Abstract
The use of co-immunoprecipitation (co-IP) to purify multi-protein complexes has contributed greatly to our understanding of the DNA damage response network associated with Fanconi anemia (FA), Bloom syndrome (BS) and breast cancer. Four new FA genes and two new protein partners for the Bloom syndrome gene product have been identified by co-IP. Here, we discuss our experience in using co-IP and other techniques to isolate and characterize new FA and BS-related proteins.
1. Introduction
Bloom syndrome is a human genetic disease characterized by growth retardation, immunodeficiency, cancer predisposition, and genomic instability (1). The disease is caused by mutations in BLM, a helicase that plays a key role in homologous recombination-dependent DNA repair (2). Fanconi anemia (FA), another human genetic disease, shares several features with Bloom syndrome, including cancer predisposition and genome instability (3). However, unlike Bloom syndrome which is always caused by mutations in a single gene, FA can result from lesions in any of multiple genes. To date, thirteen FA genes have been identified. All of their products function in a DNA damage response network that includes BLM. Three FA genes FANCD1, FANCN, and FANCJ are breast cancer susceptibility (BRCA) genes BRCA2, PALB2, and BACH1, making FA an attractive model to investigate the mechanism of action by BRCA proteins (4).
Research on Bloom syndrome and FA has been greatly accelerated using immunopurification to isolate their associated multiprotein complexes and subsequent identification of their components (5). The basic principle of this strategy is simple: components of the BLM or FA complexes will most likely have important functions similar to those of the disease proteins. Moreover, defects in these components may cause disease conditions similar to Bloom syndrome or FA. Indeed, two newly identified BLM complex components, RMI1 (BLAP75) and RMI2 (BLAP18), have been found to form a novel multi-OB-fold complex that has a critical role in protecting genome stability (6, 7). Moreover, each of three novel FA core complex components have been found to be mutated to cause some cases of FA (FANCL, FANCB and FANCM) (8–10). Furthermore, another FA gene, FANCN (PALB2), was first identified as a component of the FANCD1 (BRCA2) complex (11). Interested readers can find references to these discoveries in other reviews (4).
Here we discuss our experience using co-IP to immunopurify BLM and FA protein complexes. We also describe several approaches to verify and analyze the newly identified proteins.
2. Developing a high-quality antibody
Crucial to our success in immunopurification of the FA core complex and the BLM complex has been the generation of highly specific antibodies against these disease proteins. In our experience, a good antibody is critical for the success of purification. One can find detailed protocols of developing antibodies in a previously published method paper (12). We usually make rabbit polyclonal antibodies against maltose-binding protein (MBP; New England Biolabs) fused to different regions of the target protein. We also use the same MBP-fusion protein to affinity-purify antibodies. The purified antibodies are always more specific and bring down fewer contaminants than crude anti-serum. These antibodies allowed us to co-IP cognate target complexes from HeLa nuclear extract in a single step (5). This is consistent with our experience in immunopurification of several chromatin-remodeling complexes (SWI/SNF, NURD, and ATRX complexes), all of which have been isolated using highly specific antibodies against the endogenous proteins.
3. Immunopurification of protein complexes and identification of their components
3.1 Preparation of nuclear extract (NE)
We normally start purification by determining the distribution of the target protein in the cellular extracts. We typically prepare cytosolic and nuclear extract, and determine the location of the target protein. If the nuclear extract contains the target, we can further prepare soluble and chromatin sub-fractions to further localize the target protein. Immunoprecipitation using subcellular fractions rather than whole cell lysates can reduce co-purification of contaminating proteins. Since FA and BLM complexes function in nuclear or chromatin fractions, we normally prepare extracts from these sources as starting material. It should be mentioned, however, that certain FA proteins are present in the cytosol, and FA subcomplexes have been isolated from this source (13).
We often use human HeLa cells as the starting material for purification. One advantage of using this cell line is that it contains higher levels of proteins that protect genome stability than other lines (such as transformed lymphoblastoid and fibroblast cells). Moreover, the cells can be purchased in large quantity from the National Cell Culture Center.
Nuclear extract from human HeLa cells is prepared essentially as described (14). To avoid degradation of proteins, all procedures were performed at 4°C.
Cell pellets were washed twice with phosphate-buffered saline (PBS) and once with the hypotonic buffer A (10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2) supplemented with 0.5 mM PMSF, 1 mM DTT and proteinase inhibitor cocktail tablet (Roche). They were collected by low-speed centrifugation (3000rpm, BECKMAN Allegra 6KR Centrifuge).
Two volumes of buffer A were then added to the cell pellet. The pellet was homogenized about 10 times using a dounce homogenizer, and the nuclei were collected by centrifugation. Cells should be completely lysed in this buffer, which can be tested by staining with dyes such as Trypan Blue. The supernatant is saved as the cytosol fraction.
The nuclei were washed once with 5–10 volumes of buffer A to remove contaminating cytosol proteins, and collected by centrifugation.
The nuclear extract was prepared by extracting the nuclei two consecutive times using two volumes of buffer C each time (20 mM HEPES pH 7.9, 0.42 M NaCl, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, supplemented with 0.5 mM PMSF, 1 mM DTT and cocktail). Because the nuclei are in one volume of Buffer A, the final salt concentration in the mixture is lower than that of Buffer C: the salt concentration of the first nuclear extract (NE1) is about 0.28 M, and that of the second extract (NE2) is about 0.37 M. This differential salt concentration results in differential extractions of the proteins: those that loosely associate with chromatin are enriched in the first extract (NE1), whereas those tightly bound to chromatin are in the second extract (NE2). We found that the full FA core complex is mainly present in NE2, whereas FA subcomplexes are largely present in NE1.
The nuclei were homogenized about 10 times in to improve the efficiency of extraction. We usually rotate the nuclei in Buffer C for 30 min at 4°C, followed by centrifugation at high speed (15,000 rpm) for 15 min. The supernatant is saved as the nuclear extract. Its protein concentration should be between 6–8 mg/ml. One could perform immunoblotting analysis of the nuclear extract to ensure that the target protein is not significantly degraded. The nuclear pellet should also be saved as it contains many proteins that tightly associate with chromatin and can be used as a source for additional purification.
3.2 Preparing chromatin fractions
Chromatin fractions can be used for purification of proteins that bind chromatin so tightly that they cannot be extracted efficiently by Buffer C using the above protocol. To extract these proteins, the chromatin can be digested with DNase I as described previously (15).
Cells were first washed with PBS, and then incubated for 3 min at 4°C in cytoskeleton buffer (CSK): 10 mM Pipes, pH 6.8, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, supplemented with 0.5 mM PMSF, 1 mM DTT, cocktail and 0.5% Triton X-100.
The cytoskeletal proteins and membranes were removed from the soluble protein fractions by centrifugation at 5,000 g for 3 min.
The pellet, which contains chromatin, was solubilized by digestion with RNase-free DNase I (Roche, 100 units/ml) in CSK buffer supplemented with protease inhibitors for 15 min at 37°C.
Ammonium sulfate (from 1M stock) was then added to the mixture to make final concentration of 0.25 M. The mixture was incubated for 5 min at 4°C, and then centrifuged. The supernatant is the chromatin fraction containing solubilized chromatin-bound proteins (and DNA).
The pellet was further extracted with 2 M NaCl in CSK buffer for 5 min at 4°C, and then centrifuged. The remaining pellet is solubilized in urea buffer and is considered as the nuclear matrix–containing fraction.
3.3 Co-immunoprecipitation (co-IP)
Co-IP has been widely used to isolate a target protein and its associated complexes from cell extracts. This method is based on the highly specific interactions between the antibody and the antigen (i.e., the target protein). Such specific interactions often allow about 1000-fold purification of the target protein from cell lysate in a single step, often a purity sufficient for identification purpose. However, the success of IP often depends on the abundance of protein in the extract, the quality of the antibody, and the accessibility of the epitope on the target protein to the antibody. As the concentration of each protein in the lysate and their antibody qualities vary, it may be necessary to optimize conditions for each IP. For example, if the concentration of a target protein is too low in the extract, one may need to fractionate the extract to enrich the target. If the antibody is not of good quality, one may need to affinity-purify the antibody to improve its specificity and titer. If the epitope is not accessible to the antibody, or if the antibody cannot recognize the native form of the protein, one may have to develop a different antibody against a different region of the protein.
The basic IP protocol includes incubation of the antibody with the cell lysate to allow formation of the antibody-antigen complex. This complex is then captured on Protein A or Protein G Sepharose beads. The unbound proteins are removed by washing with various buffers. Finally, the target protein and its associated polypeptides are eluted off the beads with reagents that disrupt the antibody-antigen interactions. Our lab usually combines the first two steps by incubating antibody, cell lysate and Protein A/G beads together. Here is one procedure commonly used in our group for co-IP:
HeLa nuclear extract (0.2–0.5 ml) was diluted 4-fold with IP buffer (20 mM HEPES [pH 7.9], 200 mM NaCl, 1 mM DTT, 0.2 mM PMSF, 10% glycerol).
The diluted extract was centrifuged at 100,000 rpm using BECKMAN Optima TLX Ultracentrifuge. This step removes the insoluble membrane and cytoskeletal structures that non-specifically associate with protein A beads. This step is critical for purity of the complex, and can be repeated to ensure that the extract is clean of the any insoluble debris.
After ultracentrifugation, the supernatant was carefully transferred to another tube containing the antibody and protein A-beads (Amersham Pharmacia). No insoluble debris should be transferred as this will contaminate the IP. The amounts of the antibody and the protein A beads should be determined experimentally for each individual protein. For small scale IP (less than 0.2 ml of nuclear extract), one can use 100–1000 ng of antibody (rabbit IgG) and 10 μl of Protein A beads as a starting point.
The mixture was rotated in the cold room on a rotator for 2 to 12 hours. The longer incubation may increase the yield of the IP, as well as the amount of contaminating polypeptides. One may need to balance these factors in deciding how long to incubate the antibody with the lysate.
The immunoprecipitate on the beads was collected by centrifugation (1000 rpm, 1 min). The supernatant should be saved for immunoblotting analysis to determine how efficient the IP was, and whether the target protein was depleted from the extract by IP. If the target protein is completely depleted, one may consider increasing the amount of the extract.
The beads were washed four times with the IP buffer (at least 10 volumes each time). The target protein and its associated complex on the beads were eluted from the beads by using 1 to 2 volumes of 100 mM glycine-HCl buffer (pH 2.5). The eluate should be neutralized immediately by adding 1/10th volume of Tris.HCl (1M, pH 8.0). It can then be analyzed by SDS-PAGE and immunoblotting.
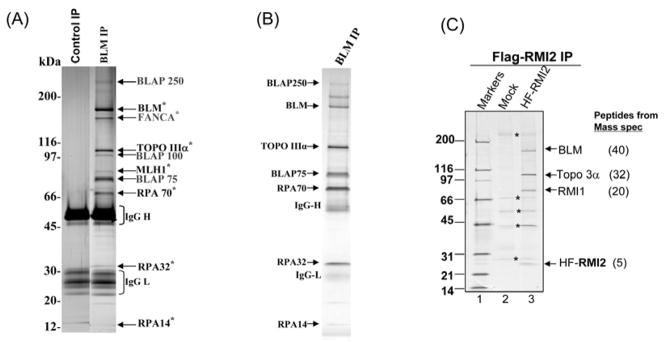
We often perform the following procedures to improve IP. First, we often include negative controls by performing a mock IP with Protein A/G beads alone (without adding antibody to the extract). This is because that Protein A/G beads can sometimes non-specifically pull down cellular proteins (actin, myosin and tubulin, etc) especially when the IP conditions are not optimal. These non-specific proteins, if present at high levels, can render identification of true target proteins difficult. One way to reduce contamination is to pre-incubate the cellular lysate with protein A/G-beads for 1–2 hours prior to IP. Proteins that bind non-specifically to the beads can be removed by centrifugation. Second, we sometimes optimize IP conditions by adjusting salt concentrations in the mixture. A high salt concentration may disrupt the interaction between the target and its associated complex, whereas low salt concentration may increase non-specific protein interactions and contamination. We normally use 200 mM NaCl for most IP. However, if the target protein complex is highly stable, we often use higher salt concentration (0.3 or 0.5 M NaCl) to reduce non-specific interactions. Third, one limitation of the above IP protocol is that the immunoprecipitate often contains immunoglobulin heavy and light chains (IgG-H and IgG-L), which may overlap with components of the target protein complex on the SDS gel, hindering their identification. To solve this problem, dimethylpimelimidate (DMP) can be used to covalently crosslink antibody to Protein A beads, so that IgG will not be eluted by the elution buffer (see Figure 1A and 1B as examples). However, DMP crosslinking often reduces or sometimes inactivates activity of an antibody, so that it may require the use of 3 to 10 –fold more antibody than the standard IP protocol. The increased amount of antibody may yield more non-specific proteins in the IP. However, because this method can significantly reduce the level of IgG in the IP, we use it frequently. We follow a published protocol to crosslink antibody to Protein A beads (16). Briefly:
Figure 1. Immunoprecipitation of the BLM complex by different methods.
(A)(B) silver-stained SDS gels showing the BLM complex immunoprecipitated by a polyclonal BLM antibody that was uncrosslinked (A) or crosslinked (B) to protein A beads by DMP. The complex was eluted from beads using 0.1 M Glycine (pH 2.5). Different BLM complex components are indicated by arrows. Notably, the IgG heavy and light chains are present in large quantity in A but not B. (C) A silver-stained SDS-gel showing polypeptides immunoprecipitated by Flag antibody against RMI2 that is linked to both Flag and 6xHistidine tags. A mock IP was performed to show contaminating polypeptides isolated due to crossreactivity with Flag antibody. Notably, RPA was missing in the co-IP, suggesting that the tagged approach may not isolate the full BLM complex. These figures were all reproduced from previous publications: Fig. 1A was from Figure 1A of reference (5); Fig. 1B was from Figure 1A, lane 1, of reference (6); and Fig. 1C was from Fig. 1C of reference (7).
Protein A beads (100 μl) was incubated with 10–30 μg of rabbit polyclonal antibody in 500 μl of PBS at room temperature for 30 min by rotating. This allows binding of antibody to Protein A beads.
The beads were washed 3 times with PBS, at least 10 volumes each time, to remove unbound antibody. They were then washed twice with 0.1 M sodium Borate, pH 9.0.
The beads were resuspended in 1 ml of 0.1 M sodium Borate, pH 9.0 and 5.2 mg DMP (Pierce), and the mixture was rotated at room temperature for 45 min. This allows crosslinking of antibody to Protein A beads.
The beads were washed once with 1M Ethanolamine, pH 8.0, and then incubated overnight at 4°C or 2 hours at room temperature in the same solution on a rotator to block the remaining reactive DMP, so that it does not crosslink cellular proteins during IP.
The beads were then washed 4 times with glycine (0.1M, pH 2.5), and twice with PBS and kept at 4°C.
3.4 Mass spectrometry analysis
For mass spectrometric analysis, the co-immunoprecipitated proteins were visualized by staining with Coomassie blue or silver. They were then excised from the gel, digested with trypsin, and analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) and/or liquid chromatography-mass spectrometry (LC/MS) analyses. Proteins that are visible by Coomassie staining are usually in sufficient quantity for identification by mass spectrometry, whereas those that can only be detected by silver-staining may not be identified consistently.
The entire immunoprecipitate can also be analyzed by mass spectrometry without fractionation by SDS-PAGE. This alternative has some advantages. For example, it may reveal all polypeptides that associate with the target protein in a single experiment. This feature is particularly desirable for some polypeptides that are difficult to stain. However, one disadvantage of the method is that it cannot conclusively identify individual polypeptides on the SDS-gel. In addition, if the immunoprecipitate is contaminated by other polypeptides, they may obscure identification of the target protein and its associated complex. Furthermore, if the amount of target protein in the mixture is too high, which usually happens for epitope-tagged protein, the identification of low-abundance associated proteins would be difficult.
It should be emphasized that all antibodies have some degree of cross-reactivity, and they frequently co-immunoprecipitate cross-reactive polypeptides in IP. In our hands, a number of polypeptides frequently contaminate IP from HeLa nuclear extract (Table 1). These contaminants not only include cytoskeletal proteins (actin, myosin, and tubulin), but also protein chaperones, RNA-interacting proteins, ribosomal proteins, and polypeptides involved in transcription (BCLAF1, TAF15, MYCBP, NONO), RNA splicing, chromatin-remodeling (PRMT5), and DNA repair (TOP2A, PRKDC, RIF1). If one finds proteins listed in Table 1 in an IP, one should proceed carefully and perform additional experiments to determine whether these proteins are not contaminants. It should be emphasized that proteins on this list could neverthless be components of a protein complex. For example, actin and actin-related proteins are components of SWI/SNF and other chromatin remodeling complexes. One should not overlook these proteins just because they are common contaminants in IP with many different antibodies.
Table 1. Common contaminants in immunoprecipitation from HeLa nuclear extract.
Proteins identified by mass spectrometry in the immunoprecipitate from HeLa nuclear extract by rabbit polyclonal antibodies against a variety unrelated proteins. Some of these (highlighted by bold characters) are also identified in the immunoprecipitate by Flag antibody from nuclear extract of HeLa cells that do not express any tagged proteins. We believe that these are common contaminants that interact non-specifically with antibodies.
| Official Symbol | GeneID | Official Full Name | Official Symbol | GeneID | Official Full Name |
|---|---|---|---|---|---|
| ACTA1 | 58 | actin, alpha 1, skeletal muscle | PRKDC | 5591 | protein kinase, DNA-activated, catalytic polypeptide |
| ACTA2 | 59 | actin, alpha 2, smooth muscle, aorta | PRMT5 | 10419 | protein arginine methyltransferase 5 |
| ACTBL2 | 345651 | actin, beta-like 2 | PRPF31 | 26121 | PRP31 pre-mRNA processing factor 31 homolog (S. cerevisiae) |
| ACTBL3 | 440915 | actin, beta-like 3 | RBM10 | 8241 | RNA binding motif protein 10 |
| ACTG1 | 71 | actin, gamma 1 | RBM39 | 9584 | RNA binding motif protein 39 |
| AHNAK | 79026 | AHNAK nucleoprotein | RBMX | 27316 | RNA binding motif protein, X-linked |
| ANXA2 | 302 | annexin A2 | RIF1 | 55183 | RAP1 interacting factor homolog (yeast) |
| BAT2D1 | 23215 | BAT2 domain containing 1 | RPL13 | 6137 | ribosomal protein L13 |
| BCLAF1 | 9774 | BCL2-associated transcription factor 1 | RPL29 | 6159 | ribosomal protein L29 |
| C11orf84 | 144097 | chromosome 11 open reading frame 84 | RPL38 | 6169 | ribosomal protein L38 |
| C1QBP | 708 | complement component 1, q subcomponent binding protein | RPLP2 | 6181 | ribosomal protein, large, P2 |
| CAPZA1 | 829 | capping protein (actin filament) muscle Z-line, alpha 1 | RPS4Y1 | 6192 | ribosomal protein S4, Y-linked 1 |
| CCAR1 | 55749 | cell division cycle and apoptosis regulator 1 | RPS8 | 6202 | ribosomal protein S8 |
| CLK3 | 1198 | CDC-like kinase 3 | SF3B1 | 23451 | splicing factor 3b, subunit 1, 155kDa |
| CLNS1A | 1207 | chloride channel, nucleotide-sensitive, 1A | SF3B2 | 10992 | splicing factor 3b, subunit 2, 145kDa |
| CMBL | 134147 | carboxymethylenebutenolidase homolog (Pseudomonas) | SF3B3 | 23450 | splicing factor 3b, subunit 3, 130kDa |
| CORO1C | 23603 | coronin, actin binding protein, 1C | SF3B4 | 10262 | splicing factor 3b, subunit 4, 49kDa |
| DDX17 | 10521 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 17 | SFPQ | 6421 | splicing factor proline/glutamine-rich (polypyrimidine tract binding protein associated) |
| DDX20 | 11218 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 20 | SFRS1 | 6426 | splicing factor, arginine/serine-rich 1 |
| DDX3X | 1654 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, X-linked | SFRS10 | 6434 | splicing factor, arginine/serine-rich 10 |
| DDX5 | 1655 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 5 | SFRS3 | 6428 | splicing factor, arginine/serine-rich 3 |
| DHX15 | 1665 | DEAH (Asp-Glu-Ala-His) box polypeptide 15 | SFRS4 | 6429 | splicing factor, arginine/serine-rich 4 |
| DHX9 | 1660 | DEAH (Asp-Glu-Ala-His) box polypeptide 9 | SFRS5 | 6430 | splicing factor, arginine/serine-rich 5 |
| DNAJA1 | 3301 | DnaJ (Hsp40) homolog, subfamily A, member 1 | SFRS6 | 6431 | splicing factor, arginine/serine-rich 6 |
| EEF1A2 | 1917 | eukaryotic translation elongation factor 1 alpha 2 | SFRS7 | 6432 | splicing factor, arginine/serine-rich 7, 35kDa |
| EEF1AL3 | 158078 | eukaryotic translation elongation factor 1 alpha-like 3 | SFRS9 | 8683 | splicing factor, arginine/serine-rich 9 |
| EIF4B | 1975 | eukaryotic translation initiation factor 4B | SLTM | 79811 | SAFB-like, transcription modulator |
| FLNA | 2316 | filamin A, alpha (actin binding protein 280) | SNRP70 | 6625 | small nuclear ribonucleoprotein 70kDa polypeptide (RNP antigen) |
| FMR1 | 2332 | fragile X mental retardation 1 | SNRPB | 6628 | small nuclear ribonucleoprotein polypeptides B and B1 |
| FUS | 2521 | fusion (involved in t(12;16) in malignant liposarcoma) | SNRPD1 | 6632 | small nuclear ribonucleoprotein D1 polypeptide 16kDa |
| FUSIP1 | 10772 | FUS interacting protein (serine/arginine-rich) 1 | SNRPD3 | 6634 | small nuclear ribonucleoprotein D3 polypeptide 18kDa |
| FXR1 | 8087 | fragile X mental retardation, autosomal homolog 1 | SPATS2 | 65244 | spermatogenesis associated, serine-rich 2 |
| FXR2 | 9513 | fragile X mental retardation, autosomal homolog 2 | SPIN1 | 10927 | spindlin 1 |
| GAPDH | 2597 | glyceraldehyde-3-phosphate dehydrogenase | SPTAN1 | 6709 | spectrin, alpha, non-erythrocytic 1 (alpha-fodrin) |
| GEMIN4 | 50628 | gem (nuclear organelle) associated protein 4 | SPTB | 6710 | spectrin, beta, erythrocytic |
| HNRNPH1 | 3187 | heterogeneous nuclear ribonucleoprotein H1 (H) | SPTBN1 | 6711 | spectrin, beta, non-erythrocytic 1 |
| HNRNPK | 3190 | heterogeneous nuclear ribonucleoprotein K | SR140 | 23350 | U2-associated SR140 protein |
| HNRNPM | 4670 | heterogeneous nuclear ribonucleoprotein M | STK38 | 11329 | serine/threonine kinase 38 |
| HNRNPR | 10236 | heterogeneous nuclear ribonucleoprotein R | STK38L | 23012 | serine/threonine kinase 38 like |
| HNRNPU | 3192 | heterogeneous nuclear ribonucleoprotein U | SYNCRIP | 10492 | synaptotagmin binding, cytoplasmic RNA interacting protein |
| HSPA1A | 3303 | heat shock 70kDa protein 1A | TAF15 | 8148 | TAF15 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 68kDa |
| HSPA1L | 3305 | heat shock 70kDa protein 1-like | THRAP3 | 9967 | thyroid hormone receptor associated protein 3 |
| HSPA2 | 3306 | heat shock 70kDa protein 2 | TOP2A | 7153 | topoisomerase (DNA) II alpha 170kDa |
| HSPA5 | 3309 | heat shock 70kDa protein 5 (glucose-regulated protein, 78kDa) | TPM1 | 7168 | tropomyosin 1 (alpha) |
| HSPA6 | 3310 | heat shock 70kDa protein 6 (HSP70B’) | TRA2A | 29896 | transformer-2 alpha |
| HSPA8 | 3312 | heat shock 70kDa protein 8 | TUBA1C | 84790 | tubulin, alpha 1c |
| IGKV2-40 | 28916 | immunoglobulin kappa variable 2–40 | TUBA3C | 7278 | tubulin, alpha 3c |
| KHDRBS1 | 10657 | KH domain containing, RNA binding, signal transduction associated 1 | TUBB | 203068 | tubulin, beta |
| KIF11 | 3832 | kinesin family member 11 | TUBB1 | 81027 | tubulin, beta 1 |
| LMO7 | 4008 | LIM domain 7 | TUBB2C | 10383 | tubulin, beta 2C |
| MATR3 | 9782 | matrin 3 | TUBB4Q | 56604 | tubulin, beta polypeptide 4, member Q |
| MYCBP | 26292 | c-myc binding protein | TUBB6 | 84617 | tubulin, beta 6 |
| MYH10 | 4628 | myosin, heavy chain 10, non-muscle | VIM | 7431 | vimentin |
| MYH11 | 4629 | myosin, heavy chain 11, smooth muscle | WDHD1 | 11169 | WD repeat and HMG-box DNA binding protein 1 |
| MYH9 | 4627 | myosin, heavy chain 9, non-muscle | WDR77 | 79084 | WD repeat domain 77 |
| NONO | 4841 | non-POU domain containing, octamer-binding | YBX1 | 4904 | Y box binding protein 1 |
| PABPC4 | 8761 | poly(A) binding protein, cytoplasmic 4 (inducible form) | YLPM1 | 56252 | YLP motif containing 1 |
| PPP1CC | 5501 | protein phosphatase 1, catalytic subunit, gamma isoform |
3.5 Superose 6 gel-filtration chromatography
Superose 6 gel-filtration chromatography can be used both in analysis and in purification of a target protein and its associated complexes. This method fractionates proteins based on their size, and has been widely used to estimate the molecular weight of the endogenous globular proteins. We normally fractionate HeLa extracts before IP to determine if the target protein in the extract fractionates as part of a high molecular weight complex. For example, the calculated molecular weight of BLM is about 180 kDa, whereas its endogenous form in HeLa extract fractionates as a complex of 1 MDa by Superose 6 column (Fig. 2A), suggesting that BLM is likely present in a large complex. In contrast, if the calculated molecular weight matches that determined by Suprose 6 column, the protein may not form a stable complex with other proteins in HeLa extract. In the latter case, one is unlikely to find a complex associating with the target protein by standard IP.
Figure 2. Superose 6 chromatography fractionates RMI into two complexes: one contains BLM and the other one does not.
(A) Immunoblotting shows that RMI2 in HeLa nuclear extract fractionates in 2 major peaks by Superose 6 gel-filtration chromatography, which correspond to two complexes designated as Complex 1 and 2 (marked by arrows on top). Notably, the elution profiles of RMI2, RMI1 and Topo 3α are coincident, suggesting that they always associate in complexes. In addition, BLM overlaps with the peak of complex 1 but not complex 2, suggesting that BLM is absent in complex 2. (B) Silver-stained SDS gels show that while Complex 1 contains all BLM complex components, complex 2 consists of only Topo 3 α, RMI1 and RMI2. Immunoprecipitation was done by using RMI1 antibody from crude nuclear extract (NE), Superose 6 fractions containing either Complex 1 or Complex 2, or Complex 2-containing fractions after immuno-depletion of BLM (Complex 2-BLM), as indicated on top of the figure. Notably, RMI1 co-immunoprecipitated with Topo 3 α and RMI2 but not BLM (lane 4 in B), indicating that the two RMI proteins and Topo 3α form a stable subcomplex. Figures 2A and 2B were reproduced from Supplementary Figures 1A and 1B, respectively, of a previous publication(7).
Superose 6 chromatography can also reveal whether a target protein is in one or several complexes. If the target protein fractionates in several peaks, the protein may be present in multiple complexes, with each peak corresponding to a unique complex. To purify each complex, one may need to do IP using each peak fraction. For example, RMI1, RMI2 and topoisomerase 3a in HeLa extract fractionate in two major peaks, suggesting that they are present in two different complexes (Fig. 2A). Co-IP from each peak obtained two complexes, one containing BLM and the other one not (Fig. 2B).
Superose 6 chromatography can also be used as a purification step to improve IP when direct IP from nuclear extract fails to achieve desired purity. The purity of IP should be considered as unsatisfactory when silver-staining analysis of the immunoprecipitate reveals the presence of hundreds of polypeptides, or when the target protein is a minor polypeptide. In this regard, Gel-filtration columns have an advantage over other columns (Ion exchange, for example) because they can be run using mild salt and detergent buffers, so that the potential target complex remains intact.
Superose 6 columns can be used to verify if two proteins are components of the same complex. If the column profiles of two proteins are coincident, the two proteins may well be components of the same complex. In contrast, if peak fractions of the two proteins are completely separated on the Superose 6, the majority of the two proteins are certainly not present in the same complex.
Here is a common protocol that we use for Superose 6 column fractionation of nuclear extract.
We control the Superose 6 column with an FPLC system (AKTA Purifier, Amersham/GE). The column should be calibrated with proteins of known molecular weight to ensure that it works properly and has good resolution. For purification purpose, the HeLa nuclear extract (up to 2 ml) was cleaned of debris by ultracentrifugation and filtering. It was then applied to a Superose-6 column (HR 16/50; Amersham) equilibrated with the IP buffer described above at 0.3–0.5ml/min. Fractions were collected (1.5 ml each) and analyzed by SDS-PAGE and immunoblotting. The peak fractions containing the target protein were collected, and used for immunoprecipitation as described above. For analytical purpose, one can fractionate HeLa nuclear extract (up to 200 μl) through a smaller Superose 6 column (HR 10/30). Fractions (0.5 ml) were collected and analyzed by immunoblotting.
3.6 Co-IP using epitope-tagged protein
Sometimes one cannot generate high quality antibodies against a target protein for IP purpose. One way to circumvent this problem is to link the target protein with one or more epitope tags. One can then express the tagged protein in mammalian cell lines (such as HeLa), and use the antibody against the tag to purify the target complex. One can find detailed information regarding expression vectors and protocols in a previous publication (12).
There are many affinity tags to choose from, including Myc, Flag, His (6xHistidine), TAP, and HA. Our lab frequently uses Flag and HA tags, which are small and less likely to interfere with assembly of a target protein into a complex. Both antibodies are commercially available (Sigma and Upstate). We sometimes link two tags on a target protein (Flag and 6xHis), which allows affinity purification of the target protein using both tags, resulting in better purification than that with a single tag (Ling et al. 2007).
One example of purification by Flag antibody is shown in Figure 1C. In this case, antibody against RMI2 is not specific enough for affinity purification purpose (data now shown). We therefore linked RMI2 to Flag and 6xHistindine tags, and stably expressed the tagged RMI2 in HeLa cells. IP with the Flag antibody obtained Flag-tagged RMI2, BLM, Topoisomerase 3a, and RMI1. All these proteins are identified by mass spectrometry and immunoblotting (data not shown). We also included a mock IP from a HeLa extract that lacks tagged RMI2. This allows us to distinguish the BLM complex components from contaminating proteins that were isolated due to cross reactivity with the Flag antibody.
Compared to the BLM complex immunoprecipitated by using antibodies against endogenous proteins, the complex isolated by the Flag antibody is missing RPA (compare Fig. 1B and 1C; data not shown). Therefore, one may not be able to obtain the entire complex using the tagged-protein approach.
4. Verification of protein association
After the target protein and its co-immunoprecipitated polypeptides are identified by mass spectrometry, it is imperative to perform subsequent experiments to verify if their association is real and functionally relevant. This is because non-specific proteins can sometimes be co-immunoprecipitated, due to antibody crossreactivity. We suggest the following experiments for verification purpose. First, we often perform additional co-IP experiments using antibodies against different regions of the target protein. If different antibodies isolate the same protein(s), such proteins are less likely to be contaminants. Second, we always perform reciprocal IP—immunoprecipitation using antibody against newly identified proteins. If the reciprocal IP yields the original target protein, based on silver-staining and mass spectrometry analysis, the data provide compelling evidence that these proteins are components of a single complex. Third, we often perform Superose 6 gel-filtration chromatography to analyze if the newly identified proteins co-fractionate with the original target protein in the extract (see Section 3.5 above). Fourth, we often do co-IP in the presence of ethidium bromide (100 μg/ml) to rule out the possibility that association between the proteins is due to their interactions with DNA.
5. Functional analysis of newly identified components
The experiments above can only provide evidence for physical association between these proteins. Functional studies are necessary to determine if such association occurs in cells, and whether it is biologically relevant.
5.1 Study protein co-stabilization—an indicator for protein association in vivo
In cells, proteins that constitute the same complex often depend on one another for their stability. When one component of the complex is removed, either by siRNA depletion or by genetic mutation, its partners often lose their stability and show reduced levels in cells, which can be detected by immunoblotting. One reason could be that proteins need to interact with their partners within the complex for proper folding and compartmentalization. Without their partners, proteins may fold improperly and become targets for rapid degradation by proteosomes. This feature of protein co-stabilization can be used to judge whether two or more proteins are components of the same complex in vivo. For example, when the RMI2 subunit of the BLM complex is depleted by siRNA or inactivated by knockout in chicken DT40 cells, the other components of the BLM complex (BLM, RMI1, and Topo 3α) show reduced levels in cells as measured by immunoblotting (7). Similarly, when FANCB component of the FA core complex is depleted by siRNA or by genetic mutations, some of the other FA core complex components (FANCL and FAAP100) show reduced stability (9, 13). The data provide further evidence for association between these proteins in vivo.
As siRNA depletion is easier and faster than gene knockout, it is preferable to investigate protein co-stabilization by siRNA. We normally choose ON-TARGETplus SMARTpool siRNA oligos (Dhamacon Inc.), which have reduced off-target effects and satsifactory depletion efficiency. The siRNA knockdown procedure using Oligofectamine follows manufacturer’s instructions (Invitrogen) with minor modification:
Hela cells (about 4 × 105) were plated in 6 cm cell culture dish one day prior to siRNA transfection. The cells should reach 20–30% confluency at the time of transfection.
Oligofectamine (8 μl) was mixed with of Opti-MEM I medium (22 μl), and incubated at room temperature for 5–10 min.
Control or gene-targeting siRNA oligos (400 pmol) were added to 370 μl of Opti-MEM I.
The mixtures in b and c were combined and incubated for 15–20 min at room temperature to allow formation of complexes between siRNA oligoes and Oligofectamine.
The cells were removed of its medium, washed once with 2 ml of Opti-MEM I. Opti-MEM I medium (1.6 ml) was then added to the cells.
The siRNA mixture in d (400 μl) was added to each dish (the final concentration of siRNA is 200 nM), and incubated with cells for 4 hours at 37°C. New medium (1 ml) containing 30% FBS was then added to cells.
Cells were grown for 48 to 72 hours before harvest. Cells were lysed either by SDS-gel loading buffer or 1x RIPA buffer. The cell lysate was then analyzed by immunoblotting to determine the efficiency of depletion. We normally achieve 80–90% of depletion of the target protein.
5.2 DNA-damage drug sensitivity assay
Cell deficient in BLM and FA proteins show hypersensitivity to several DNA damaging drugs: BLM-deficient cells are sensitive to MMS, whereas FA cells are highly sensitive to DNA-crosslinking reagents, such as MMC (mitomycin C), cisplatin and DEB (diepoxybutane). In fact, MMC-sensitivity assay has been used as a diagnostic test for FA patients. Thus, a quick way to assess if a protein is involved in the FA-associated DNA damage response pathway is to perform MMC sensitivity assay for HeLa cells depleted of the target protein (10). Briefly,
HeLa cells were transfected with either siRNA target oligo or a control oligo, and grown for 60 hours.
The cells were then plated at a density of 500 cells/10 cm dish and grown for 8 hours.
DNA damaging drugs were then added to the cells at different concentration either in duplicates or triplicates. We normally use MMC at final concentration up to 1000 ng/ml, and cisplatin up to 10 μM.
Cells were grown in drug-containing media for 2 hours. The media was then washed away with PBS, followed by addition of fresh medium.
Cells were grown for 9 to 12 days until the colonies are visible. The colonies are then washed with PBS and stained with Crystal Violet solution (Sigma).
5.3 Sister-chromatid exchange (SCE) assay
The hallmark feature of Bloom syndrome cells is elevated SCE levels, typically about 10-fold higher than those in normal cells (1, 17). Increased SCE levels have also been observed in HeLa cells depleted of RMI1 and chicken DT40 cells inactivated of RMI2, suggesting that the entire BLM complex is involved in suppressing SCE (6, 7). Although DNA damage hypersensitivity has been observed in some BLM-deficient cells, this feature is absent in BLM mutant cells from some species. Thus, the SCE assay is the most reliable way to determine whether a new protein is important for BLM function.
Although SCE assays have been performed in HeLa cells depleted of BLM or RMI1, only a two-fold increase of SCE level has been observed. This is in contrast to a 10-fold increase of SCE in BLM patient cells, and 6-fold increase in chicken DT40 cells with inactivated RMI2. This difference is likely due to the inability of siRNA to completely remove endogenous BLM or RMI1; the residual amount of protein can partially suppresses SCE. Therefore, we recommend performing this assay using cells in which the candidate gene is inactivated. The chicken DT40 cell line is particularly suitable for this purpose, because one can readily knock out the candidate gene and examine its effect on the SCE level. Both BLM and Topoisomerase 3a have been inactivated in this cell line, and the analyses of their phenotypes have provided important insight to the function of the BLM complex. Our group has inactivated RMI2 in this cell line, and found that RMI2 suppresses SCE through the same pathway as BLM. Protocols for inactivating genes in DT40 cells can be found elsewhere (18). Here we describe the SCE assay used in our group for DT40 cells, which is modified based on previous publications (19, 20). One can also adapt this protocol to other cells.
DT40 cells were cultured in T25 flask containing RPMI medium supplemented with 10 % FBS, 1% chicken serum, 10 mM Hepes and 1% Penicillin/Streptomycin. Cells were grown at 39.5°C, in 5% CO2, until reaching density of 0.5–1 million cells/ml.
One million cells were incubated in 10 ml fresh medium containing 10 μM BrdU for 2 cell cycles (about 16 to 18 hours for wild type cells). The time of incubation is critical, as longer growth period causes insufficient staining of sister chromatids, whereas shorter period results in over-staining of both sister chromatids, both of which will prevent distinction of SCE.
Cells were pulsed by adding colcemid (0.1 μg/ml) to the medium and allowed to grow for the last 2 hours of BrdU treatment.
Cells were collected by centrifugation (1000 rpm, 5 min), resuspended in 2 ml of KCl (75 mM), and incubated for 15–30 min at room temperature.
Cells were fixed by adding 5 ml of fixing solution (methanol/acetic acid mixture, ratio 3:1), and collected by centrifugation. They were then resuspended in 5 ml of new fixing buffer, and incubated for 30 min at room temperature.
Cells were collected by centrifugation, and resuspended in 100–200 μl of fixing buffer, and dropped onto 50% ethanol pre-wet slide from 1 feet height. The slides were aged for 1–2 days before staining.
The slides were soaked for 5 min in 50 mM phosphate buffer (pH 6.8), and stained for 20 min at room temperature using Hoechst 33258 dye (10 μg/ml in 50 mM phosphate buffer, pH 6.8)
The slides were then rinsed in Macllvaine solution (164 mM Na2HPO4, pH 7.0 and 16 mM citric acid), covered with coverslips, and exposed to UV light (UVP, 15watt, 365 nM) for 30–60 min at a distance approximately 1 inch from the bulb. The time of exposure is critical, as shorter time makes sister chromatids indistinguishable, whereas longer exposure prevents one sister chromatid from staining by Giemsa.
The slides were removed of their coverslips and incubated in 2×SSC solution (0.3M NaCl, 0.03M Sodium Citrate) at 62°C for 1 hour. They were rinsed once with 50 mM phosphate buffer (pH 6.8), and stained with 3% Giemsa in 50mM phosphate solution (pH 6.8) for 20 min. Afterward, they were rinsed with water and air dried.
The slides were mounted in EUKITT mounting fluid and then can be viewed with a microscope (100× objective lens). As SCE is counted by individual scientist, the results are subject to human errors. To reduce human bias, we strongly recommend doing this experiment in a double-blind way: one researcher marks the slides with codes (to hide the genotypes of cells), whereas the second researcher counts the SCEs. At least 50 metaphase cells should be counted.
6. Concluding remarks
Co-IP has been successfully used in several groups to identify components of the BLM and Fanconi anemia complexes. Several components of these complexes have been shown to play critical roles in mediating function of BLM and FA proteins in DNA damage response. A few of them have also been found mutated in patients with the same disease. Therefore, this approach is a powerful way to study DNA damage response pathways, and could be applied to study proteins involved in other vital cellular processes. However, co-IP may not be able to isolate and identify all the proteins that mediate BLM and FA protein functions particularly those that have transient and weak interactions. Other methods, such as 2-hybrid screening or crosslinking associated proteins prior to co-IP by protein crosslinkers (for example DSP), may be used to identify such proteins.
Identification of proteins by co-IP is just the first step in elucidating their mechanism. Further experiments (reciprocal-IP and Superose 6 chromatography) are necessary to exclude the possibility that they are contaminants in the co-IP. Functional analysis (by siRNA or by genetic knockout) can provide evidence of whether these proteins act in the same pathway as BLM and FA proteins. Immunofluorescence analyses can reveal whether these proteins co-localize with BLM or FA proteins in DNA damage sites in cells (focus formation). And biochemical experiments can show whether these proteins can stimulate the activity of BLM and FA proteins in vitro. A range of approaches (some of them not described here) can facilitate our understanding of the mechanism of action of BLM and FA complexes.
Acknowledgments
We thank Drs. D. Schlessinger and R. Nagaraja for critical reading of the manuscript. This work was supported in part by the Intramural Research Program of the National Institute on Aging (Z01 AG000657-08), National Institute of Health; and by the Canadian Institutes of Health Research (MOP-79368). Figure 1A was reproduced from a previous publication, copyright © American Society of Microbiology, Molecular and Cellular Biology, Vol 23, Page 3417–3426, 2003. Figure 1B was reproduced from a previous paper in EMBO J. (6). Figure 1C and Figure 2 were reproduced from a previous publication in Genes & Development(7), Copyright © 2008, Genes & Development Online by Cold Spring Harbor Laboratory Press.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.German J. Medicine (Baltimore) 1993;72:393–406. [PubMed] [Google Scholar]
- 2.Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, Ciocci S, Proytcheva M, German J. Cell. 1995;83:655–66. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 3.Joenje H, Patel KJ. Nat Rev Genet. 2001;2:446–57. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 4.Wang W. Nat Rev Genet. 2007;8:735–48. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 5.Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, Hoatlin ME, Wang W. Mol Cell Biol. 2003;23:3417–26. doi: 10.1128/MCB.23.10.3417-3426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W. EMBO J. 2005;24:1465–76. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W. Genes Dev. 2008;22:2843–55. doi: 10.1101/gad.1708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, Hoatlin ME, Joenje H, Wang W. Nat Genet. 2003;35:165–70. doi: 10.1038/ng1241. [DOI] [PubMed] [Google Scholar]
- 9.Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, Rooimans MA, Bier P, Hoatlin M, Pals G, de Winter JP, Wang W, Joenje H. Nat Genet. 2004;36:1219–24. doi: 10.1038/ng1458. [DOI] [PubMed] [Google Scholar]
- 10.Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, Hoatlin M, Joenje H, de Winter JP, Wang W. Nat Genet. 2005;37:958–63. doi: 10.1038/ng1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM. Mol Cell. 2006;22:719–29. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Chi T, Yan Z, Xue Y, Wang W. Methods Enzymol. 2004;377:299–316. doi: 10.1016/S0076-6879(03)77018-9. [DOI] [PubMed] [Google Scholar]
- 13.Ling C, Ishiai M, Ali AM, Medhurst AL, Neveling K, Kalb R, Yan Z, Xue Y, Oostra AB, Auerbach AD, Hoatlin ME, Schindler D, Joenje H, de Winter JP, Takata M, Meetei AR, Wang W. EMBO J. 2007;26:2104–14. doi: 10.1038/sj.emboj.7601666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dignam JD, Martin PL, Shastry BS, Roeder RG. Methods Enzymol. 1983;101:582–98. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- 15.Reyes JC, Muchardt C, Yaniv M. J Cell Biol. 1997;137:263–74. doi: 10.1083/jcb.137.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Cold Spring Harbor Laboratory; New York: 1988. [Google Scholar]
- 17.Chaganti RS, Schonberg S, German J. Proc Natl Acad Sci U S A. 1974;71:4508–12. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahti JM. Methods. 1999;17:305–12. doi: 10.1006/meth.1999.0744. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Seki M, Narita Y, Sonoda E, Takeda S, Yamada K, Masuko T, Katada T, Enomoto T. EMBO J. 2000;19:3428–35. doi: 10.1093/emboj/19.13.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki M, Nakagawa T, Seki T, Kato G, Tada S, Takahashi Y, Yoshimura A, Kobayashi T, Aoki A, Otsuki M, Habermann FA, Tanabe H, Ishii Y, Enomoto T. Mol Cell Biol. 2006;26:6299–307. doi: 10.1128/MCB.00702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]