Abstract
Evidence from past studies indicates that adults and children with Obsessive-Compulsive Disorder (OCD) and Tourette syndrome (TS) experience subtle neuropsychological deficits. Less is known about neuropsychological functioning of children and adolescents with a symptom course consistent with the PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection) subgroup of OCD and tics. To provide such information, we administered three tests of attention control and two of executive function to 67 children and adolescents (ages 5–16) diagnosed with OCD and/or tics and a symptom course consistent with the PANDAS subgroup and 98 healthy volunteers (HV) matched by age, sex, and IQ. In a paired comparison of the two groups, the PANDAS subjects were less accurate than HV in a test of response suppression. Further, in a two-step linear regression analysis of the PANDAS group in which clinical variables were added stepwise into the model and in the second step matching variables (age, sex, and IQ) were added, IQ emerged as a predictor of performance on this task. In the same analysis, ADHD diagnosis and age emerged as predictors of response time in a continuous performance task. Subdividing the PANDAS group by primary psychiatric diagnosis revealed that subjects with TS or OCD with tics exhibited a longer response time compared to controls than subjects with OCD only, replicating previous findings within TS and OCD. This study demonstrates that children with PANDAS exhibit neuropsychological profiles similar to those of their primary psychiatric diagnosis.
Keywords: PANDAS, Executive functioning, Attention, Obsessive-compulsive disorder, Tourette syndrome
Introduction
The PANDAS subgroup (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections) is defined by five criteria: diagnosis of Obsessive-Compulsive Disorder (OCD) and/or tic disorder; prepubertal symptom onset; abrupt onset and/or episodic course; neurological abnormalities during symptom exacerbation; and evidence for a temporal association between symptom exacerbations and preceding infections with Group A beta-hemolytic streptococci (Swedo et al., 1998). The etiologic model for PANDAS is Sydenham's chorea (SC), the neurological manifestation of rheumatic fever, in which antistreptococcal antibodies cross-react with basal ganglia antigens and induce inflammatory changes (Kirvan, Swedo, Heuser, & Cunningham, 2003; Kirvan, Swedo, Kurahara, & Cunningham, 2006; Kirvan, Swedo, Snider, & Cunningham, 2006). Neuropsychological functioning in PANDAS has not been assessed previously, although the cognitive functioning of OCD and Tourette syndrome (TS) has been studied in both children and adolescents.
Attention in OCD and Tic Disorders
In OCD, research on attention has primarily focused on adults and has shown that performance on tests of attention generally has not differed from matched controls (see Greisberg & McKay, 2003 for review). Only two studies of children with OCD have administered attentional tasks. In a study comparing 21 children with OCD to 21 age-, sex-, socioeconomic-status-, and intelligence-matched controls, Beers et al. (1999) found that subjects with OCD performed better on the Stroop test and the Go/No-Go tasks (Drewe, 1975). In another study, Andrés et al. (2007) administered a battery of neuropsychological tasks to 35 children with OCD without comorbidities, and age- and sex-matched controls. They found no significant differences between the OCD and control groups on the Wechsler Intelligence Scale for Children-Revised (WISC-R) Digit Span or Coding (Wechsler, 1974), and moderate differences between groups on the Stroop Test, with the OCD group performing worse. Studies in TS have produced similarly mixed results; the only consistent attentional deficit has been a longer response time (RT) in a computerized continuous performance task.
Executive Functioning in OCD and Tic Disorders
Executive functioning is thought to include the ability to plan, to organize, to reason, to shift, and to inhibit (Baron, 2004). Kuelz, Hohagen, and Voderholzer (2004) reviewed the literature on executive functioning in OCD and tic disorders, separating test results into three categories: set-shifting ability, fluency, and conceptual thinking and planning ability. In the set-shifting domain, no consistent differences were found between controls and subjects with OCD on the Wisconsin Card Sorting Test (WCST), though subjects with OCD have shown decreased performance compared to controls on tests sensitive to orbitofrontal dysfunction, like the Object Alternation Test (OAT; Freedman, 1990). The authors found 17 controlled studies reported results for fluency tests; in 7 of these, the OCD group performed more poorly than the controls; however, in the other 10 reports, the OCD group scored as well or better than the controls. In the conceptual thinking domain, the authors reviewed three studies using the Tower of London, and four using the Tower of Hanoi. Five of these seven studies found decreased speed for the OCD group, but all found accuracy to be comparable between the OCD and control groups. In children with OCD, Andrés et al. (2007) found no differences between children with OCD and controls on the FAS or on two of three WCST variables. Beers et al. (1999) found no differences between the OCD and control groups on the WCST, Tower of Hanoi, or the FAS. In TS, executive functioning has been assessed most commonly using the WCST and the Trailmaking Test (TMT); however there have been no consistent findings (Como, 2001).
Studies of patients with focal brain lesions and brain-imaging studies support the commonly accepted connection between hyperactivity in orbitofrontal-subcortical circuits and OCD (Saxena, Bota, & Brody, 2001) and TS (Mink, 2006). Likewise, enlarged basal ganglia have been observed in periods of exacerbation through structural magnetic resonance imaging (MRI) in SC (Giedd et al., 1995), and a similar correlation between obsessions, compulsions, and/or tics and basal ganglia enlargement has been shown among children in the PANDAS subgroup (Giedd, Rapoport, Garvey, Perlmutter, & Swedo, 2000; Giedd, Rapoport, Leonard, Richter, & Swedo, 1996). Attention and executive functioning are thought to rely on similar neural circuits, suggesting that children with OCD and TS would show impairment on these tasks. Thus, neuropsychological assessment of children with PANDAS may add to knowledge of OCD and TS, as well as conferring information about the unique attributes of the subgroup.
The primary goal of this study was to compare the neuropsychological performance of children with PANDAS with healthy volunteers. As a secondary goal, we sought to distinguish the demographic and clinical variables that were related to the outcome variables.
Methods
Subjects
All subjects (Table 1) were recruited from the community for research protocols at the National Institute of Mental Health (NIMH) within the National Institutes of Health in Bethesda, MD. Neuropsychological testing was performed as part of larger baseline and follow-up assessments in those research investigations. Verbal or written assent for study participation was obtained from each of the pediatric subjects and written assent was provided by their parent(s). The NIMH Institutional Review Board approved the protocols.
Table 1.
Demographic Characteristics for the Study Sample.
| PANDAS (n = 67) M (SD) |
HV (n = 98) M (SD) |
|
|---|---|---|
| Age (yrs) | 8.6 (2.0) | 8.9 (2.1) |
| Full-scale IQ | 110.6 (13.8) | 111.5 (12.2) |
| Age of onset (yrs) | 7.4 (2.2) | |
| Time between onset and testing (yrs) | 1.2 (1.3) | |
| CY-BOCS total score | ||
| All PANDAS subjects (n = 63) | 11.6 (10.7) | |
| PANDAS with OCD and OCD + Tic Disorder (n = 38) | 17.0 (9.3) | |
| YGTSS total score | ||
| All PANDAS subjects (n = 62) | 11.9 (9.7) | |
| PANDAS with Tourette syndrome (n = 25) | 16.0 (7.9) | |
| NIMH Depression (n = 65) | 3.2 (2.1) | |
| PANDAS (n = 67) N (%) |
HV (n = 98) N (%) |
|
| Male | 44 (65%) | 70 (71%) |
| Ethnicity | ||
| White, not Hispanic | 66 (98%) | 87 (88%) |
| Asian or Pacific Islander | 1 (1%) | 2 (2%) |
| Black, not Hispanic | 9 (9%) | |
| Primary diagnosis | ||
| OCD | 15 (22%) | |
| Tourette syndrome | 27 (40%) | |
| OCD + tic disorder | 25 (37%) | |
| Comorbid ADHD | 13 (19%) | |
| Comorbid MDD | 3 (4%) | |
| Subjects taking at least one medication1 | 20 (29%) | |
Abbreviations: PANDAS, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection; HV, healthy volunteer; CY-BOCS, Children's Yale-Brown Obsessive-Compulsive Scale (range: 0–40); YGTSS, Yale Global Tic Severity Scale (range: 0–50); OCD, Obsessive-Compulsive Disorder; TS, Tourette syndrome; NIMH Depression, National Institute of Mental Health Global Depression rating scale (range: 1–15); ADHD, attention deficit/hyperactivity disorder; MDD, Major Depressive Disorder.
Some patients were taking more than one medication; see text for details.
Full-scale IQ was tested by a licensed clinical psychologist administering the WISC-R, Wechsler Intelligence Scale for Children-Third Edition (WISC-III; Wechsler, 1991), Wechsler Abbreviated Scale of Intelligence (WASI), or Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, 1967). Some subjects received a four-subtest estimate of the WISC-R (Kaufman, 1976), a four-subtest shortened version of the WISC-III (Dumont & Faro, 1993), a two-subtest estimate of the WASI (Sattler, 1992), or a short form of the WPPSI (Kaufman, 1972). Ages were rounded down to whole integers to simplify matching and analysis.
Healthy volunteers (HV; n = 98) were recruited as part of a MRI study of typically developing children. HV were screened through a telephone interview, parent and teacher rating versions of the Child Behavior Checklist (Achenbach & Ruffle, 2000), and physical and neurological assessment. Exclusion criteria included psychiatric diagnosis in the subject or a first-degree relative and head injury or other conditions that might have affected gross brain development. For this study, HV were selected from a larger pool of subjects to match the PANDAS subjects on age, sex, and full-scale IQ. Subjects with PANDAS and HV were matched one-to-one for age and for sex, and within 10 points for full-scale IQ.
Subjects with PANDAS were recruited for three separate protocols: a yearlong double-blind study comparing azithromycin and penicillin antibiotic prophylaxis (n = 8, 11.9%), an 8-month double-blind cross-over study comparing a prophylactic dose of penicillin to placebo (n = 31, 46.3%), and a comparative study of immunomodulatory treatment with random assignment to plasma exchange, intravenous immunoglobin, or placebo with intravenous saline (n = 26, 38.8%). Two subjects (3%) participated in both the second and third protocols.
Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association, 1994) diagnostic criteria and PANDAS criteria (described by Swedo et al., 1998) were assessed in extensive clinical interviews. Subjects were diagnosed with TS, OCD, or OCD with tics (i.e., this last group of subjects met criteria for a tic disorder other than full TS). Tic symptoms were assessed using the Yale Global Tic Severity Scale (YGTSS; scale range: 0–50; Leckman et al., 1989); and obsessive and compulsive symptoms using the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS; scale range: 0–40; Goodman, Price, Rasmussen, Mazure, Delgado, et al., 1989; Goodman, Price, Rasmussen, Mazure, Fleischmann et al., 1989). Although both the YGTSS and CY-BOCS contain two separate subscales (Vocal and Motors Tics, and Obsessions and Compulsions, respectively), only the total score is presented here to simplify comparisons. In addition, the NIMH Global Depression scale (scale range: 1–15; Insel et al., 1983; Murphy, Pickar, & Alterman, 1982) was used to assess depressive symptoms for 65 of the 67 subjects.
Age of onset of OCD and/or tic symptoms was determined through chart review. Of the PANDAS subjects, 20 (29.9%) were taking psychotropic medication at the time of testing, 9 were taking one medication (13.4%), 9 were taking two medications, and 2 (3%) were taking three medications. Subjects were taking serotonergic agonists, including sertraline, fluvoxamine, and fluoxetine (n = 12); methylphenidate (n = 4); dopamine antagonists, including haloperidol, risperidone, and fluphenazine (n = 4); clonidine (n = 4); tricyclic antidepressants, including clomipramine and imipramine (n = 2); clonazepam (n = 2); divalproex (n = 1); buspirone (n = 1); and trazodone (n = 1).
Apparatus and Stimuli
All tasks were programmed on either an IBM or Apple computer, and stimulus presentation, timing, and response collection were controlled by the computer.
Tests of control over attention and response suppression
These tasks were designed to evaluate suppression of different types of information and at different levels of attention processing (Casey, Tottenham, & Fossella, 2002). The paradigms have been used in previous studies of cognitive control in clinical and typically developing populations and are described in detail there (Casey, Durston, & Fossella, 2001; Casey et al., 1997; Casey, Gordon, Mannheim, & Rumsey, 1993; Casey, Vauss, & Swedo, 1994b).
The response execution task is a visual continuous performance task (CPT) used to assess ability to inhibit, to sustain attention, or to maintain vigilance. Eight letters (X, K, F, W, P, B, G, and C) were presented in random order with the nontarget, a capital X, presented on 50% of the 84 trials. This task assessed the ability of the subject to inhibit a compelling response. Stimulus duration was 500 msec and the interstimulus interval was 1500 msec. The subject responded by pressing the spacebar on a keyboard when the nontarget appeared on the screen. Performance on 10 practice trials was assessed to ensure the subject understood the task before testing began. Outcome variables included RT and accuracy of responses. The sample included 22 PANDAS and HV pairs with a mean of 9.0 years of age (SD = 1.7). Fourteen (63.6%) of the pairs were male.1 The full-scale IQ for the PANDAS subjects (M = 113.8, SD = 13.7) did not differ from the HVs (M = 114.7, SD = 13.2) in a paired-samples t-test, t(21) = −1.08, p = .29. Within the PANDAS group, 5 (22.7%) were diagnosed with OCD, 6 (27.3%) with TS, and 11 (50.0%) with OCD with tics. In addition, 6 (27.3%) were diagnosed with attention deficit/hyperactivity disorder (ADHD) and 1 (4.5%) with Major Depressive Disorder (MDD).
The response selection task is designed to measure suppression of a response to a competing response choice rule. The task consisted of the presentation of a digit, either 1, 2, 3, or 4. The subject's task was to press the corresponding 1, 2, 3, or 4 button on a keyboard whenever a digit appeared. To assess the ability to inhibit response tendencies, two conditions were run. In the first condition (“control”), the subject responded as previously described. In the second condition (“suppression”), the response set was reversed and the subject had to press the fourth button when a 1 appeared, the third button when a 2 appeared, and so forth. Variables included accuracy and RT for the control and suppression conditions. In addition, two additional variables were calculated: to determine the degree to which the differences in mean reaction time were driven by the suppression condition, we converted the two reaction times to difference scores; and to test whether greater difference scores for subjects could be explained simply by their larger overall reaction times, we scaled the difference score of each subject to his or her mean reaction time in the suppression condition. The sample included 40 PANDAS and HV pairs with a mean of 8.3 years of age (SD = 1.9). Twenty-eight (70.0%) of the pairs were male. The full-scale IQ for the PANDAS subjects (M = 108.6, SD = 13.3) did not differ from the HVs (M = 108.5, SD = 13.5) in a paired-samples t-test, t(39) = 0.11, p = .92. Within the PANDAS group, 10 (25.0%) were diagnosed with OCD, 16 (40.0%) with TS, and 14 (35.0%) with OCD with tics. In addition, 5 (12.5%) were diagnosed with ADHD and 2 (5.0%) with MDD.
In the stimulus selection task subjects were given a forced-choice visual discrimination task designed to measure suppression of response to a previously attended stimulus attribute. Three stimuli were presented in a row on a computer screen. The stimuli varied either in shape (circle, triangle, and square) or in whether they were filled or open. The subject's task was to determine which of the three stimuli was different from the other two. The stimuli remained until the subject responded by pressing the 1, 2, or 3 button of the keyboard to indicate which of the three stimuli was different. To assess deficits specific to shifts in attention set rather than to the task, the stimulus attribute on which the subject determined uniqueness was either the same for a block of trials (same trials) or changed from trial to trial within a block (mixed trials). The block pattern of each session was same-mixed-same-mixed, with each block consisting of 48 trials (192 trials total). Outcome variables for this task were the same as those for the response selection task (see above). The sample included 44 PANDAS and HV pairs with a mean of 8.5 years of age (SD = 2.2). Thirty-two (72.7%) of the pairs were male. The full-scale IQ for the PANDAS subjects (M = 108.8, SD = 12.7) did not differ from the HVs (M = 109.4, SD = 13.0) in a paired-samples t-test, t(43) = −0.69, p = .49. Within the PANDAS group, 11 (25.0%) were diagnosed with OCD, 17 (38.6%) with TS, and 16 (36.4%) with OCD with tics. In addition, 6 (13.6%) were diagnosed with ADHD and 2 (4.5%) with MDD.
Tests of executive functioning
The 64-card version of the Wisconsin Card Sorting Task (WCST; Heaton, Chelune, Talley, Kay, & Curtiss, 1993) was computer administered (Harris, 1988). Variables included categories completed, number of trials and errors, perseverative responses, and number of failures to maintain set. The sample included 40 PANDAS and HV pairs with a mean of 9.0 years of age (SD = 1.9). Twenty-nine (72.5%) of the pairs were male. Although the full-scale IQ for the PANDAS subjects (M = 113.4, SD = 13.3) differed from the HVs (M = 115.3, SD = 11.0) in a paired-samples t-test, t(39) = −2.32, p = .03, the difference between pairs (M = 1.9, SD = 5.3) was not clinically significant. Within the PANDAS group, 9 (22.5%) were diagnosed with OCD, 16 (40.0%) with TS, and 15 (37.5%) with OCD with tics. In addition, 5 (12.5%) were diagnosed with ADHD and 2 (5.0%) with MDD.
The Tower of Hanoi (TOH) is a disk-transfer task that evaluates a child's ability to plan and to organize a sequence of spatially controlled moves in an attempt to duplicate the goal state from the initial problem state. An individual's response in this task involves a sequence of spatially controlled motor moves, rather than discrete choices as in the WCST. It necessitates online representation of intermediate subgoals in working memory and is considered a measure of spatial planning ability, working memory, and behavioral suppression (Baron, 2004). The TOH condition that was used in this study is the three-disk problem that was used and described in detail by Casey, Vauss, Chused, & Swedo (1994a). In part of the paradigm, subjects are presented with two problems, each of which can be completed in a minimum of seven moves. In addition to the time and number of moves to complete both tasks, we calculated the mean time and moves for both tasks, mean number of problems solved/completed and mean number completed in the fewest moves. For subjects who successfully completed only one of the two tasks, the values from that task were used to calculate the mean time and moves. The sample included 45 PANDAS and HV pairs with a mean of 8.0 years of age (SD = 2.3). Twenty-nine (64.4%) of the pairs were male. The full-scale IQ for the PANDAS subjects (M = 113.7, SD = 12.8) did not differ from the HVs (M = 114.3, SD = 11.8) in a paired-samples t-test, t(44) = −0.79, p = .44. Within the PANDAS group, 11 (24.4%) were diagnosed with OCD, 19 (42.4%) with TS, and 15 (33.3%) with OCD with tics. In addition, 6 (13.3%) were diagnosed with ADHD and 3 (6.7%) with MDD.
Analytic Procedure
To test whether performance differed between the PANDAS and HV groups, we used a within-samples t-test, after matching the PANDAS subjects to HV one-to-one by sex, age, and full-scale IQ. Certain continuous variables (i.e., accuracy in the response execution task; accuracy of control condition in the response selection task; and accuracy of the control and mixed condition in the sensory selection task) showed a strong ceiling or floor effect, so values were dichotomized; the McNemar test was used to compare these values. The McNemar test was also used for the number of problems solved and problems completed in fewest moves in the TOH. The Bonferroni correction was applied to the significance values after analysis.
To determine which clinical and demographic variables best predicted performance for variables in which there was a significant difference between the PANDAS and HV, we used a hierarchical linear regression within the PANDAS group. In the first step, we entered clinical variables not used to match subjects in the comparison between PANDAS and HV groups in a stepwise fashion in order to select the best predictors of performance (i.e., primary psychiatric diagnosis; medication status [whether the patient was taking a medication]; ADHD or MDD comorbidity; age of onset; time since onset; and OCD, tic, and depression severity). In the second step, we entered the variables used to match the PANDAS and HV groups (i.e., age at testing, sex, and full-scale IQ) to determine what effects these variables had on the model, and reported β values.
Last, we extrapolated from the results of the regression models which variables consistently predicted performance, and controlled for those variables in the comparison between PANDAS and HV groups. We split the PANDAS-HV pairs into separate groups based on the median value of continuous predictor variables, and by group for categorical predictor variables. Within-subjects t-tests were conducted between the PANDAS-HV pairs of each subgroup to determine if there was a difference in significance between the subgroups.
All hypothesis tests were evaluated for significance at p < .05, two-tailed.
Results
Performance Compared Between PANDAS and HV
Results of the comparison between the PANDAS and HV groups are summarized in Table 2. PANDAS subjects were less accurate than HV subjects in the suppression condition of the response selection task, t(39) = −3.54, p = .001, and exhibited a slower RT on the response execution task, t(21) = 3.12, p = .005. After Bonferroni correction, only the former difference was significant.
Table 2.
Comparison of PANDAS and HV Performance on Tests of Attentional and Executive Functioning.
| Variables | PANDAS Mean (SD) |
HV Mean (SD) |
t | df | p | Effect sizea |
|---|---|---|---|---|---|---|
| Response execution (n = 22) | ||||||
| RT (msec) | 674.77 (214.85) | 527.00 (112.66) | 3.12 | 21 | .005 | 1.36 |
| Accuracyb,c | 9 | 11 | .73 | |||
| Response selection (n = 40) | ||||||
| Accuracy controlb,c | 18 | 25 | .12 | |||
| Accuracy suppression | 0.84 (0.15) | 0.93 (0.07) | −3.54 | 39 | .001 | −1.13 |
| RT control (msec) | 1216.48 (437.11) | 1132.63 (277.48) | 1.35 | 39 | .19 | 0.43 |
| RT suppression (msec) | 1627.18 (539.74) | 1533.48 (486.00) | 1.11 | 39 | .27 | 0.36 |
| Sensory selection (n = 44) | ||||||
| Accuracy controlb,c | 39 | 41 | .73 | |||
| Accuracy mixedb,c | 40 | 40 | 1.00 | |||
| RT control (msec) | 1680.55 (852.22) | 1584.66 (766.86) | 0.66 | 43 | .51 | 0.20 |
| RT mixed (msec) | 1964.41 (1028.53) | 1854.43 (773.61) | 0.63 | 43 | .53 | 0.19 |
| Tower of Hanoi (n = 45) | ||||||
| Mean time (s) | 77.24 (40.66) | 78.98 (43.97) | −0.23 | 44 | .82 | −0.07 |
| Problems solvedc,d | 44 | 38 | .07 | |||
| Mean moves | 10.73 (2.48) | 10.16 (2.04) | 1.21 | 44 | .23 | 0.36 |
| Problems completed in fewest movesc,e | 14 | 15 | 1.00 | |||
| WCST (n = 40) | ||||||
| Categories completed | 3.78 (2.02) | 4.33 (1.56) | −1.35 | 39 | .18 | −0.43 |
| Trials | 119.68 (14.56) | 121.10 (11.62) | −0.54 | 39 | .59 | −0.17 |
| Errors | 43.93 (23.80) | 44.13 (18.96) | −0.04 | 39 | .96 | −0.01 |
| Perseverative responses | 20.58 (13.31) | 21.38 (10.59) | −0.30 | 39 | .77 | −0.10 |
| Failure to maintain set | 2.25 (1.89) | 1.93 (1.70) | 0.80 | 39 | .43 | 0.26 |
Abbreviations: PANDAS, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection; HV, healthy volunteer; RT, response time; WCST, Wisconsin Card Sorting Test.
Cohen's d, using the formula d = 2t / √(df).
Values represent number of cases in which 100% accuracy was achieved.
McNemar's chi-squared test used to compare groups.
Values represent number of cases in which both problems were successfully solved.
Values represent number of cases in which at least one of the two problems was solved in the fewest number of moves.
Variables Affecting PANDAS Performance
Results of the linear regression are summarized in Table 3. For the response execution task, ADHD diagnosis emerged as a significant predictor of RT in the first step (p = .01) and remained significant in the second step (p = .003), in which age also was a significant predictor (p = .009). For the response selection task, none of the clinical variables in the first model were significant individual predictors of outcome, but, in the second model (in which sex, age, and full-scale IQ were included), full-scale IQ was a significant predictor of accuracy in the suppression condition (p = .04).
Table 3.
Regression Analyses with RT of Response Execution Task and Accuracy in Inhibition Condition of Response Selection Task.
| R2 | ΔR2 | pr2 | β | P | |
|---|---|---|---|---|---|
| Reaction time analysis: D.V. = RT on response execution task | |||||
| Step 1 | .34 | .34 | .000 | ||
| ADHD | .33 | .58 | .01 | ||
| Step 2 | .61 | .27 | .001 | ||
| ADHD | .47 | .59 | .003 | ||
| Age | .24 | −.52 | .009 | ||
| Sex | .00 | −.01 | .10 | ||
| Full-scale IQ | .11 | −.37 | .06 | ||
| Accuracy analysis: D.V. = accuracy in suppression condition on response selection task | |||||
| Step 2 | .12 | .12 | .07 | ||
| Age | .00 | .00 | .99 | ||
| Sex | .00 | −.04 | .81 | ||
| Full-scale IQ | .12 | .35 | .04 | ||
Abbreviations: D.V., dependent variable; RT, response time.
Analysis of Subgroups Based in Regression Analysis
For the RT of the response execution task, PANDAS subjects with ADHD (n = 6) exhibited a significantly longer RT than their HV matches, t(5) = 3.85, p = .01; however those without ADHD (n = 16) did not have significantly longer RTs than their HV matches, t(15) = 1.79, p = .09.
PANDAS-HV pairs were split by full-scale IQ at the median IQ score for the total PANDAS group. In the response selection task, PANDAS subjects with a full-scale IQ of 111 or lower (n = 24) were significantly less accurate than their HV matches, t(23) = −3.18, p = .004, but subjects with higher IQs (n = 16) were not significantly different in accuracy from their matches, t(15) = −1.81, p = .09.
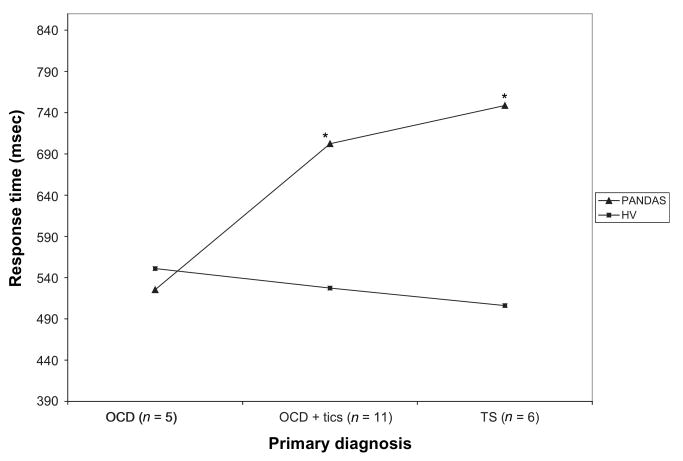
Because past literature has indicated that subjects with TS consistently show a longer RT on the CPT (similar to the response execution task), we divided the PANDAS group in the response execution task by primary psychiatric diagnosis (OCD, n = 5; OCD + tics, n = 11; TS, n = 6). As shown in Figure 1, subjects with TS or OCD + tics exhibited a significantly longer RT than their controls [TS: t(5) = 3.09, p = .03; OCD + tics: t(10) = 2.53, p = .03], and subjects with OCD did not show a significant difference from their HV matches, t(4) = −0.35, p = .75.
Figure 1.
Mean differences between PANDAS and HV subjects in separate diagnostic groups in response time of the response execution task (* indicates differences between PANDAS and HV groups are significant at p < .05).
Effects of Medication
Because nearly a third of subjects in the PANDAS subgroup were taking a psychotropic medication at the time of testing, we conducted a hierarchical linear regression within the PANDAS subgroup for all outcome variables to examine the effects of medication use. Medication status predicted RT in the suppression condition of the response selection task in model 1, β = −.42, p = .01, and in model 2, β = −.30, p = .03, which included age, sex, and full-scale IQ in the analysis. Using a paired t-test to further study this outcome variable, subjects on medication (n = 12) displayed a significantly longer RT than their HV pairs, t(11) = 2.881, p = .02; however, subjects not on medication (n = 28) did not differ from HV on this variable, t(27) = −0.384, p = .70.
Discussion
The current study was designed to assess specific aspects of neurocognitive functioning of children in the PANDAS subgroup. PANDAS subjects differed from matched controls on only one of the measures — the response accuracy in a test of attention and suppression. These results are consistent with previous studies of OCD, which have shown few or no deficits in attention (e.g., Boone, Ananth, Philpott, Kaur, & Djenderedjian, 1991; e.g., Flament et al., 1990; Hollander et al., 1993). Our results suggest that there may be an effect of age of symptom onset on attentional functioning, which has been studied directly by at least one study by Roth, Milovan, Baribeau, and O'Connor (2005), who studied children with onsets either above or below age 12. However, the results of this study are not directly applicable to our study group because the age of onset for most PANDAS subjects was below 12 years, and none were older than 15 at onset (see Table 1). The results of the present investigation are also in keeping with previous reports for the WCST, where OCD subjects consistently perform as well as matched controls (for review, see Kuelz, Hohagen, & Voderholzer, 2004). Last, studies of adults with OCD on the TOH have produced mixed results, with some (Schmidtke, Schorb, Winkelmann, & Hohagen, 1998) showing no deficits, and others (Cavedini, Cisima, Riboldi, D'Annucci, & Bellodi, 2001; Mataix-Cols et al., 1999) showing deficits compared to controls. Deficits may be a result of dysfunction within the orbitofrontal-striatal-thalamocortical circuitry, as has been demonstrated in functional imaging studies of OCD (Saxena et al., 2001), or it may represent a separate deficit in speed and special working memory (Kuelz, Hohagen, & Voderholzer, 2004).
Our results are reflective of the neuropsychological literature of TS in the executive functioning and attentional functioning domain. In a review of the literature, Como (2001) found the only reliable deficit in executive functioning (including both tests of executive functioning and attention) was increased time on the CPT. When the PANDAS group was divided by primary psychiatric diagnosis, those with tics and OCD or TS performed significantly worse than their controls, while those with OCD (without tics) did not show a significant difference in performance from their matched controls. However, caution should be taken when interpreting these results because of the small sample size of each diagnostic group. Nevertheless, these results may be explained in part by Baron-Cohen et al. (Baron-Cohen, Cross, Crowson, & Robertson, 1994), who have theorized that children with TS exhibit a dysfunction of the “Intention Editor,” explaining their inhibitory deficits, manifested in involuntary movements and utterances and obsessive thoughts. Further, results of intracortical transcranial magnetic stimulation in children with TS have shown reduced intracortical inhibition and a shortened cortical silent period, providing a neurological basis for decreased inhibitory control (Moll et al., 2001). However, Ozonoff, Strayer, McMahon, and Filloux (1998) have shown that ADHD comorbidity may account for attentional dysfunction in TS. Studies of executive functioning in children with TS have shown no significant deficits (Ozonoff & Jensen, 1999; Verté, Geurts, Roeyers, Oosterlaan, & Sergeant, 2005; Yeates & Bornstein, 1996).
Within the response execution task, ADHD and age were significant predictors of RT; subjects with PANDAS and ADHD and younger subjects exhibited a significantly longer RT than their matched HV. Both of these results are supported by previous findings using this task by Casey, Durston, and Fossella (2001). In their study of 108 typically developing children, age and RT were negatively correlated. In addition, in a group of 26 nonmedicated children with ADHD between the ages of 6 and 16 years, the authors found decreased performance on the response execution task as compared to matched controls. With regards to the response selection task, the differences between low- and high-IQ subjects may be explained, in part, by the small sample sizes. Previous studies of cognitive functioning in OCD and TS have not sustained effects of IQ on attention or executive functioning (Como, 2001; Greisberg & McKay, 2003).
Results of our analyses on the effects of medication agree with previous studies that have found little to no neuropsychological differences between medicated and unmedicated pediatric subjects with OCD and/or TS. For TS, one study (Bornstein & Yang, 1991) found no differences in performance on a wide battery of neuropsychological tests (including the WCST) between medicated and unmedicated children, even though most of the medicated children were taking a neuroleptic (clonidine, pimozide, or haloperidol) at the time of testing. A similar study (Channon, Pratt, & Robertson, 2003) found that medicated children scored lower on only one test of multitasking. Likewise, a study (Mataix-Cols, Alonso, Pifarré, Menchón, & Vallejo, 2002) that compared SRI-medicated SRI-free children diagnosed with OCD found no significant differences between groups in a continuous performance task and the WCST; the groups only differed significantly in the number of reversions in the TOH. The literature and results of our current investigation generally argue against a deleterious effect of medication use on neuropsychological performance; however, to our knowledge, there have been no long-term outcome studies evaluating this question.
Strengths of our study included a well-defined set of subjects in the PANDAS subgroup, which were carefully matched to control subjects. Although these tests may not be specific enough to assess important facets of cognitive functioning in subjects with PANDAS, they have proven useful in assessing deficits in attention in subjects with ADHD (Casey, Durston, & Fossella, 2001). Our results from the executive functioning task reveal effects of comorbid ADHD on cognitive functioning in subjects with OCD and/or TS, which warrant further study.
Limitations and Future Directions
This study is limited by the small sample sizes for some measures, and the secondary analyses are further limited by the division of the cohort into smaller samples. The limited sample size can be explained by two factors: (a) some patients may not have been available to complete all tests because of time limitations imposed by the schedule of their primary treatment protocol, and (b) the battery of administered tests differed across the primary protocols into which the subjects were enrolled; the tests presented here represent those for which we had the largest sample sizes. In addition, data for some patients were no longer available at the time of analysis, so that sample sizes for individual tasks vary. Of particular note, the sample size for the response execution task was considerably smaller (n = 22) than those of the other tasks. The power of this analysis and several others, including the regression analysis, to detect small differences is thereby reduced, requiring additional caution in interpreting the results.
Lack of functional neuroimaging data also limited the specificity of our results. Otto (1992) points out that it is difficult to draw conclusions about specific dysfunction within the frontostriatal circuitry from cognitive test results without imaging data because caudate dysfunction can mimic frontal lobe dysfunction, other compensatory mechanisms in the brain may mask basal ganglia or prefrontal dysfunction, and cognitive dysfunctions may be secondary to the disorder itself.
Future investigations would be strengthened by the addition of neuroimaging data, such as event-related brain potential (ERP). Studies of ERP in subjects with OCD have consistently shown hyperarousal of the cortex during attentional tasks (de Groot, Torello, Boutros, & Allen, 1997; Gohle et al., 2008; Herrmann, Jacob, Unterecker, & Fallgatter, 2003; Towey et al., 1990). In subjects with TS, ERP has begun to help unravel the neurological substrates that separate subjects with and without comorbid ADHD (Drake et al., 1992; Oades, Dittmann-Balcar, Schepker, Eggers, & Zerbin, 1996; Zhu et al., 2006). Other methods of functional imaging, such as functional Magnetic Resonance Imaging (fMRI), may also be considered.
Conclusions
Our results are mainly consistent with past neuropsychogical findings in OCD and TS. The results of this study reveal that subjects in the PANDAS subgroup do not exhibit specific executive functioning or attentional deficits, which may reflect diffuse, nonfocal dysfunction, similar to subjects with typical (i.e., non-PANDAS) OCD or TS. These findings may add weight to the suggestion by Swedo and Grant (2005) to treat patients with PANDAS according to standard practices for their primary psychiatric diagnosis.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Mental Health (NIMH), National Institutes of Health (NIH). The views expressed in this article do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government.
The authors thank Drs. Liv S. Clasen and Joseph Snow for their assistance in organizing the neuropsychological data, and Dr. Teresa Huggins for her aid in the statistical analysis. In addition, we extend our gratitude to the children and their families who volunteered their time and efforts during these research protocols.
Footnotes
Summary statistics for age and sex are given for pairs, and not for the PANDAS and HV groups separately, because pairs were matched exactly for age and sex.
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington DC: Author; 1994. [Google Scholar]
- Andrés S, Boget T, Lázaro L, Penadés R, Morer A, Salamero M, et al. Neuropsychological performance in children and adolescents with obsessive-compulsive disorder and influence of clinical variables. Biological Psychiatry. 2007;61(8):946–951. doi: 10.1016/j.biopsych.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Baron IS. Neuropsychological evaluation of the child. New York: Oxford University Press; 2004. [Google Scholar]
- Baron-Cohen S, Cross P, Crowson M, Robertson M. Can children with Gilles de la Tourette syndrome edit their intentions? Psychological Medicine. 1994;24(1):29–40. doi: 10.1017/s0033291700026805. [DOI] [PubMed] [Google Scholar]
- Beers SR, Rosenberg DR, Dick EL, Williams T, O'Hearn KM, Birmaher B, et al. Neuropsychological study of frontal lobe function in psychotropic-naive children with obsessive-compulsive disorder. American Journal of Psychiatry. 1999;156(5):777–779. doi: 10.1176/ajp.156.5.777. [DOI] [PubMed] [Google Scholar]
- Boone KB, Ananth J, Philpott L, Kaur A, Djenderedjian A. Neuropsychological characteristics of nondepressed adults with obsessive-compulsive disorder. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1991;4(2):96–109. [Google Scholar]
- Bornstein RA, Yang V. Neuropsychological performance in medicated and unmedicated patients with Tourette's disorder. American Journal of Psychiatry. 1991;148(4):468–471. doi: 10.1176/ajp.148.4.468. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clinical Neuroscience Research. 2001;1:267–282. [Google Scholar]
- Casey BJ, Gordon CT, Mannheim GB, Rumsey JM. Dysfunctional attention in autistic savants. Journal of Clinical and Experimental Neuropsychology. 1993;15(6):933–946. doi: 10.1080/01688639308402609. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40(3):237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Vauss YC, Chused A, Swedo SE. Cognitive functioning in Sydenham's chorea: Part 2. Executive functioning. Developmental Neuropsychology. 1994a;10(2):89–96. [Google Scholar]
- Casey BJ, Vauss YC, Swedo SE. Cognitive functioning in Sydenham's chorea: Part 1. Attentional processes. Developmental Neuropsychology. 1994b;10(2):75–88. [Google Scholar]
- Cavedini P, Cisima M, Riboldi G, D'Annucci A, Bellodi L. A neuropsychological study of dissociation in cortical and subcortical functioning in obsessive-compulsive disorder by Tower of Hanoi task. Brain and Cognition. 2001;46(3):357–363. doi: 10.1006/brcg.2001.1293. [DOI] [PubMed] [Google Scholar]
- Channon S, Pratt P, Robertson MM. Executive function, memory, and learning in Tourette's syndrome. Neuropsychology. 2003;17(2):247–254. doi: 10.1037/0894-4105.17.2.247. [DOI] [PubMed] [Google Scholar]
- Como PG. Neuropsychological function in Tourette's syndrome. In: Cohen DJ, Jankovic J, Goetz CG, editors. Tourette's Syndrome. Vol. 85. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 103–111. [PubMed] [Google Scholar]
- de Groot CM, Torello MW, Boutros NN, Allen R. Auditory event-related potentials and statistical probability mapping in obsessive-compulsive disorder. Clinical EEG. 1997;28(3):148–154. doi: 10.1177/155005949702800306. [DOI] [PubMed] [Google Scholar]
- Drake MEJ, Hietter SA, Padamadan H, Bogner JE, Andrews JM, Weate S. Auditory evoked potentials in Gilles de la Tourette syndrome. Clinical EEG. 1992;23(1):19–23. doi: 10.1177/155005949202300106. [DOI] [PubMed] [Google Scholar]
- Drewe EA. Go - no go learning after frontal lobe lesions in humans. Cortex. 1975;11(1):8–16. doi: 10.1016/s0010-9452(75)80015-3. [DOI] [PubMed] [Google Scholar]
- Dumont R, Faro C. A WISC-III short form for learning-disabled students. Psychology in the Schools. 1993;30:212–219. [Google Scholar]
- Flament MF, Koby E, Rapoport JL, Berg CJ, Zahn T, Cox C, et al. Childhood obsessive-compulsive disorder: A prospective follow-up study. Journal of Child Psychology and Psychiatry. 1990;31(3):363–380. doi: 10.1111/j.1469-7610.1990.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Freedman M. Object alternation and orbitofrontal system dysfunction in Alzheimer's and Parkinson's disease. Brain and Cognition. 1990;14(2):134–143. doi: 10.1016/0278-2626(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. American Journal of Psychiatry. 2000;157(2):281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Kruesi MJP, Parker C, Schaprio MB, Allen AJ, et al. Sydenham's chorea: Magnetic resonance imaging of the basal ganglia. Neurology. 1995;45(12):2199–2202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE. Case study: Acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(7):913–915. doi: 10.1097/00004583-199607000-00017. [DOI] [PubMed] [Google Scholar]
- Gohle D, Juckel G, Mavrogiorgou P, Pogarell O, Mulert C, Rujescu D, et al. Electro-physiological evidence for cortical abnormalities in obsessive-compulsive disorder: A replication study using auditory event-related P300 subcomponents. Journal of Psychiatric Research. 2008;42(4):297–303. doi: 10.1016/j.jpsychires.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Archives of General Psychiatry. 1989;46(11):1012–1216. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Greisberg S, McKay D. Neuropsychology of obsessive-compulsive disorder: A review and treatment implications. Clinical Psychology Review. 2003;23(1):95–117. doi: 10.1016/s0272-7358(02)00232-5. [DOI] [PubMed] [Google Scholar]
- Harris ME. Wisconsin Card Sorting Test: Scoring Program (Version 2.0) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay G, Curtiss G. Wisconsin Card Sorting Test Manual. revised. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Herrmann MJ, Jacob C, Unterecker S, Fallgatter AJ. Reduced response-inhibition in obsessive-compulsive disorder measured with topographic evoked potential mapping. Psychiatry Research. 2003;120(3):265–271. doi: 10.1016/s0165-1781(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Hollander E, Cohen L, Richards M, Mullen L, DeCaria C, Stern Y. A pilot study of the neuropsychology of obsessive-compulsive disorder and Parkinson's disease: Basal ganglia disorders. Journal of Neuropsychiatry & Clinical Neurosciences. 1993;5(1):104–107. doi: 10.1176/jnp.5.1.104. [DOI] [PubMed] [Google Scholar]
- Insel TR, Murphy DL, Cohen RM, Alterman I, Kilts C, Linnoila M. Obsessive-compulsive disorder. A double-blind trial of clomipramine and clorgyline. Archives of General Psychiatry. 1983;40(6):605–612. doi: 10.1001/archpsyc.1983.04390010015002. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. A short form of the Wechsler Preschool and Primary Scale of Intelligence. Journal of Consulting and Clinical Psychology. 1972;39:361–369. [Google Scholar]
- Kaufman AS. A four-test short form of the WISC-R. Contemporary Educational Psychology. 1976;1:180–196. [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nature Medicine. 2003;9(7):914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity. 2006;39(1):21–29. doi: 10.1080/08916930500484757. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. Journal of Neuroimmunology. 2006;179(1–2):173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Kuelz AK, Hohagen F, Voderholzer U. Neuropsychological performance in obsessive-compulsive disorder: A critical review. Biological Psychology. 2004;65(3):185–236. doi: 10.1016/j.biopsycho.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Alonso P, Pifarré J, Menchón JM, Vallejo J. Neuropsychological performance in medicated vs. unmedicated patients with obsessive-compulsive disorder. Psychiatry Research. 2002;109(3):255–264. doi: 10.1016/s0165-1781(02)00024-0. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Junque C, Sanchez-Turet M, Vallejo J, Verger K, Barrios M. Neuropsychological functioning in a subclinical obsessive-compulsive sample. Biological Psychiatry. 1999;45(7):898–904. doi: 10.1016/s0006-3223(98)00260-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. Neurobiology of basal ganglia and Tourette syndrome: Basal ganglia circuits and thalamocortical outputs. Advances in Neurology. 2006;99:89–98. [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott GE, Wirth S, Bock N, Rothenberger A. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: Evidence for additive inhibitory deficits within the motor system. Annals of Neurology. 2001;49(3):393–396. [PubMed] [Google Scholar]
- Murphy DL, Pickar D, Alterman IS. Methods for quantitative assessment of depressive and manic behavior. In: Burdock E, Sudilovsky A, Gershon S, editors. The behavior of psychiatric patients. New York: Marcel Dekker; 1982. [Google Scholar]
- Oades RD, Dittmann-Balcar A, Schepker R, Eggers C, Zerbin D. Auditory event-related potentials (ERPs) and mismatch negativity (MMN) in healthy children and those with attention-deficit or tourette/tic symptoms. Biological Psychology. 1996;43(2):163–185. doi: 10.1016/0301-0511(96)05189-7. [DOI] [PubMed] [Google Scholar]
- Otto MW. Normal and abnormal information processing: A neuropsychological perspective on obsessive compulsive disorder. Psychiatric Clinics of North America. 1992;15(4):825–848. [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 1999;29(2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL, McMahon WM, Filloux F. Inhibitory deficits in Tourette syndrome: A function of comorbidity and symptom severity. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39(8):1109–1118. [PubMed] [Google Scholar]
- Roth RM, Milovan D, Baribeau J, O'Connor K. Neuropsychological functioning in early- and late-onset obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(2):208–213. doi: 10.1176/jnp.17.2.208. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of Children. 3rd. J. M. Sattler; San Diego, CA: 1992. [Google Scholar]
- Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Seminars in Clinical Neuropsychiatry. 2001;6(2):82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Schmidtke K, Schorb A, Winkelmann G, Hohagen F. Cognitive frontal lobe dysfunction in obsessive-compulsive disorder. Biological Psychiatry. 1998;43(9):666–673. doi: 10.1016/s0006-3223(97)00355-7. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Grant PJ. Annotation: PANDAS: A model for human autoimmune disease. Journal of Child Psychology and Psychiatry. 2005;46(3):227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. American Journal of Psychiatry. 1998;155(2):264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- Towey J, Bruder G, Hollander E, Friedman D, Erhan H, Liebowitz M, et al. Endogenous event-related potentials in obsessive-compulsive disorder. Biological Psychiatry. 1990;28(2):92–98. doi: 10.1016/0006-3223(90)90626-d. [DOI] [PubMed] [Google Scholar]
- Verté S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Development and Psychopathology. 2005;17(2):415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1967. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children Revised. New York: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Yeates K, Bornstein R. Neuropsychological correlates of learning disability subtypes in children with Tourette's syndrome. Journal of the International Neuropsychological Society. 1996;2(5):375–382. doi: 10.1017/s1355617700001442. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu PZ, Leung KM, Su LY, Wu DX, Zhou M. P300 differences exist between Tourette's syndrome with and without attention deficiency and hyperactivity disorder in children. World Journal of Biological Psychiatry. 2006;7(2):91–98. doi: 10.1080/15622970500492723. [DOI] [PubMed] [Google Scholar]