Nov-Dec;4(6):626–631. doi: 10.1097/00006454-198511000-00006. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Furukawa T., Bell L. M., Offit P. A., Perrella P. A., Plotkin S. A. Immune response of infants and children to low-passage bovine rotavirus (strain WC3). Am J Dis Child. 1986 Apr;140(4):350–356. doi: 10.1001/archpedi.1986.02140180084030. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Offit P. A., Dolan K. T., Tezza A., Gogalin K., Twist E. M., Plotkin S. A. Response of adult human volunteers to oral administration of bovine and bovine/human reassortant rotaviruses. Vaccine. 1986 Mar;4(1):25–31. doi: 10.1016/0264-410x(86)90094-0. [DOI] [PubMed] [Google Scholar]
- Dolan K. T., Twist E. M., Horton-Slight P., Forrer C., Bell L. M., Jr, Plotkin S. A., Clark H. F. Epidemiology of rotavirus electropherotypes determined by a simplified diagnostic technique with RNA analysis. J Clin Microbiol. 1985 May;21(5):753–758. doi: 10.1128/jcm.21.5.753-758.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L. Rotaviral gastroenteritis--some recent developments. J Infect Dis. 1984 May;149(5):663–666. doi: 10.1093/infdis/149.5.663. [DOI] [PubMed] [Google Scholar]
- Ebina T., Sato A., Umezu K., Ishida N., Ohyama S., Oizumi A., Aikawa K., Katagiri S., Katsushima N., Imai A. Prevention of rotavirus infection by oral administration of cow colostrum containing antihumanrotavirus antibody. Med Microbiol Immunol. 1985;174(4):177–185. doi: 10.1007/BF02123694. [DOI] [PubMed] [Google Scholar]
- Flewett T. H. Rotavirus vaccines--achievements and prospects. Arch Dis Child. 1986 Mar;61(3):211–212. doi: 10.1136/adc.61.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul S. K., Simpson T. F., Woode G. N., Fulton R. W. Antigenic relationships among some animal rotaviruses: virus neutralization in vitro and cross-protection in piglets. J Clin Microbiol. 1982 Sep;16(3):495–503. doi: 10.1128/jcm.16.3.495-503.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Kornstein M. J., Plotkin S. A. A murine model for oral infection with a primate rotavirus (simian SA11). J Virol. 1984 Jul;51(1):233–236. doi: 10.1128/jvi.51.1.233-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J Infect Dis. 1985 Dec;152(6):1152–1158. doi: 10.1093/infdis/152.6.1152. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Plotkin S. A. Response of mice to rotaviruses of bovine or primate origin assessed by radioimmunoassay, radioimmunoprecipitation, and plaque reduction neutralization. Infect Immun. 1983 Oct;42(1):293–300. doi: 10.1128/iai.42.1.293-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Shinozaki T., Fujii R., Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69(2):155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Taniguchi K., Chiba S. Serotype determination of human rotavirus isolates and antibody prevalence in pediatric population in Hokkaido, Japan. Arch Virol. 1984;81(1-2):1–12. doi: 10.1007/BF01309292. [DOI] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25(10):1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Losonsky G. A., Vonderfecht S., Leister F., Wee S. B. Antibody to human rotavirus in cow's milk. N Engl J Med. 1985 Mar 7;312(10):605–610. doi: 10.1056/NEJM198503073121002. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Zissis G., Brandt C. D., Rodriguez W. J., Kim H. W., Parrott R. H., Urrutia J. J., Mata L., Greenberg H. B. Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med. 1978 Nov 23;299(21):1156–1161. doi: 10.1056/NEJM197811232992103. [DOI] [PubMed] [Google Scholar]
- de Zoysa I., Feachem R. G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull World Health Organ. 1985;63(3):569–583. [PMC free article] [PubMed] [Google Scholar]