Abstract
The objective of this study was to test the hypothesis that 4 weeks of lithium administration would be associated with changes in brain gray and white matter volumes in healthy individuals. Thirteen right-handed healthy volunteers (6 females, mean age = 25.9 ± 10.0 y) were studied. 3D SPGR MRIs (TR = 25ms, TE = 5ms, slice-thickness = 1.5mm) were acquired using a 1.5 T GE Signa Imaging System, at baseline and after 4 weeks of lithium administration at therapeutically relevant doses. Optimized voxel based morphometry (VBM) analyses were conducted. Left and right dorsolateral prefrontal cortex and left anterior cingulate gray matter volumes increased significantly following lithium administration. Total white matter volume was increased, whereas total brain volume and total gray matter volume were not significantly changed following 4 weeks of lithium. Lithium treatment resulted in prefrontal regional gray matter volume increases in healthy volunteers, as well as increases in total white matter volume. Whether these changes are mediated by neurotrophic/neuroprotective or osmotic effects remains unknown.
Keywords: lithium, neuroprotection, neuroimaging, cingulate gyrus, white matter
Introduction
Lithium is the most widely used mood stabilizer for the treatment of bipolar disorder. It affects multiple cellular signaling pathways in the brain [1] and has neurotrophic and neuroprotective effects [2]. Studies utilizing anatomical region-of-interest [3,4] and cortical pattern matching [5] methods, as well as neurochemical imaging [6], demonstrated detectable brain changes related to lithium treatment in bipolar patients. Specifically, both cross-sectional [4,5] and longitudinal prospective [3] studies demonstrated increased total gray matter volumes in bipolar patients treated with lithium compared to both untreated patients and age- and sex-matched healthy comparisons. Furthermore, proton magnetic resonance spectroscopy studies demonstrated increased levels of total brain N-acetyl-aspartate, a putative marker of neuronal viability, myelin formation and maintenance, after 4 weeks of lithium in bipolar patients and healthy volunteers [6]. The effect of lithium on the healthy human brain is an intriguing finding, and it may help clarify the mechanisms by which lithium exerts its effects in bipolar patients.
We previously measured the metabolite levels for N-acetyl-aspartate, glycerophosphocholine plus phosphocholine and myo-inositol in the left and right dorsolateral prefrontal cortices (DLPFC) in a sample of healthy individuals before and after 4 weeks of lithium administration, and found no significant changes [7]. The objective of this study was to complement these neurochemical results with neuroanatomical measurements, using voxel-based morphometry (VBM), a technique that aims to identify differences in the local composition of brain tissue, after discounting large scale differences in gross anatomy and position [8] and BRAINS2 [9]. We hypothesized that chronic lithium administration would be associated with changes in brain gray and white matter volumes in the prefrontal subregions (i.e. DLPFC, anterior cingulate) in healthy individuals.
Materials and Methods
Subjects
This study protocol was approved by the University of Pittsburgh Biomedical IRB. All subjects gave their informed consent for participation in the study. The sample consisted of 13 right-handed healthy volunteers (6 females, 7 males; mean age = 25.9 ± 10.0 y, range 18-52 y). They were evaluated with the Structured Clinical Interview for DSM-IV non-patient version. The subjects had no current major medical illnesses, no past or current DSM-IV axis I disorders, or first-degree relative with any psychiatric disorders. MRI scans were done at baseline and after 4 weeks of lithium given at doses (600-1500 mg/day) that produced a mean blood level of 0.6 mEq/l throughout the 4 weeks, and 0.83 mEq/l at the end of the 4th week (mean blood lithium levels for weeks 1, 2 and 3 were 0.34 mEq/l, 0.53 mEq/l, 0.67 mEq/l, respectively). We started lithium at 300 mg/day, increased to 600 mg/day on the second day and maintained the dose at 600 mg/day for the first week. The 12-h lithium blood levels were obtained at the end of the first week and weekly thereafter, and doses were adjusted accordingly to maintain a minimum blood level of 0.6 mEq/l through the following 3 weeks.
Image Acquisition
MRIs were performed on a 1.5T GE Signa Imaging System running version Signa 5.4.3 software (General Electric Medical Systems, Milwaukee, WI). We used 3D gradient echo imaging (Spoiled Gradient Recalled Acquisition, SPGR), performed in the coronal plane (TR = 25 ms, TE = 5 ms; flip angle = 40°; FOV = 24 cm; slice thickness = 1.5 mm; voxel size = 1× 1 × 1.5 mm; NEX = 1; matrix size = 256 × 192), to obtain 124 images covering the entire brain.
Data Analysis
Image analysis was performed using a Linux (Fedora Core 4) workstation with MATLAB 7.1 (MathWorks, Natick, MA). Optimized VBM analyses were conducted with Statistical Parametrical Mapping (SPM2, Wellcome Department of Cognitive Neurology, University College London, UK). The images were preprocessed following the optimized VBM protocol [8]. The T1-weighted images were realigned and segmented into gray, white and CSF compartments, using the customized template from all T1 MRI scans by an optimized VBM script (http://dbm.neuro.uni-jena.de/vbm.html). The segmented images were modulated and smoothed with a 12-mm full-width half-maximum Gaussian kernel. Contrasts were performed to identify changes in gray and white matter volumes from baseline to lithium conditions using two-sided paired t-tests. We defined the cingulate, dorsolateral prefrontal cortex (DLPFC), amygdala and hippocampus bilaterally as regions of interest (ROI). We used small volume correction for ROI analysis. Significant results are reported at p < 0.05 corrected for multiple comparisons. All results are reported as Talairach coordinates using Talairach Daemon (ric.uthscsa.edu/projects/talairachdaemon.html). Talairach coordinates were obtained by applying Brett’s transformation (www.mrc=cbu.cam.ac.uk/Imaging/Common/mnispace.shtml) to the Montreal Neurological Institute (MNI) coordinates output by SPM2.
Total brain volume and total gray and white matter volumes were manually traced by one rater using BRAINS2 software, developed at the University of Iowa Hospitals and Clinics [9].
We used paired samples t test to calculate the change in total brain, total gray matter and white matter volumes following 4 weeks of lithium. Nonparametric correlations were used to calculate the correlation between mean lithium blood levels and the observed changes in total brain, total gray matter and white matter volumes and the significant ROIs.
Results
The mean lithium blood level at the end of four weeks was 0.83 ± 2.0 mEq/l, with a mean lithium dosage of 1281 ± 218 mg/day. In the paired samples t test, we found that total white matter volumes were increased by 2%, whereas total brain volume and total gray matter volume remained unchanged following 4 weeks of lithium administration (Tables 1 and 2).
Table 1.
Individual changes in total brain, total gray and white matter volumes (ml) before and after lithium administration as measured by BRAINS2.
| Subject | Total brain volume (pre- lithium) |
Total brain volume (post- lithium) |
Total gray volume (pre- lithium) |
Total gray volume (post- lithium) |
Total white volume (pre- lithium) |
Total white volume (post- lithium) |
|---|---|---|---|---|---|---|
| 1 | 1374.09 | 1379.97 | 696.29 | 706.50 | 471.22 | 476.23 |
| 2 | 1307.57 | 1305.66 | 661.56 | 662.00 | 432.91 | 446.09 |
| 3 | 1060.02 | 1069.65 | 531.74 | 531.94 | 348.38 | 350.26 |
| 4 | 1104.33 | 1092.64 | 572.22 | 567.84 | 353.30 | 359.67 |
| 5 | 1236.77 | 1253.88 | 636.10 | 650.40 | 397.41 | 406.40 |
| 6 | 1272.72 | 1272.72 | 679.85 | 684.43 | 402.51 | 406.74 |
| 7 | 1309.66 | 1309.63 | 694.57 | 703.04 | 413.88 | 427.14 |
| 8 | 1236.59 | 1219.12 | 633.29 | 633.65 | 396.66 | 399.52 |
| 9 | 1140.34 | 1137.05 | 592.80 | 589.99 | 362.55 | 369.83 |
| 10 | 1297.86 | 1300.66 | 718.39 | 713.83 | 409.39 | 419.91 |
| 11 | 1386.47 | 1379.70 | 708.84 | 705.68 | 467.75 | 451.39 |
| 12 | 1400.82 | 1393.43 | 753.41 | 723.53 | 478.45 | 505.76 |
| 13 | 1222.35 | 1231.67 | 638.16 | 643.23 | 398.04 | 402.21 |
Table 2.
Mean ± s.d. (ml) of total brain volume and total gray and white matter volumes before and after 4 weeks of lithium administration (paired samples t test).
| Region | Pre-lithium | Post-lithium | t | df | p |
|---|---|---|---|---|---|
| Total brain volume |
1257.7 ± 106.5 | 1257.4 ± 105.9 | 0.112 | 12 | 0.913 |
| Total gray volume |
655.2 ± 63.0 | 655.1 ± 61.0 | 0.029 | 12 | 0.977 |
| Total white volume |
410.2 ± 43.0 | 417.0 ± 45.0 | -2.552 | 12 | 0.025 |
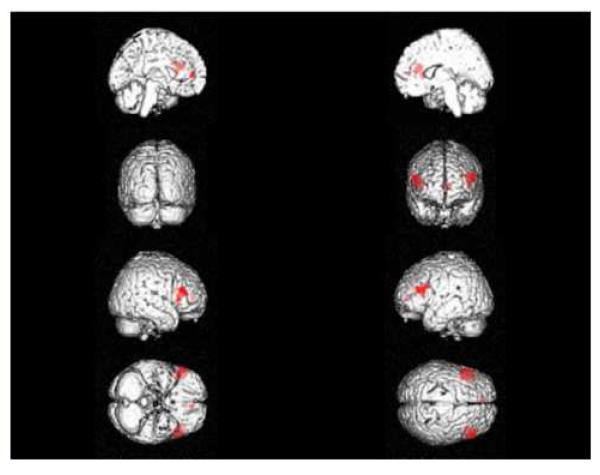
Left DLPFC (Brodmann’s area 46) gray matter volume was significantly increased following lithium administration (Talairach coordinates: x = -36, y = 32, z = 20, Z = 3.57, k = 349, pcorrected = 0.040) (Figure 1). Right DLPFC (Talairach coordinates: x = 54, y = 30, z = 23, Z = 4.02, k = 1212, pcorrected = 0.007) and left anterior cingulate (ACC - Brodmann 10) gray matter volumes also increased significantly following lithium administration (Talairach coordinates: x = -8, y = 46, z = 6; borderline between ACC and medial frontal gyrus, Z = 3.63, k = 479, pcorrected = 0.038) (Figure 1). None of the ROIs showed statistically significant change in white matter content, and none of the other ROIs showed statistically significant change in gray matter content with lithium administration.
Figure 1.
Regions of increased gray matter after 4 weeks of lithium administration are shown. The red areas show the significant increases in gray matter volumes in the left and right DLPFC and left ACC in 13 healthy volunteers after 4 weeks of lithium administration at therapeutically relevant doses. Right to left (and from top to bottom): left medial, right medial, posterior, anterior, right lateral, left lateral, ventral and dorsal views of the brain with the significant regions marked in red color.
Mean lithium blood levels were not significantly correlated with left and right DLPFC and left ACC gray matter changes, or with the changes observed in the brain and total white and gray matter volumes (nonparametric correlations, all p>0.05).
Discussion
VBM analyses revealed significant increases the left and right DLPFC and left ACC gray matter volumes in healthy individuals after 4 weeks of lithium administration. Also, total white matter volumes were increased by 2%, whereas total brain volume and total gray matter volume remained unchanged.
DLPFC and ACC are subdivisions of the prefrontal cortex, which is a primary brain region of interest in the pathophysiology of bipolar disorder. Both regions have been shown to be affected in bipolar disorder. Left ACC gray matter volume is reduced in adult [10] and juvenile [11] bipolar patients. Research employing a statistical parametric mapping approach also revealed reduced gray matter density in the fronto-limbic cortex, particularly in the cingulate [12,13], and in Bearden and colleagues’ study [5] greatest differences in gray matter density were found in the bilateral cingulate and paralimbic cortices in lithium-treated bipolar patients (n=20) compared to those not taking lithium (n=8) and healthy controls (n=28). The DLPFC is an understudied region in bipolar disorder, with one study [14] showing significantly smaller gray matter volumes in the left middle and superior, and the right middle and inferior prefrontal regions in 17 bipolar patients hospitalized for a manic episode and receiving various psychotropic medications, and another study demonstrating decreased gray matter volume in the left DLPFC in pediatric bipolar patients [15]. Bearden and colleagues [5] also noted greater gray matter density in the left DLPFC in lithium-treated bipolar patients compared to healthy controls. Taken together, the detected gray matter increases in these regions may be associated with the therapeutic effects of lithium, although we should note that our subjects were all healthy controls.
Apart from the regional changes in the ACC and DLPFC, total brain white matter volumes were increased after 4 weeks of lithium administration. One longitudinal [3] study showed an average 3% increase in total brain gray matter in eight out of ten bipolar patients treated with 4 weeks of lithium, while there were no changes in the white matter, or the regional cerebral water content (measured by magnetic resonance spectroscopy). Another study, although cross-sectional, showed that bipolar patients treated with lithium had greater total gray matter volumes than untreated bipolar patients and healthy controls, while there were no significant differences in total white matter volumes across three groups [4]. Also in Bearden and colleagues’ study [5], no white matter changes were detected. It is hard to explain why we found total white matter changes in healthy subjects who took lithium for four weeks, as most other studies failed to identify any effects in white matter. One explanation is that lithium may have different generalized effects in healthy and diseased brains. This global white matter increase is also of interest considering recent evidence for white matter pathology as an early marker for bipolar disorder [16] and lithium’s effect of causing formation of multiple axons in rat hippocampus neurons through inhibition of glycogen synthase kinase-3β [17].
These gray and white matter findings may reflect neuroprotective and neurotrophic effects of lithium, as suggested possibly by neuropil increase [2,3], as lithium also enhances neurogenesis and prevents injury to nerve cells via various insults [18]. Recently, Yucel and colleagues [19] examined the effects of lithium on hippocampal volume in a medication-naïve sample of bipolar patients, treated with 1-8 weeks of lithium, and showed that there was a bilateral increase in total hippocampus and hippocampal head volumes in patients treated with lithium, compared to unmedicated patients.
The osmotic effects of lithium, which may lead to neuron swelling, may provide an alternative explanation for the increased brain volumes following lithium administration. Phatak and colleagues [20] demonstrated that chronic (5 weeks, at 0.82 mEq/L blood lithium concentrations) lithium administration resulted in 3.1% elevation of tissue water content in frontal cortex and hippocampus and 0.8% elevation of tissue water content in cerebellum in rat brains [20]. This effect was statistically significant only in frontal gray matter (white matter was not sampled), and was not observed in rats treated with 11 days of lithium. Although the global white matter volume increase we observed may be due to an osmotic effect, we found significant gray matter effects only in certain prefrontal ROIs (cingulate and DLPFC), but not globally or in other ROIs (i.e. amygdala and hippocampus), a finding that makes the osmotic explanation unlikely.
One of the limitations of our study is that the lithium levels obtained were in the low range (∼0.6 mEq/l) throughout 4 weeks, although at the end of 4th week the mean lithium blood level was 0.83 mEq/l. Also, the small number of subjects involved in the study with the lack of a control group (untreated or with administration of placebo or another drug), and the absence of data on the test re-test reliability of the MRI machine are other potential limitations of this study. Although 7-17 days of lithium treatment does not seem to produce any significant changes in either T1 or T2 relaxation times in rat brains [21], the presence of lithium in the healthy human brain altering the T1 properties of cerebrospinal fluid, gray and white matter, so that one may be classified as the other in the repeat scan, is yet another point that must be kept in mind when interpreting our results.
In conclusion, 4 weeks of lithium administration led to significant increases in the left and right DLPFC and left ACC gray matter volumes and total white matter volume in healthy individuals. Whether these effects are due to increased neuropil or osmotic effects of lithium needs to be determined.
Acknowledgements
This work was partly supported by MH 01736, MH 30915, MH 68766, MH 068662, RR 20571, Veterans Administration (VA Merit Review), The Krus Endowed Chair in Psychiatry (UTHSCSA), and CAPES Foundation (Brazil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Corbella B, Vieta E. Molecular targets of lithium action. Acta Neuropsychiatrica. 2003;15:316–340. doi: 10.1046/j.1601-5215.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- [2].Manji HK, Moore GJ, Chen GG. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol. Psychiatry. 2000;48:740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- [3].Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- [4].Sassi RB, Nicoletti M, Brambilla PP, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci. Lett. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- [5].Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol. Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.10.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, Faulk MW, Koch S, Glitz DA, Jolkovsky L, Manji HK. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol. Psychiatry. 2000;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- [7].Brambilla P, Stanley JA, Sassi RB, Nicoletti MA, Mallinger AG, Keshavan MS, Soares JC. 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–1924. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- [8].Mechelli A, Price CJ, Friston KJ, Ashburner JJ. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- [9].Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput. Med. Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- [10].Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol. Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [11].Kaur S, Sassi RB, Axelson DD, Nicoletti M, Brambilla P, Monkul ES, Hatch JP, Keshavan MS, Ryan N, Birmaher B, Soares JC. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am. J. Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- [12].Doris A, Belton E, Ebmeier KP, Glabus MF, Marshall I. Reduction of cingulate gray matter density in poor outcome bipolar illness. Psychiatry Res. 2004;130:153–159. doi: 10.1016/j.pscychresns.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [13].Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biol. Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- [14].Lopez-Larson MP, DelBello MP, Zimmerman ME, Schweirs ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol. Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- [15].Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch. Gen. Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- [16].Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am. J. Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- [17].Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [18].Wada A, Yokoo H, Yanagita T, Kobayashi H. Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J. Pharmacol. Sci. 2005;99:307–321. doi: 10.1254/jphs.crj05009x. [DOI] [PubMed] [Google Scholar]
- [19].Yucel K, Taylor VH, McKinnon MC, MacDonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301405. in press. [DOI] [PubMed] [Google Scholar]
- [20].Phatak P, Shaldivin A, King LS, Shapiro P, Regenold WT. Lithium and inositol: effects on brain water homeostasis in the rat. Psychopharmacology (Berl) 2006;186:41–47. doi: 10.1007/s00213-006-0354-y. [DOI] [PubMed] [Google Scholar]
- [21].Komoroski RA, Pearce JM. Localized 7Li MR spectroscopy and spin relaxation in rat brain in vivo. Magn. Reson. Med. 2004;52(1):164–168. doi: 10.1002/mrm.20112. [DOI] [PubMed] [Google Scholar]