SUMMARY
The Sir2 family of proteins are broadly conserved NAD+-dependant deacetylases that are implicated in diverse biological processes including DNA regulation, metabolism and longevity. Sir2 proteins are regulated in part by the cellular concentrations of a noncompetitive inhibitor, nicotinamide, that reacts with a Sir2 reaction intermediate via a base exchange reaction to reform NAD+ at the expense of deacetylation. To gain a mechanistic understanding of nicotinamide inhibition in Sir2 enzymes, we captured the structure of nicotinamide bound to a Sir2 homologue, yeast Hst2, in complex with its acetyl-lysine 16 histone H4 substrate and a reaction intermediate analog, ADP-HPD. Together with related biochemical studies and structures, we identify a nicotinamide inhibition and base exchange site that is distinct from the so-called “C pocket” binding site for the nicotinamide group of NAD+. These results provide insights into the Sir2 mechanism of nicotinamide inhibition and have important implications for the development of Sir2-specific effectors.
INTRODUCTION
The class III family of histone deacetylases, silent information regulator 2 (Sir2) proteins, require NAD+ to remove an acetyl moiety from the ε-amino group of lysine residues within protein targets (Imai et al., 2000; Landry et al., 2000; Smith et al., 2000) to yield the deacetylated protein target, nicotinamide, and 2′-O-acetyl-ADP-ribose (Jackson and Denu, 2002; Sauve et al., 2001). Sir2 proteins are broadly conserved from bacteria to humans (Brachmann et al., 1995), and they are able to deacetylate numerous proteins in addition to histones, including acetyl-coA synthetase (Starai et al., 2002), α-tubulin (North et al., 2003), myoD (Fulco et al., 2003), p53, FOXO, Ku70, and NF-κB (Longo and Kennedy, 2006). Their ability to deacetylate such a wide range of substrates has implicated them to play a stimulatory role in a wide spectrum of biological functions including DNA recombination (Gottlieb and Esposito, 1989) and repair (Bennett et al., 2001), longevity, transcriptional silencing, apoptosis, axonal protection, insulin signaling and fat mobilization (Longo and Kennedy, 2006). In addition, increased dosage or expression of Sir2 has been shown to increase lifespan in yeast, worms, flies, and mice, and increased longevity due to a calorie restricted diet has been shown in most of these animals to be Sir2 dependant (Longo and Kennedy, 2006). Conversely, decreased Sir2 activity due to gene deletion or enzyme inhibition shortens yeast lifespan (Kaeberlein et al., 1999). A recent study also reports that deletion of the mammalian SIRT6 homologue in mice results in genomic instability and an aging-like phenotype (Mostoslavsky et al., 2006).
Sir2 proteins couple the removal of the acetyl moiety of acetyl-lysine to cleavage of the high energy glycosidic bond between nicotinamide and ADP-ribose in β-NAD+. Nicotinamide, a reaction product and noncompetitive inhibitor of Sir2 proteins (Bitterman et al., 2002; Landry et al., 2000), has also been shown to be a physiological regulator of this family of proteins (Schmidt et al., 2004). Yeast cells grown in the presence of nicotinamide show a dramatic reduction in silencing, an increase in rDNA recombination, and a shortening of replicative lifespan (Bitterman et al., 2002). Nicotinamide can also inhibit Sir2 deacetylation of p53 in mouse embryonic fibroblast cells upon DNA damage (Luo et al., 2001), and of histones H3 and H4 in human embryonic kidney cells, which leads to loss of repression by COUP transcription factor-interacting proteins 1 and 2 (Senawong et al., 2003). Depletion of nicotinamide by overexpression of PCN1, a gene that encodes a nicotinamide deaminase, is sufficient to activate Sir2 and extend yeast lifespan (Anderson et al., 2003; Gallo et al., 2004).
Structural studies of the catalytic core region of Sir2 homologues reveal a large and conserved Rossmann fold domain, a smaller and more structurally diverse zinc binding domain, and a series of loops connecting the two domains, forming the catalytic cleft where the substrates bind (Min et al., 2001). Several structures of Sir2 proteins in complex with NAD+ in a nonproductive conformation (Avalos et al., 2004; Chang et al., 2002; Min et al., 2001; Zhao et al., 2003b) make clear that simultaneous acetyl-lysine binding is required for NAD+ to adopt a productive conformation where it is catalytically competent (Avalos et al., 2004; Zhao et al., 2004). In this productive conformation, the nicotinamide group of NAD+ is bound in a highly conserved “C pocket” (Min et al., 2001). It has been a matter of debate as to whether this highly conserved pocket (Avalos et al., 2004) or an alternative pocket that we call the “D pocket,” identified by modeling studies (Zhao et al., 2004), is the binding site for the inhibitory nicotinamide molecule. Although structures of A. fulgidus Sir2-Af2 bound to NAD+ or ADP-ribose and T. maritima SirTm bound to acetyl-lysine in the presence of high concentrations of nicotinamide show nicotinamide bound in the highly conserved C pocket (Avalos et al., 2005), neither of these are the complexes to which an inhibitory nicotinamide molecule is expected to bind.
To address the structural basis for nicotinamide inhibition and exchange in the context of a relevant Sirtuin complex, we report the structure of nicotinamide bound to the Sir2 homologue, yeast Hst2, in complex with its acetyl-lysine 16 histone H4 substrate and a reaction intermediate analogue, ADP-HPD. This structure reveals a nicotinamide inhibition and base exchange “D pocket” that is distinct from the C pocket binding site for the nicotinamide group of NAD+. We also present related biochemical studies and structures that support this site as physiologically relevant for nicotinamide regulation of sirtuins.
RESULTS AND DISCUSSION
Structure of Nicotinamide Bound to an yHst2/Acetyllysine/ADP-HPD Ternary Complex
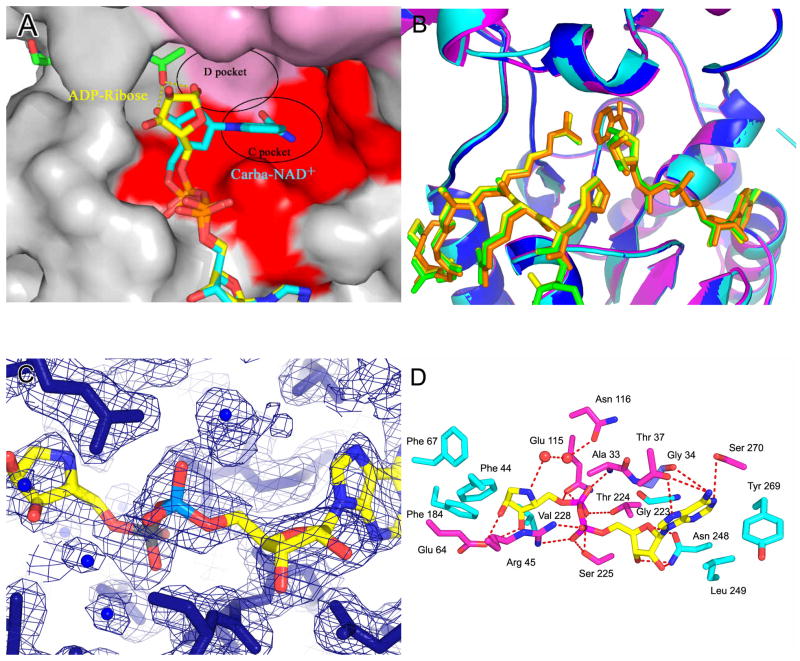
We had previously reported on the structure of a ternary complex of yHst2 bound to an acetyl-lysine 16 histone H4 derived peptide and carba-NAD+, a non-hydrolysable NAD+ analogue (Slama and Simmons, 1988; Slama and Simmons, 1989; Zhao et al., 2004) (Supplemental Figure 1A). The structure reveals that the acetyl group of the acetyl-lysine substrate hydrogen bonds to the 2′ and 3′ hydroxyl groups of the cyclopentane ring, presumably to help position the nicotinamide group in the highly conserved C pocket for hydrolysis, leaving the acetyl group inappropriately positioned for nucleophilic attack of the 1′ carbon. A comparison of this structure with a ternary complex in which ADP-ribose replaces carba-NAD+, reveals that the ribose ring is rotated by about 90° relative to its corresponding position in carba-NAD+ with the 1′-hydroxyl group of the ADP-ribose ring pointing into another highly conserved, hydrophobic “D pocket” that could accommodate an incoming nicotinamide group for a transglycosidation reaction to reform β-NAD+ (Zhao et al., 2004) (Figure 1A).
Figure 1. Structures of the free yHst2/ADP-HPD/histone H4 complex.
(A) Ternary yHst2 (gray) complex, highlighting strictly conserved (pink) and conserved (red) residues, the binding sites of acetyl-lysine (green), carba-NAD+ (cyan) and ADP-ribose (yellow) and the conserved C and D pockets. Hydrogen bonds between the acetyl-lysine and carba-NAD+ are shown as yellow dotted lines. Residues 43–48 of the flexible loop and residue 64 were omitted for clarity.
(B) Superimposition of the yHst2/ADP-ribose/H4 complex (magenta) with the yHst2/ADP-HPD/H4 complex (cyan) and the yHst2/ADP-HPD/H4 complex bound to nicotinamide (blue). The intermediate analogue, acetylated histone H4 ligands, and nicotinamide are shown in green for the ADP-ribose complex, yellow for the free ADP-HPD complex, and orange for the nicotinamide bound ADP-HDP complex.
(C) Simulated annealing omit density contoured at 1.0 sigma showing density for the protein (blue) and ADP-HPD (atoms individually colored). Water molecules are shown as blue spheres.
(D) yHst2 bound to ADP-HPD (atoms individually colored) and highlighting residues that make hydrogen bonds (red dashed lines) or van der Waals contacts with ADP-HPD. Hydrogen bonding residues are colored pink, residues that make van der Waals interactions are colored cyan, and residues that make both interactions are colored purple.
To trap free nicotinamide bound to a relevant sirtuin complex, we prepared crystals of a ternary complex containing yHst2, an acetyl-lysine 16 histone H4 peptide and the intermediate analogue ADP-HPD, and soaked these crystals with high concentrations of nicotinamide. We chose ADP-HPD as an intermediate analogue for these studies because of its similarity to the proposed positively charged oxocarbenium ion reaction intermediate that is expected to form directly after cleavage of the glycosidic bond between nicotinamide and ADP-ribose (Supplemental Figure 1A) (Slama et al., 1995).
Crystals of the ternary yHst2/ADP-HPD/histone H4 complex in the presence and absence of nicotinamide were isomorphous to the previously described yeast Hst2/ADP-ribose/histone H4 crystals (Zhao et al., 2004) and formed in the space group P3221 containing one molecule per asymmetric unit. The structures were determined by molecular replacement and refined to resolutions of 2.05 Å and 2.00 Å, respectively (Table 1).
Table 1.
Data collection and refinement statistics.
| yHst2/ADP-HPD/H4 | yHst2/ADP-HPD/H4 + nicotinamide | yHst2 I117F/carba-NAD+/H4 | |
|---|---|---|---|
| Data Collection | |||
| Space Group | P3221 | P3221 | P3221 |
| Cell parameters, Å | a = b= 106.76, c = 67.7 | a = b = 105.94, c = 67.1 | a = b = 106.65, c = 67.7 |
| Resolution, Å | 50-2.00 | 50-2.05 | 50-2.07 |
| Unique reflections | 28626 | 27580 | 25169 |
| Completeness, %* | 94.2 (94.0) | 99.9 (99.6) | 91.9 (94.5) |
| Multiplicity* | 6.5 (6.1) | 6.1 (5.8) | 6.0 (5.9) |
| I/σ* | 53.3 (6.6) | 20.0 (5.6) | 31.5 (10.6) |
| Rmerge, %*† | 6.4 (39.6) | 11.1 (32.0) | 4.6 (11.3) |
| Refinement Statistics | |||
| Rwork, %‡ | 22.5 | 21.9 | 22.5 |
| Rfree, %§ | 23.3 | 24.6 | 23.3 |
| Number of atoms | |||
| Protein# | 2312 | 2286 | 2313 |
| H4 peptide | 56 | 61 | 56 |
| Carba-NAD+# | 44 | ||
| ADP-HPD | 35 | 35 | |
| Nicotinamide | 9 | ||
| Water | 116 | 133 | 180 |
| Zn ions# | 1 | 1 | 1 |
| Rms deviations | |||
| Bond length, Å | 0.007 | 0.006 | 0.007 |
| Bond angles, ° | 1.2 | 1.2 | 1.3 |
| B-factors, Å2 | 34.5 | 36.9 | 41.5 |
Values in parentheses are from the highest resolution shell.
Values for each molecule in the asymmetric unit.
Rmerge = Σ|I − ‹I›|/Σ‹I›.
Rworking = Σ||Fo| − |Fc||/Σ|Fo|.
Rfree = ΣT||Fo| − |Fc||/ΣTb|Fo|, where T is a test set of a percentage (5% for all structures) of the total reflections randomly chosen and set aside before refinement.
The structure of the nicotinamide-bound and free complexes are very similar to each other, with an rms deviation of 0.235 Å for Cα atoms and the acetyl-lysine and ADP-HPD ligands are also almost identical between these two structures and the previously described ADP-ribose containing complex (Figure 1B). The ribose-ring mimic of ADP-HPD is bound in a largely hydrophobic pocket and makes only one polar interaction in which a network of water molecules bridge a hydrogen bond between the ring nitrogen of ADP-HPD and N116 (Figure 1D).
The Nicotinamide Binding Site
The most significant difference between the nicotinamide-bound and free complexes is the presence of a nicotinamide molecule bound in the highly conserved D pocket, adjacent to the β-face of the ADP-HPD molecule in the nicotinamide-bound complex (Figures 2A, 2B and 2C). This pocket is distinct from the C pocket of the nicotinamide moiety of the substrate NAD+. It is mostly hydrophobic and formed by residues E64, F184, and F67 around the pyridine ring, and F44 and F117 proximal to the carboxyamide moiety (Figures 2C). Of the residues that form pocket D, F44, F67, N116 and F184 are strictly conserved, and E64 and I117 are only conservatively substituted, implying that this binding site may be important for nicotinamide regulation of Sir2 proteins. Since ADP-HPD is analogous to the proposed oxocarbenium intermediate, this conformation of bound nicotinamide is consistent with a nicotinamide molecule binding to the protein complex after the initial nicotinamide cleavage and reacting with the oxocarbenium intermediate, reforming β-NAD+. Importantly, the conformation of nicotinamide as described would only be consistent with the exclusive formation of β-NAD+.
Figure 2. The nicotinamide binding pocket.
(A) Fo-Fc map contoured at 2.0 sigma (blue) prior to the addition of nicotinamide for protein refinement, and simulated annealing omit density contoured at 1.0 sigma (pink) showing nicotinamide density for the ternary yHst2/ADP-HPD/H4 complex soaked with nicotinamide. The protein is shown in blue and the acetyl-lysine, ADP-HPD and nicotinamide atoms are shown individually colored.
(B) Corresponding 2 sigma Fo-Fc map for the unsoaked crystals.
(C) Interactions between nicotinamide (atoms individually colored), yHst2 (white backbone) and acetyl-lysine 16 of histone H4 (green backbone). Residues that make van der Waals interactions and hydrogen bonds (red) to nicotinamide are shown.
(D) yHst2 bound to nicotinamide, acetyllysine, and ADP-HPD highlighting residues that make van der Waals or hydrogen bonding interactions with nicotinamide.
(E) yHst2 in a view roughly orthogonal to and with the same color coding as (D), but highlighting residues that form the tunnel (red) through which nicotinamide is proposed to diffuse.
(F) yHst2 (gray) shown in surface representation with tunnel residues from the yHst2/carba-NAD+ (pink), yHst2/2′-O-acetyl ADP-ribose/Acetyl-lysine (cyan), yHst2/carba-NAD+/Acetyl-lysine (yellow) and yHst2/ADP-HPD/Acetyl-lysine (purple) structures highlighted.
The nicotinamide molecule has a slightly higher B factor, 74 Å2, than the average B factor for the protein atoms in that complex, and the density for the carboxyamide better defined than for the pyridine ring (Figure 2A), suggesting greater flexibility of the pyridine ring. The carboxyamide oxygen of nicotinamide makes a water mediated hydrogen bond to the backbone nitrogen of I117, while the carboxyamide nitrogen makes water mediated hydrogen bonds to the backbone nitrogen of I117, and the sidechain carbonyl oxygen of N116 and a phosphate oxygen of the ADP-HPD ligand (Figure 2C). The pyridine ring of the nicotinamide molecule also makes van der Waals interactions with F44 and F67 (Figure 2C). Residues F44, F67, N116, and I117 are strictly conserved across Sir2 homologues, highlighting the significance of these interactions. Since the nicotinamide pyridine ring makes exclusively van der Waals interactions in the binding pocket and the density for this region is not as clear as for the carboxyamide moiety of nicotinamide, the ring is probably free to rotate in the binding pocket. We propose that when acetyl-lysine is bound, the pyridine ring flips away from the acetyl group and towards the β face of the ribose ring where it can carry out nucleophilic attach of the 1′ carbon of the ribose ring of the oxocarbenium ion intermediate. The kinetics of both the cleavage of nicotinamide from NAD+ to form the oxocarbenium intermediate, and the attack of the 2′-OH on the acetyl carbonyl carbon are fast as compared to the overall reaction rate (Smith and Denu, 2006). Although enzyme where the general base, H135, is mutated to alanine accumulates the α-1′-O-alkylamidate intermediate, wild-type enzyme accumulates neither the α-1′-O-alkylamidate intermediate or the oxocarbenium intermediate (Smith and Denu, 2006), indicating that the presence of nicotinamide in its binding site when or immediately after the intermediate forms will determine whether base exchange or deacetylation chemistry will occur. Our structure clearly shows that both nicotinamide and the oxocarbenium intermediate are capable of binding simultaneously to the enzyme active site in a conformation suitable for base exchange chemistry.
In order for nicotinamide to bind the enzyme/oxocarbenium/acetyl-lysine complex, there must be a tunnel that leads from the enzyme active site to solvent in this complex. Indeed, in the nicotinamide bound complex there is a hydrophobic tunnel from the nicotinamide binding site to bulk solvent (Figures 2D and 2E). The tunnel is formed by residues 37–43 of the flexible β1-α2 loop and is approximately 9×8 Å in diameter, a sufficient size to accommodate a nicotinamide molecule. In the nicotinamide bound complex, the tunnel is occupied by a number of water molecules that make hydrogen bonds mainly to backbone atoms. We propose that after cleavage of NAD+ and the formation of the oxocarbenium intermediate, nicotinamide diffuses from solvent or the C pocket through this hydrophobic tunnel to the enzyme active site where it participates in base exchange. This hypothesis is supported by a superposition of residues that form this tunnel in a number of yHst2 complexes, as substrate or intermediate ternary complexes show an open conformation that would allow nicotinamide to diffuse out or into the tunnel, respectively, while, binary or product complexes show a more closed tunnel (Figure 2F).
Mutations that Affect Nicotinamide Inhibition
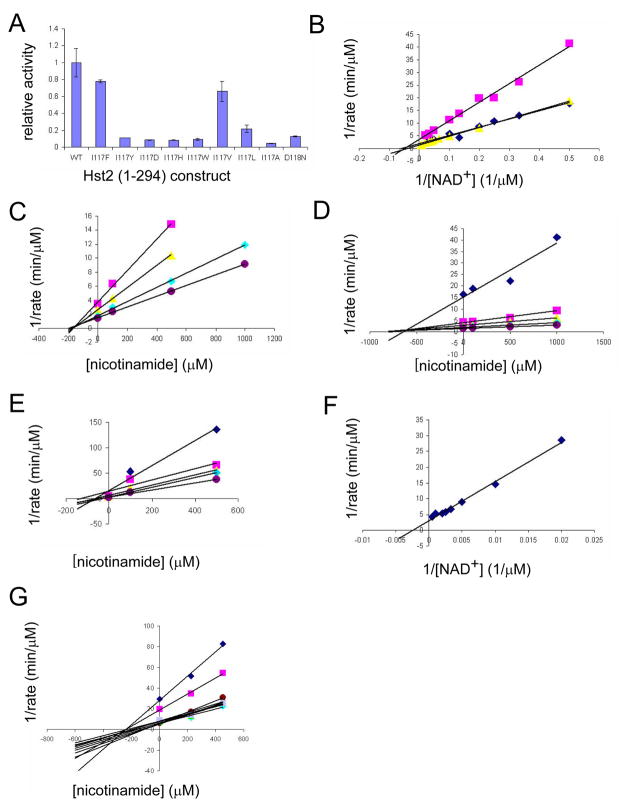
The sidechain of residue I117 sits in the back of the hydrophobic D pocket to which nicotinamide is bound in the yHst2/ADP-HPD/histone H4 nicotinamide bound complex structure, and participates in van der Waals interactions with nicotinamide (Figure 2D). In order to further probe the physiological relevance of this free nicotinamide binding site, we mutated yHst2 residue I117 to several residues and measured the effect of these mutations on nicotinamide inhibition. We found that mutations to very large (Y, W), small (A), or charged (H, D) residues rendered the enzyme nearly or completely catalytically inactive, while the mutations I117V or I117F were nearly as active as the wild-type protein (Figure 3A). Bisubstrate kinetic analysis of the I117V or I117F mutants revealed that they had slightly higher Km values for NAD+ relative to the wild-type enzyme, 25.5 μM, 25.8 μM, and 16.1 μM, respectively (Figure 3B).
Figure 3. Activity, kinetic, and inhibition data for wild-type and mutant yHst2.
(A) Relative activity of wild-type and mutant yHst2 enzymes based on their ability to deacetylate a fluorescently-labeled acetylated peptide in duplicate. Error bars represent one SD of experiments done at least in triplicate.
(B) Lineweaver-Burk plot describing the NAD+ binding kinetics of wild-type yHst2 (blue diamonds), the yHst2 I117F and I117V mutants (pink squares, yellow triangles, respectively) done in triplicate.
(C) Dixon plot describing the nicotinamide inhibition of wild-type yHst2 at varying concentrations of NAD+, 15 μM (pink squares), 25 μM (yellow triangles), 50 μM (cyan diamonds), and 80 μM (purple circles). Each line is fit to an equation for a noncompetitive inhibitor with obtained in triplicate.
(D) Dixon plot describing the nicotinamide inhibition of yHst2 I117F with conditions as described in (C), but also including 5 μM NAD+ (blue diamonds).
(E) Dixon plot describing the nicotinamide inhibition of yHst2 I117V with conditions as described in (D).
(F) Lineweaver-Burk plot describing the NAD+ binding kinetics of yHst2 D118N (blue diamonds) with data obtained in triplicate.
(G) Dixon plot describing the nicotinamide inhibition of yHst2 D18N at varying concentrations of NAD+, 50 μM (blue diamonds), 100 μM (pink squares), 250 μM (yellow squares), 450 μM (cyan diamonds), 600 μM (purple circles), 800 μM (maroon circles), 1000 μM (blue minus signs), 1250 μM (green triangles), 1500 μM (cyan plus signs), 2000 μM (gray diamonds).
Since the I117V and I117F yHst2 mutations had near wild-type activity for deacetylation, we compared their ability to be inhibited by nicotinamide. We found the Kii for nicotinamide to be 1000 ± 30 μM (Kis = 720 ± 40 μM) for the I117F mutant enzyme, a nearly 6-fold increase over the Kii for nicotinamide of wild-type enzyme, 170 ± 28 μM (Kis = 140 ± 3 μM) (Figures 3C and 3D). This finding is consistent with the bulkier phenylanine residue partially occluding the nicotinamide binding site within the D pocket. Notably, this increase is much larger than would be expected if free nicotinamide bound in the C pocket because the increased Km for NAD+ is less than 2-fold for the I117F mutant enzyme as compared to the wild-type enzyme. Conversely, we found the Kii for nicotinamide to be 65 ± 40 μM (Kis = 65 ± 1.1 μM) for the I117V mutant enzyme (Figure 3E), a nearly 2.5-fold decrease over the Kii for nicotinamide of the wild-type enzyme. This result is also consistent with the notion that the smaller valine residue slightly expands the D pocket, therefore making this pocket slightly more sensitive to nicotinamide inhibition. Together, these functional studies supports the structural finding that the D pocket is the site for nicotinamide inhibition.
An earlier study had reported that nicotinamide can also inhibit Sir2 enzymes through the same binding pocket that binds the nicotinamide group of NAD+, the C pocket (Avalos et al., 2005). In support of this hypothesis, the authors reported that a D101N mutation in Sir2Tm, a Sir2 homologue from the thermaphilic bacterium Thermotoga maritima, resulted in an enzyme that was significantly compromised for both NAD+ binding and sensitivity to nicotinamide inhibition. The native aspartic acid residue participates in hydrogen bonding interactions with the amide group of the nicotinamide moiety of NAD+, an interaction that is also observed via the corresponding aspartic acid 118 of yHst2 in the yHst2/carba-NAD+/acetyl-lysine histone H4 complex. In order to address the significance of the C pocket in nicotinamide inhibition of yHst2, we prepared the corresponding D118N mutation in yHst2 and characterized its enzymatic properties. As illustrated in Figure 3A, the D118N mutant retained approximately 13% of the activity of the wild-type protein, with an apparent Km for NAD+ of 453.5 μM, a more than 28-fold increase over the wild-type Km for NAD+ (Figures 3B and 3F). This increase in Km for NAD+ is expected because substitution of aspartic acid with asparagine disrupts the important hydrogen bond between the amide group of NAD+ and the Asp118 side chain. Next, we measured the Kii for nicotinamide of the D118N mutant. Strikingly, we found the Kii for nicotinamide for D118N to be 180 ± 50 μM (Ki = 320 ± 30 μM) (Figure 3G), very similar to the wild-type value of 170 ± 28 μM. This result indicates that a yHst2, mutation of the C pocket residue, Asn118, has a negligible effect on the sensitivity of the enzyme to nicotinamide inhibition, leading us to the conclusion that the D pocket plays a more significant role that the C pocket for nicotinamide inhibition of yHst2 and likely other eukaryotic deacetylases.
Structure of yHst2 I117F in Ternary Complex with Acetyl-lysine 16 histone H4 and Carba-NAD+
To further support the conclusion that the sidechain of residue 117 occupies the free nicotinamide binding site, pocket D, and when mutated to phenylalanine, occludes a physiologically relevant nicotinamide binding site, we determined the structure of yHst2 I117F bound to an acetylated histone H4-derived peptide and carba-NAD+. This complex was isomorphous to the previously described wild-type complex (Zhao et al., 2004) and to the ADP-HPD containing yHst2 complexes described here. The structure was solved by molecular replacement and refined to 2.0 Å (Table 1).
The yHst2 I117F complex superimposes very well with the wild-type complex. Notably, the hydrogen bonding distance between the carboxyamide carbonyl oxygen of carba-NAD+ and the backbone nitrogen of residue 117 is virtually identical, 2.94 Å and 2.89 Å, respectively, between the mutant complex and the wild-type complex, supporting the conclusion that a mutation to residue I117 has a very minor effect on NAD+ binding, and would be expected to have a similarly minor effect on nicotinamide binding, if free nicotinamide bound in the site previously occupied by the nicotinamide moiety of NAD+, as has been proposed (Avalos et al., 2005; Avalos et al., 2004).
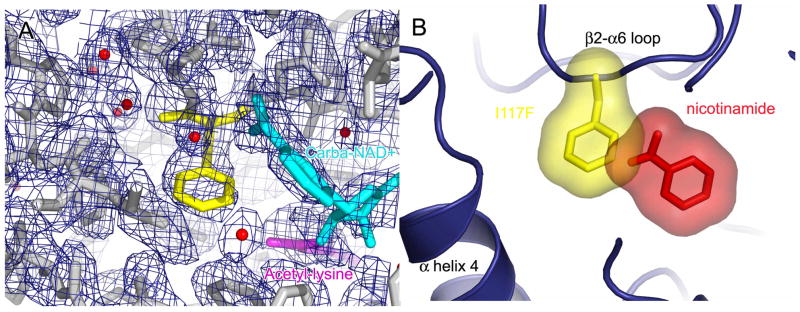
The structure of the yHst2 I117F complex shows clear density for the phenylalanine side chain of residue 117 protruding into the free nicotinamide binding pocket described here (Figure 4A), and not into the C pocket that would be occupied by the nicotinamide moiety of NAD+. The phenylalanine side chain does not seem to alter the conformation of the nicotinamide moiety of carba-NAD+, nor disrupt the interactions this moiety makes with any protein residues. Since the yHst2 I117F/carba-NAD+/histone H4 complex is isomorphous with the nicotinamide bound yHst2/ADP-HPD/histone H4 complex, and their protein molecules have an rms deviation of 0.25 Å2 for Cα atoms, the two complex structures were superimposed. A view of the nicotinamide binding site of the superimposed complexes shows that the I117F sidechain indeed protrudes into the free nicotinamide binding site, pocket D, in a way that is incompatible with nicotinamide binding as it is seen in the wild-type enzyme (Figure 4B), consistent with the accompanying structural and biochemical studies pointing to the importance of the D pocket for nicotinamide inhibition and base exchange in Sir2 enzymes.
Figure 4. The overall structure of yHst2 I117F bound to carba-NAD+ and acetyl-lysine.
(A) Simulated annealing omit density contoured at 1.0 sigma showing density for the protein (gray), carba-NAD+ (cyan), acetyllysine (magenta) and the mutated I117F residue (yellow).
(B) The superposition of the nicotinamide bound yHst2/ADP-HPD/H4 and the yHst2 I117F/carba-NAD+/H4 structures. The yHst2 protein is shown in blue while the mutated I117F sidechain (yellow) and the nicotinamide molecule (red) are shown both in stick and modeled in terms of the van der Waals radius of each atom of the molecules.
Implications for the Modulation of Sir2 Proteins by Small Molecules
The involvement of Sir2 proteins in a growing number of cellular processes makes them attractive therapeutic drug targets for the development of small molecule effectors. The structure reported here with bound nicotinamide suggests a rational approach for the development of both Sir2 specific inhibitors and activators. Compounds that bind in the nicotinamide binding site, D pocket, and either sterically block the subsequent acetylation reaction or react with the oxocarbenium ion intermediate may act as effective Sir2-specific inhibitors, while compounds that cannot react with the reaction intermediate and do not perturb the acetylation reaction, would function as Sir2 activators by alleviating nicotinamide inhibition. Since endogenous levels of nicotinamide limit Sir2 activity in yeast cells (Sauve et al., 2005), relief of nicotinamide inhibition is a physiologically viable approach to Sir2 activation. Using a similar approach, isonicotinamide was tested as a potential Sir2 activator (Sauve et al., 2005), although activation by isonicotinamide was found to require high concentrations (10–100 mM) (Sauve et al., 2005). Taken together, we anticipate that the structural elucidation of the nicotinamide inhibitory and base exchange site for Sir2 enzymes reported here will pave the way for a new generation of Sir2 effectors that exploit this site.
EXPERIMENTAL PROCEDURES
Protein preparation, crystallization and structure determination
The 64-residue C-terminal deletion construct of yHst2 (residues 1–294) and point mutants were purified and expressed as previously described (Zhao et al., 2003a). Point mutations in yHst2 (1–294) were generated from the pRSET-A plasmid overexpressing the N-terminal His6-tagged fusion protein with site directed mutagenesis based on the QuikChange protocol from Stratagene (Papworth et al., 1996).
Crystals of the yHst2/ADP-HPD/H4 and the yHst2 I117F/carba-NAD+/H4 complexes were grown using the vapor diffusion method at room temperature and were obtained by equilibrating about 0.15 mM of the respective complex against a reservoir solution containing 2.0 M (NH4)2SO4, 100 mM Na citrate, pH 5.6, 200 mM K/Na tartrate or 2.0 M (NH4)2SO4 and 100 mM Na citrate, pH 5.5. To obtain nicotinamide bound complex, yHst2/ADP-HPD/H4 crystals were soaked with reservoir solution supplemented with 50 mM nicotinamide. All crystals were flash frozen in reservoir solution supplemented with 25% (vol/vol) glycerol for data collection.
All crystallographic data was collected on the A1 beamline at CHESS, and processed with the HKL2000 suite (HKL Research, Charlottesville, VA). The structures were solved with the program AMoRe (Navaza, 1994) using the yHst2/ADP-ribose/H4 structure (PDB code 1SZD) as a molecular replacement model for the yHst2/ADP-HPD/H4 and yHst2/ADP-HPD/H4 + nicotinamide complex structures and the yHst2/carba-NAD+/H4 structure (PDB code 1SZC) as a molecular replacement model for the yHst2 I117F/carba-NAD+/H4 complex structure. Structures were refined with CNS (Brunger et al., 1998) with model building with O (Jones et al., 1991) with the nicotinamide built into 1Fo-Fc difference density of the yHst2/ADP-HPD/H4 + nicotinamide structure at the end of the refinement. The final models were checked with composite-simulated annealing omit maps.
Kinetic Assays
All enzymatic assays were carried out at room temperature in a buffer containing 25 mM Tris-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, and 1 mM MgCl using a deacetylase fluorescent activity assay kit (AK-555, Biomol Research Laboratories). 1 μM protein, and 500 μM of both NAD+ and fluorogenic acetyl-lysine substrate were used and reactions were quenched after 15 min. by the addition of 10 mM nicotinamide inhibitor and fluorogenic-lysine developer. For NAD+ Km measurements, 1 μM protein, saturating fluorogenic acetyl-lysine substrate (100 μM), and varying concentrations of NAD+ (0.5–5000 μM) were used. Data taken in triplicate were fitted to the equation 1/v = (Km/Vmax)(1/[S]) + 1/Vmax, using a root mean least squares approach in the form of a double reciprocal Lineweaver-Burk plot where the x-intercept is equal to −1/Km. For nicotinamide Ki measurements, 1 μM protein, saturating fluorogenic acetyl-lysine substrate (100 μM) and varying amounts of NAD+ (5–2000 μM) and nicotinamide (0–1mM) were used. Data taken in triplicate were fitted to the equation 1/v = (1+Km)/VmaxKii * [I] + 1/Vmax(1+Km/[S]), using a root mean least squares approach in the form of a Dixon plot for a strict noncompetitive inhibitor where Kii is equal to −x at the x-intercept. The slopes of the Dixon Plot were replotted versus 1/[NAD+], and the slope of the corresponding line was set equal to Km/(Vmax*Ki), in order to calculate Ki.
Supplementary Material
Acknowledgments
We thank M. Fitzgerald and R. Anand for useful discussions. This work was supported by a NIH grants (CA107107) to R.M. and B.S. (CA 09171) and a grant from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health awarded to the Wistar Institute. This work is based upon research conducted, in part, at CHESS, which is supported by the NSF (DMR 0225180) and NIH (RR-01646).
Footnotes
ACCESSION NUMBERS Coordinates for the structures yHst2/ADP-HPD/H4, yHst2/ADP-HPD/H4 + nicotinamide, and yHst2 I117F/carba-NAD+/H4 have been deposited in the Protein Data Bank (PDB) under the accession numbers 2OD7, 2OD9, and 2OD2, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Avalos JL, Boeke JD, Wolberger C. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol Cell. 2004;13:639–648. doi: 10.1016/s1097-2765(04)00082-6. [DOI] [PubMed] [Google Scholar]
- Bennett CB, Snipe JR, Westmoreland JW, Resnick MA. SIR functions are required for the toleration of an unrepaired double-strand break in a dispensable yeast chromosome. Mol Cell Biol. 2001;21:5359–5373. doi: 10.1128/MCB.21.16.5359-5373.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Chang JH, Kim HC, Hwang KY, Lee JW, Jackson SP, Bell SD, Cho Y. Structural basis for the NAD-dependent deacetylase mechanism of Sir2. J Biol Chem. 2002;277:34489–34498. doi: 10.1074/jbc.M205460200. [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Gallo CM, Smith DL, Jr, Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol. 2004;24:1301–1312. doi: 10.1128/MCB.24.3.1301-1312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Jackson MD, Denu JM. Structural identification of 2′- and 3′-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta -NAD+-dependent histone/protein deacetylases. J Biol Chem. 2002;277:18535–18544. doi: 10.1074/jbc.M200671200. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallographica Section A . 1991;A47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Min J, Landry J, Sternglanz R, Xu RM. Crystal structure of a SIR2 homolog-NAD complex. Cell. 2001;105:269–279. doi: 10.1016/s0092-8674(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Navaza J. AMoRe: an automated package for molecular replacement. Acta Crystallographica Section A . 1994;A50:157–163. [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Papworth C, Braman J, Wright DA. Strategies. 1996;9:3–4. [Google Scholar]
- Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL. Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions. Biochemistry. 2001;40:15456–15463. doi: 10.1021/bi011858j. [DOI] [PubMed] [Google Scholar]
- Sauve AA, Moir RD, Schramm VL, Willis IM. Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell. 2005;17:595–601. doi: 10.1016/j.molcel.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Schmidt MT, Smith BC, Jackson MD, Denu JM. Coenzyme specificity of Sir2 protein deacetylases: implications for physiological regulation. J Biol Chem. 2004;279:40122–40129. doi: 10.1074/jbc.M407484200. [DOI] [PubMed] [Google Scholar]
- Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. J Biol Chem. 2003;278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama JT, Aboul-Ela N, Goli DM, Cheesman BV, Simmons AM, Jacobson MK. Specific inhibition of poly(ADP-ribose) glycohydrolase by adenosine diphosphate (hydroxymethyl)pyrrolidinediol. J Med Chem. 1995;38:389–393. doi: 10.1021/jm00002a021. [DOI] [PubMed] [Google Scholar]
- Slama JT, Simmons AM. Carbanicotinamide adenine dinucleotide: synthesis and enzymological properties of a carbocyclic analogue of oxidized nicotinamide adenine dinucleotide. Biochemistry. 1988;27:183–193. doi: 10.1021/bi00401a028. [DOI] [PubMed] [Google Scholar]
- Slama JT, Simmons AM. Inhibition of NAD glycohydrolase and ADP-ribosyl transferases by carbocyclic analogues of oxidized nicotinamide adenine dinucleotide. Biochemistry. 1989;28:7688–7694. doi: 10.1021/bi00445a025. [DOI] [PubMed] [Google Scholar]
- Smith BC, Denu JM. Sir2 protein deacetylases: evidence for chemical intermediates and functions of a conserved histidine. Biochemistry. 2006;45:272–282. doi: 10.1021/bi052014t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- Zhao K, Chai X, Clements A, Marmorstein R. Structure and autoregulation of the yeast Hst2 homolog of Sir2. Nat Struct Biol. 2003a;10:864–871. doi: 10.1038/nsb978. [DOI] [PubMed] [Google Scholar]
- Zhao K, Chai X, Marmorstein R. Structure of the yeast Hst2 protein deacetylase in ternary complex with 2′-O-acetyl ADP ribose and histone peptide. Structure. 2003b;11:1403–1411. doi: 10.1016/j.str.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Zhao K, Harshaw R, Chai X, Marmorstein R. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD(+)-dependent Sir2 histone/protein deacetylases. Proc Natl Acad Sci U S A. 2004;101:8563–8568. doi: 10.1073/pnas.0401057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.