Abstract
Purpose
Glycoconjugates regulate a variety of biological events in mucosal surfaces, such as differentiation of postmitotic epithelial cells and maintenance of the wet-surfaced phenotype. This study aimed to identify the repertoire of genes (glycogenes) involved in biosynthesis of glycoconjugates in conjunctiva of normal subjects and dry eye patients.
Methods
RNA from conjunctival impression cytology samples was amplified and hybridized to a custom-designed glycogene microarray. Intensity data were converted to expression values and analyzed by ANOVA. Microarray data for selected Notch glycogenes were confirmed by quantitative real-time PCR. Notch receptors and ligands were immunolocalized on conjunctival biopsies by confocal microscopy.
Results
By microarray, 424 glycogenes were identified in normal conjunctival epithelium; galectins, glycosyltransferases, mucins, Notch signaling molecules, and proteoglycans were among the most highly expressed. In dry eye, 46 glycogenes were significantly downregulated, and included five members of the Notch signaling pathway (Notch1, -2, -3, Jagged1, Delta1), four Wnt signaling molecules (Wnt4, -5A, Frizzled6, -7), and three heparan sulfate glycotransferases (HS2ST1, HS3ST6, EXTL2). Only interferon-induced transmembrane protein 1 was upregulated. By real-time PCR, expression ratios of Notch1, -3, and Jagged1 in dry eye were 0.43, 0.56 and 0.50, respectively, compared to controls (p<0.05). Notch1, -3, and Jagged1 immunolocalized throughout the conjunctival epithelium, whereas Notch2 and Delta1 were distributed apically.
Conclusions
This study revealed the differential glycogene expression profiles in normal subjects and dry eye patients. Downregulation of Notch signaling in dry eye may result in abnormal differentiation of the conjunctival epithelium and have implications in the pathogenesis of the disease.
Keywords: conjunctiva, glycogene, dry eye, microarray, Notch signaling
Introduction
Glycosylation is the most common form of posttranscriptional modification of proteins, with over half of all proteins estimated to contain one or more glycan chains.1 In wet-surfaced epithelia, the roles of glycan chains are varied. Glycans confer a hydrophilic character to mucins on epithelial cell surfaces,2 are essential in maintaining epithelial barrier function,3 and modulate cell surface receptor activation.4 An extensive list of genes—generically named glycogenes—are responsible for the biosynthesis of glycoconjugates and include glycosyltransferases, glycolytic enzymes, sugar nucleotide synthetases, sugar nucleotide transporters and, in a broader sense, sugar-chain recognizing molecules, and glycoconjugates themselves.5 Despite the importance of glycans to maintaining a wet-surfaced phenotype, little is known about the glycogene profile of normal human ocular surface epithelia or whether there is an alteration of this profile in drying ocular surface diseases.
Within the extensive repertoire of glycoconjugates present in self-renewing epithelia, glycosylated cell surface receptors, such as the Notch family of single-pass transmembrane proteins, have received attention in recent years because of their involvement in cell differentiation. Notch receptors contain a large extracellular domain with many epidermal growth factor-like repeats that are glycosylated with O-fucose and O-glucose glycans as well as N-glycans.4 To date, four Notch receptors (Notch1-4) have been identified in mammals, with five corresponding ligands, including Delta1, Delta3, Delta4, Jagged1, and Jagged2.6 Notch-mediated intracellular signaling is triggered by direct cell-cell interaction between Notch receptors and their ligands on adjacent cells.7 In mucosal surfaces such as the gastrointestinal tract, the Notch signaling pathway is fundamental to cell lineage commitment and appears to regulate the differentiation of postmitotic epithelial cells.8 Inactivation of all Notch/ligand interactions by specific deletion of the O-fucosyltransferase 1 gene (Pofut1) in intestinal and colonic epithelial cells in the mouse results in enterocolitis.9
Dry eye is a multifactorial disease of the ocular surface that is prevalent in women and that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to ocular surface epithelia.10 At the histopathological level, the ocular surface of dry eye patients shows increased proliferative activity,11 reduced number of conjunctival goblet cells,12 and, in late stages, the loss of the wet-surfaced phenotype and keratinization.10 Several reports have identified alterations in specific glycosyltransferases and glycoconjugates on the ocular surface epithelia of patients with dry eye,13-15 but to date no comprehensive study has been carried out on the overall expression of glycogenes in these patients. The purpose of this study was to identify the glycogene expression profile of human conjunctiva in normal subjects and patients with dry eye disease, using a custom-designed glycogene microarray.
Methods
Subject Selection
This study was performed in accordance with the Declaration of Helsinki and The Schepens's Institutional Review Board. Informed consent was obtained from all participants. All prospective subjects completed an institutional review board-approved questionnaire regarding the presence, type, and frequency of their dry eye symptoms. Two groups of subjects were studied. The first group consisted of 15 normal subjects (12 females, 3 males; 40±3 years old) who had no dry eye symptoms, no eye diseases, and no history of eye surgery or contact lens wear. The second group consisted of 15 patients with non-Sjögren's dry eye (14 females, 1 male, 49±3 years old) who fit the inclusion criteria for non-Sjögren's dry eye: (1) moderate to severe dry eye symptoms, (2) Schirmer I reading of less than 8 mm, without anesthesia, (3) tear breakup time of less than 5 seconds, and (4) positive corneal and conjunctival staining with rose bengal. Patients taking topical or systemic medications, as well as patients with history of ocular surgery and/or contact lens wear, ocular allergies, diabetes, Sjögren's syndrome, or other autoimmune diseases, were excluded. All subjects were blood type O to avoid variation in terminal glycosylation.16,17
Sample Collection and RNA Extraction
Conjunctival epithelial cells were obtained by impression cytology from the bulbar conjunctiva, using nitrocellulose membranes as described.18 Total RNA was extracted from the membranes using TRIzol (Invitrogen Corp; Carlsbad, CA) and purified using RNeasy columns (Qiagen, Inc.; Valencia, CA). Amount of total RNA was determined using a Nanodrop® ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies; Wilmington, DE). Samples with a minimum 30 ng/μl and 260/280 ratio between 2 and 2.1 were considered adequate for microarray analysis. Nine (9) normal samples were divided into three groups, each group containing 3 individual samples. The samples in each group were then pooled and used for microarray analysis. Nine (9) dry eye samples were divided and pooled for microarray in the same manner. Additionally, 6 normal and 6 dry eye samples were used in real-time PCR experiments.
RNA Labeling and Chip Hybridization
The RNA sample quality was checked with an Agilent Bioanalyzer (Agilent Technologies; Palo Alto, CA). RNA from each preparation was labeled using the MessageAmp II-Biotin Enhanced Amplification kit (Ambion Inc.; Austin, TX). Hybridization to the custom-designed GLYCOv3 gene chip microarray was performed according to Affymetrix's recommended protocols. The GLYCOv3 microarray was developed by the Consortium for Functional Glycomics and includes probes for 1,268 human gene transcripts. A complete description and annotation for the GLYCOv3 microarray is available at: http://www.functionalglycomics.org/static/consortium/resources.shtml. The Affymetrix chips were scanned using the Affymetrix GeneChip Scanner 3000. Chips had a background less than 50 intensity units and a GAPDH 3′/5′ ratio of less than three. Robust Multichip Average (RMA) was used to convert the intensity values to expression values.19,20 RMA consists of a three-step approach that uses a background correction on the Perfect Match (PM) data, a quantile normalization, and summarizes the probe set information by using Tukey's median polish algorithm. ANOVA was performed using BRB Array Tools; BRB utilizes multivariate permutation tests to ensure that the number or proportion of false discoveries is controlled; it is effective when the number of samples is limited. The list of significant genes from the ANOVA was filtered such that the p-value cutoff was lower than 0.05, and the ratio of expression (dry eye:normal) would be greater than 1.2 for upregulated genes and 0.8 for downregulated genes. The False Discovery Rate (FDR) was calculated using the Benjamini-Hochberg method. FDR is an approach to the multiple comparisons problem. Multiple testing is a classic problem for high-dimensional data sets, such as microarrays (which monitor the expression level of thousands of genes simultaneously) that could give rise to many false positive genes. The FDR of a set of predictions is the expected percentage of false predictions in the prediction set.
Reverse Transcription (RT) and Real-time PCR
First-strand cDNA was synthesized from total RNA using oligo dT primers and reverse transcriptase (Superscript III RT; Invitrogen Corp). The relative expression of Notch1, Notch3, and Jagged1 was determined on a sequence detection system (ABI Prism 7900HT; PE Applied Biosystems; Foster City, CA), using previously described primers and TaqMan probes.21 The average threshold cycle (Ct) values for GAPDH were used as an endogenous reference to correct for differences in the amount of total RNA added to each reaction. To validate the relative quantitation, the efficiency of the target gene amplification was compared with the efficiency of the GAPDH amplification, as described in the manufacturer's protocol (PE Applied Biosystems). The comparative Ct method was used for relative quantitation of the number of transcripts in normal subjects and patients with dry eye, with the relative mRNA levels in normal subjects selected as the calibrator. Statistical comparisons of the real-time PCR results were performed using unpaired Student's t test (InStat; GraphPad Software, Inc.; La Jolla, CA) and p<0.05 was considered significant. A non-template control was included in all the experiments performed with real-time PCR to evaluate DNA contamination of the reagents used for amplification. None of the experiments resulted in a non-template control-positive signal, indicating that there was no DNA contamination in the assays. Conventional PCR experiments were performed to confirm that only a single band is obtained when amplifying conjunctival cDNA with the Notch1, Notch3, and Jagged1 primers used in this study.
Confocal Microscopy
For confocal microscopy experiments, sections from two conjunctival biopsy specimens collected for a previous study11 were used. Cryostat sections (7 μm) were incubated for 2 hours at room temperature with primary antibodies (diluted 1:50 in PBS) directed against the extracellular domain of Notch1, -2, -3, Delta1 and Jagged1 (Santa Cruz Biotechnology; Santa Cruz, CA), and for 1 hour in their corresponding FITC-labeled secondary antibodies (Jackson Immunoresearch Laboratories Inc.; West Grove, PA) diluted 1:100 in PBS. Sections were mounted in Vectashield mounting medium (Vector Laboratories; Burlingame, CA) with propidium iodide to localize the cell nuclei. Images were acquired with a TSC-SP2 laser confocal microscope (Leica Microsystems; Heidelberg, Germany) and digitalized using Leica confocal software LCS v2.61. Sections of normal colon served as positive controls. Sections incubated with secondary antibody alone served as negative controls.
Results
Glycogene Profile of the Normal Human Conjunctival Epithelium
The GLYCOv3 microarray identified 424 glycogenes expressed in the normal conjunctival epithelium (for complete list, view http://www.functionalglycomics.org/glycomics/publicdata/microarray.jsp, ref. MAEXP_310_052307). A pie chart showing the relative distribution (percentage) of the gene ontology groups is shown in Figure 1. The largest glycogene groups included several families of glycosyltransferases (29%), growth factors (14%), glycan degradation proteins (10%) and carbohydrate-binding proteins (9%). Mucins and Notch signaling molecules, which accounted for 4% and 3% of the glycogenes, respectively, were among the most highly expressed in human conjunctiva (Table 1).
Figure 1.
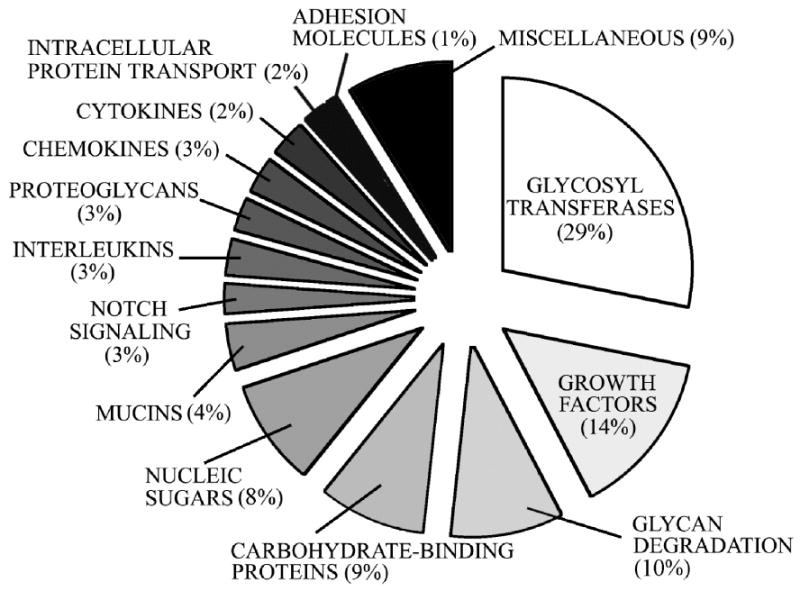
Distribution of gene ontology groups in normal conjunctiva. Glycogenes (n=424) were categorized according to gene function as defined in the GLYCOv3 microarray database.
Table 1.
Fifty most highly expressed glycogenes in the human conjunctival epithelium as determined by glycogene microarray analysis.
| Category | Accession number | Common name | Geometric mean of intensities |
|---|---|---|---|
| Carbohydrate-binding proteins | NM_02306 | Galectin 3 | 3,938 |
| NM_005567.2 | Galectin 6 binding protein | 1,043 | |
| NM_006499.3 | Galectin 8 | 579 | |
| Cytokines & chemokines | NM_006435.1 | INF-induced transmembrane protein 2 | 997 |
| NM_003641.1 | INF-induced transmembrane protein 1 | 814 | |
| NM_000416.1 | INF-gamma receptor 1 | 544 | |
| Glycan degradation | NM_005561.2 | Lysosomal-associated membrane protein | 850 |
| NM_000521.2 | Hexosaminidase B preprotein | 526 | |
| Glycosyltransferases | NM_004751.1 | GlcNAc transferase 3, mucin-type | 1,090 |
| NM_001344.1 | Defender against cell death (DAD1) | 939 | |
| NM_004776.2 | Beta4-Gal transferase 5 (B4GALT5) | 850 | |
| NM_002951.2 | Ribophorin II | 753 | |
| NM_005216.3 | Dolichyl-diphospho oligosaccharide transferase (DDOST) | 588 | |
| NM_054013 | GlcNAc transferase IVb | 530 | |
| Growth factors & receptors | M31159.1 | IGF 3 | 2,112 |
| X07868 | IGF 2 (Somatomedin A) | 958 | |
| NM_000142.2 | FGF receptor 3 | 788 | |
| NM_001982.1 | Erythroblastic leukemia viral oncogene homolog 3 | 680 | |
| Miscellaneous | M25915.1 | Clusterin | 4,272 |
| NM_001402.1 | Eukariotic translation elongation factor 1 alpha 1 | 3,940 | |
| NM_021103.1 | Thymosin beta 10 | 3,695 | |
| NM_005566.1 | LDHA | 2,818 | |
| NM_001961.1 | Eukariotic translation elongation factor 2 | 2,605 | |
| BF686442 | Prothymosin alpha | 2,182 | |
| AF028832.1 | Hsp89-alpha-delta-N | 1,960 | |
| NM_003564.1 | Transgelin 2 | 1,445 | |
| NM_002966.1 | S100 calcium binding protein A10 | 1,383 | |
| NM_001418.1 | Eukariotic translation initiation factor 4 gamma 2 | 1,276 | |
| NM_003752.2 | Eukariotic translation initiation factor 3 subunit 8 | 1,239 | |
| NM_006263.1 | Proteasome activator subunit1 (PA28 alpha) | 1,104 | |
| AL037557 | Laminin receptor 1 | 854 | |
| NM_012286.1 | MORF-related gene X | 847 | |
| NM_002795.1 | Proteasome subunit beta type 3 | 841 | |
| BF697964 | Destrin | 687 | |
| NM_002802.1 | Proteasome subunit 26S | 658 | |
| M69148.1 | Midkine | 646 | |
| X57198.1 | Transcription elongation factor | 602 | |
| NM_000454.1 | Superoxide dismutase 1 | 514 | |
| Mucins | NM_152673 | MUC20 | 2,019 |
| NM_002456.1 | MUC1 | 1,493 | |
| AF106518 | CD164 antigen, sialomucin | 852 | |
| AF361486 | MUC16 | 717 | |
| AJ298317 | MUC5AC | 713 | |
| Notch signaling | NM 024408 | Notch2 | 725 |
| NM 000214 | Jagged1 | 546 | |
| Nucleic sugars | NM_006098.1 | Guanine nucleotide binding protein | 2,734 |
| S73498.1 | AgX-1 antigen | 538 | |
| Proteoglycans | NM_002999.1 | Syndecan 4 (Ryudocan) | 1,205 |
| K01144.1 | CD74 | 1,167 | |
| NM_002997. | Syndecan 1 | 1,150 |
Alteration of Glycogene Expression in Dry Eye
In our study, the amount of total RNA extracted from impression cytology samples of conjunctiva from normal subjects and dry eye patients was comparable (3.7±0.8 μg and 2.8±0.5 μg, respectively) and varied between 1 and 10 μg per sample. The expression of 46 glycogenes was significantly altered in dry eye (Table 2). Interestingly, all Notch receptors expressed in human conjunctiva—Notch1, -2, and -3—were downregulated in dry eye as compared to normal conjunctiva (ratios 0.71, 0.70, and 0.74, respectively). Notch ligands Jagged1 and Delta1 were also downregulated (0.76 and 0.59, respectively). In addition to Notch, four members of the Wnt signaling pathway were downregulated: Wnt4 (ratio 0.73) and Wnt5A (0.72), and their cell-surface receptors Frizzled6 (0.63) and Frizzled7 (0.64). Other glycogenes downregulated in dry eye included 11 glycosyltransferases: three of these (HS2ST1, HS3ST6, EXTL2) involved in the biosynthesis of heparan sulfate, a glycosaminoglycan synthesized on cell surface proteins, primarily syndecans and glypicans. The only glycogene upregulated in dry eye was interferon-induced transmembrane protein 1 (ratio 2.19).
Table 2.
Glycogenes differentially expressed in dry eye. The expression ratio represents the geometric mean signal in conjunctiva of dry eye patients versus normal subjects. FDR: False Discovery Rate.
| Category | Accession number | Common name | Ratio | p value | FDR |
|---|---|---|---|---|---|
| Carbohydrate-binding proteins | AL132773 | Attractin | 0.77 | 0.01 | 0.26 |
| AF106861.1 | Attractin 2 | 0.70 | 0.02 | 0.32 | |
| NM_002307.1 | Galectin 7 | 0.69 | 0.02 | 0.35 | |
| NM_000297.1 | Polycystin 2 | 0.72 | 0.01 | 0.26 | |
| NM_000361.1 | Thrombomodulin | 0.72 | 0.01 | 0.27 | |
| Cytokines & chemokines | U20350.1 | CX3 chemokine receptor 1 (V28) | 0.56 | 0.0009 | 0.21 |
| NM_003641.1 | Interferon-induced transmembrane protein 1 | 2.19 | 0.03 | 0.36 | |
| Glycosyltransferases | AK025456 | Asparagine-linked glycosylation 11 (ALG11) | 0.63 | 0.003 | 0.21 |
| NM_014863.1 | Chondroitin GalNAc4-O-sulfotransferase (GalNAc4S6ST) | 0.72 | 0.003 | 0.21 | |
| NM_001439.1 | Exostoses-like 2 (EXTL2) | 0.64 | 0.004 | 0.21 | |
| NM_173540.1 | Fucosyl transferase 11 (FUT11) | 0.74 | 0.006 | 0.21 | |
| NM_012262 | Heparan sulfate 2-O-sulfotransferase 1 (HS2ST1) | 0.69 | 0.002 | 0.21 | |
| NM_001009606.1 | Heparan sulfate 3-O-sulfotransferase 6 (HS3ST6) | 0.73 | 0.04 | 0.38 | |
| BF001665 | O-linked N-acetylglucosamine transferase (OGT) | 0.68 | 0.006 | 0.21 | |
| NM_030965.1 | Sialyltransferase 6 GalNAc 5 (ST6GalNAc5) | 0.49 | 0.02 | 0.30 | |
| NM_000463.2 | UDP glucuronosyltransferase 1A1 (UGT1A1) | 0.66 | 0.004 | 0.21 | |
| NM_021027.1 | UDP glucuronosyltransferase 1A9 (UGT1A9) | 0.64 | 0.002 | 0.21 | |
| NM_020121.2 | UDP glucose ceramide glucosyltransferase 2 (UGCGL2) | 0.76 | 0.009 | 0.26 | |
| Growth factors & receptors | NM_020328.1 | Activin A receptor, type IB | 0.70 | 0.03 | 0.36 |
| X59065 | Fibroblast growth factor 1 | 0.46 | 0.006 | 0.22 | |
| NM_003506.1 | Frizzled6 | 0.64 | 0.01 | 0.26 | |
| NM_003507.1 | Frizzled7 | 0.64 | 0.005 | 0.21 | |
| NM_000597.1 | Insulin-like growth factor binding protein 2 | 0.52 | 0.01 | 0.29 | |
| NM_020998.1 | Macrophage stimulating 1 | 0.69 | 0.008 | 0.25 | |
| AF336376 | Platelet derived growth factor D | 0.59 | 0.04 | 0.37 | |
| NM_003243.1 | TGF beta receptor 3 | 0.34 | 0.004 | 0.21 | |
| NM_004257.1 | TGF beta receptor-associated protein 1 | 0.74 | 0.001 | 0.21 | |
| NM_030761.1 | Wnt4 | 0.73 | 0.05 | 0.38 | |
| NM_003392.1 | Wnt5A | 0.72 | 0.03 | 0.36 | |
| Interleukins | BE856546 | Interleukin 6 signal transducer | 0.66 | 0.004 | 0.21 |
| Intracellular protein transport | NM_031431 | Component of oligomeric Golgi complex 3 (COG 3) | 0.76 | 0.008 | 0.24 |
| Miscellaneous | NM_003752.2 | Eukaryotic translation initiation factor 3, subunit 8 | 0.75 | 0.03 | 0.36 |
| NM_000153.1 | Galactosylceramidase precursor | 0.75 | 0.04 | 0.38 | |
| U72069.1 | Karyopherin beta2 | 0.73 | 0.04 | 0.38 | |
| J03263.1 | Lysosomal-associated membrane protein 1 | 0.62 | 0.0009 | 0.21 | |
| NM_013995.1 | Lysosomal-associated membrane protein 2B | 0.75 | 0.004 | 0.21 | |
| AL042588 | Paternally expressed 3 | 0.68 | 0.003 | 0.21 | |
| NM_006445 | Pre-mRNA processing factor 8 (PRP8) homolog | 0.76 | 0.01 | 0.26 | |
| Notch signaling | NM_005618 | Delta1 | 0.76 | 0.02 | 0.35 |
| NM_000214 | Jagged1 | 0.59 | 0.01 | 0.26 | |
| NM_017617 | Notch1 | 0.71 | 0.003 | 0.21 | |
| NM_024408 | Notch2 | 0.70 | 0.03 | 0.36 | |
| NM_000435 | Notch3 | 0.75 | 0.02 | 0.35 | |
| Nucleic sugars | NM_003838.1 | Fucose-1-phosphate guanyltransferase | 0.65 | 0.0003 | 0.21 |
| AF033026.1 | Phosphoadenosine-phosphosulfate (PAPS) synthetase 1 | 0.72 | 0.03 | 0.36 | |
| Proteoglycans | AF020043 | Bamacan | 0.73 | 0.005 | 0.21 |
Real-time PCR Analysis of Notch Expression in Normal and Dry Eye Patients
Previous results using tissue-specific gene targeting have demonstrated that Notch inactivation results in keratinization of the corneal epithelium in rodents, suggesting that Notch signaling is necessary to maintain the wet-surfaced phenotype of the ocular surface.22 Therefore, our studies focused on the downregulation of Notch receptors and ligands in dry eye patients. The differential expression of components of the Notch signaling pathway observed by microarray in these patients was confirmed by real-time PCR (Figure 2A). Three Notch genes were selected because they were the most significantly downregulated by microarray, and included two receptors (Notch1 and Notch3) and one ligand (Jagged1). As shown by real-time PCR, the expression ratios of Notch1, Notch3, and Jagged1 mRNA in the conjunctiva of dry eye patients versus normal subjects was 0.43 (p=0.04), 0.56 (p=0.03) and 0.50 (p=0.02), respectively. This downregulation in Notch expression was in agreement with that observed by microarray analysis, with real-time PCR data showing more pronounced differences between dry eye patients and controls (Figure 2B).
Figure 2.
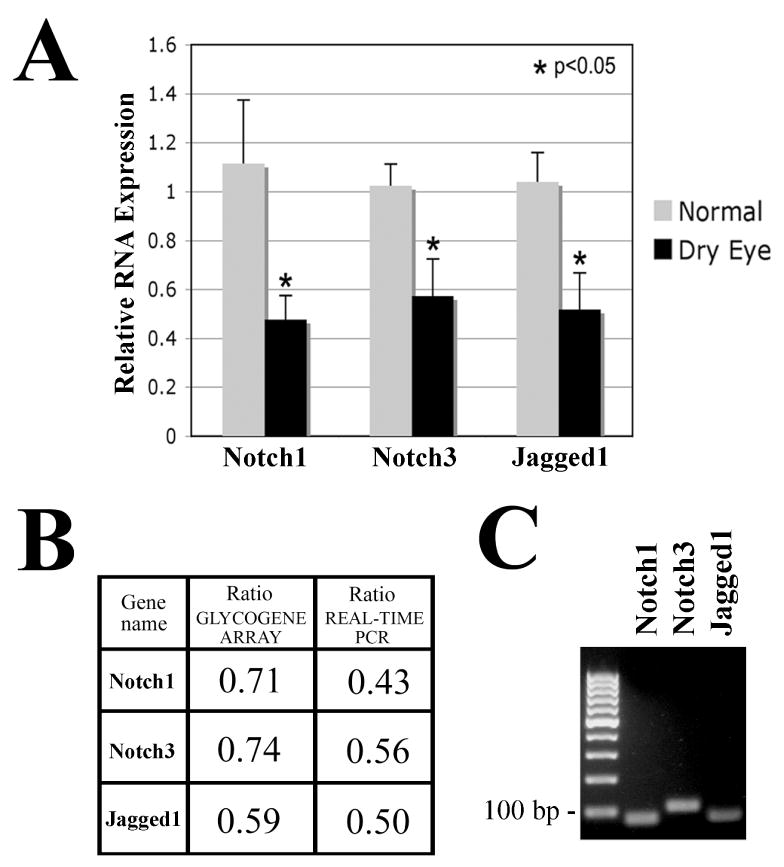
Real-time PCR analysis of Notch1, Notch3 and Jagged1. (A) By real-time PCR, Notch1, Notch3, and their ligand Jagged1 were significantly downregulated in the conjunctiva of patients with dry eye (p<0.05). (B) Comparison of microarray and real-time PCR showed downward trends in the results, with real-time PCR analyses resulting in more pronounced differences in expression of Notch signaling genes between normal subjects and dry eye patients. (C) Conventional PCR after 30 cycles of cDNA amplification produced a unique band corresponding to the predicted size for Notch1 (75 bp), Notch3 (105 bp) and Jagged1 (76 bp).
Immunolocalization of Notch in Human Conjunctiva
The distribution of Notch1, -2, -3, and their ligands Delta1 and Jagged1 was determined by confocal microscopy on human conjunctival biopsies (Figure 3). Antibodies against Notch1, Notch3 and Jagged1 bound throughout the entire normal conjunctival epithelium. Notch3 appears, therefore, to be specific to the conjunctiva at the ocular surface, since it has not been detected in corneal epithelium.23 Antibodies to Notch2 and Delta1 bound predominantly to the apical layer of the conjunctival epithelium. In these experiments, intense cytoplasmic binding was observed, consistent with the intracellular trafficking of Notch receptors and ligands.24 Binding of antibodies against Notch1, -2, -3, Delta1, and Jagged1 to sections of conjunctival epithelium from non-Sjögren's dry eye samples was weaker and did not differ in distribution from that observed in normal samples (data not shown).
Figure 3.
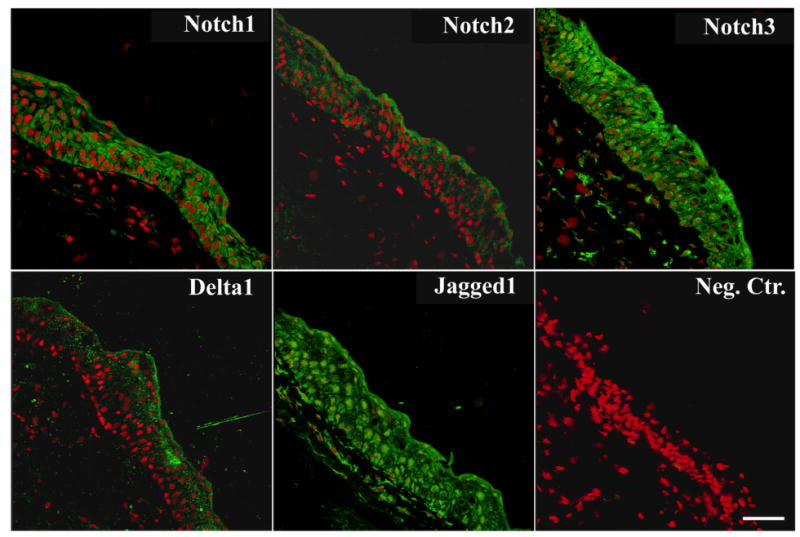
Immunolocalization of Notch receptors and ligands in normal conjunctiva. By confocal microscopy, Notch1, -3, and Jagged1 were present throughout conjunctival epithelium. Antibodies to Notch2 and Delta1 bound primarily to apical cells. Propidium iodide was included in the mounting medium to localize the position of the nuclei in all sections (red). In control experiments, antibodies to Notch1, -2, -3, Delta1, and Jagged1 bound to epithelial cells in normal colon (data not shown). Negative control corresponds to secondary antibody only. Scale bar = 75 μm.
Discussion
In this study, a custom-designed glycogene chip microarray was used to identify glycogenes relevant to the synthesis of glycan structures and glycoconjugates in the conjunctival epithelium of normal subjects and patients with dry eye. In normal conjunctiva, 424 glycogenes encoding glycosyltransferases, growth factors, glycosidases, carbohydrate-binding proteins, nucleic sugar transporters, mucins, members of the Notch signaling pathway, and proteoglycans were identified. Patients with dry eye had an altered glycogene profile in their conjunctival epithelium. The expression of 46 genes was significantly reduced and included members of the Notch and Wnt signaling pathways, as well as heparan sulfate glycotransferases—glycogenes known to be involved in the maintenance of a wet-surfaced phenotype.
The human conjunctiva consists of a nonkeratinized squamous epithelium with goblet cells interspersed among the layers of stratified cells. Progression into dry eye disease is commonly characterized by increased proliferation in the conjunctival epithelium, goblet cell deficiency, and in severe cases, keratinization due to abnormal differentiation of the epithelium.11,25,26 Recent evidence showed that cell fate decisions and differentiation processes in mucosal surfaces is determined by the Notch pathway. Histological examination of the mouse cornea after inducible ablation of Notch1 showed extensive hyperplasia and keratinization of the epithelium, suggesting that Notch is necessary for the proper differentiation of the corneal epithelium.22,27 Subsequent human and murine studies using tissue localization and in vitro models to test the function of Notch and their immediate downstream targets (e.g., Hes1) are consistent with the role of Notch pathway in regulating differentiation and proliferation activities in the cornea.23,28,29 To date, however, there is no data on the role of Notch signaling in conjunctival non-goblet and goblet cell epithelial differentiation. In the intestine of gut-inducible mutant mice, inactivation of Notch receptors -1 and -2, Notch O-fucosylation, and the Notch transcription factor CSL/RBP-J resulted in complete conversion of proliferating crypt progenitors into postmitotic goblet cells.9,30,31 On the other hand, it has also been reported that activation of Notch1 in gut-inducible mutant mice increases the numbers of goblet cells.8 This discrepancy seems to be explained by the role of Notch acting in opposing ways at two points in goblet cell development—during differentiation of progenitor and of postmitotic cells.8 In the conjunctival epithelium, we hypothesize that decreases in Notch receptors and ligands play a role in the pathogenesis of dry eye by altering the development of non-goblet and goblet cells.
Notch is known to interact with at least two other signaling pathways, Wnt and vitamin A.7 Wnt proteins are secreted glycoproteins that elicit cellular responses through their assembly to a membrane receptor complex that includes the Frizzled receptors.32 In our experiments, four members of the Wnt signaling pathway, Wnt4, Wnt5A, Frizzled6, and Frizzled7, were downregulated in dry eye. In addition to Wnt, Notch signaling is also linked to vitamin A metabolism by regulating the expression of cellular retinol binding protein 1 (CRBP1), required for retinol metabolism into retinoic acid.27 It is well known that vitamin A levels influence the programme of terminal differentiation of the cornea. It is, therefore, possible to speculate that Notch, Wnt and vitamin A are part of a web of intersecting signaling pathways whose downregulation in dry eye alters the differentiation of the conjunctival epithelium.
Among other genes significantly downregulated in dry eye, 11 were glycosyltransferases. Three of those, HS2ST, HS3ST and EXTL2, are involved in the modification of heparan sulfate, a glycosaminoglycan known to be present on epithelial cell surfaces.33 On the cell surface, heparan sulfate can sequester secreted soluble ligands and modulate their activity, thus, activating and inhibiting cell proliferation, motility, and differentiation.33 Interestingly, HS3ST seems to be involved in the regulation of Notch signaling in Drosophila melanogaster. Reduction of HS3ST by siRNA compromised Notch signaling and affected the number and size of endosomal/lysosomal compartments, suggesting the role of 3-O sulfated heparan sulfate in intracellular trafficking of Notch.34
In our study, we did not detect changes in overall expression of glycosyltransferases involved in mucin-type O-glycosylation. Mucins play a role in maintaining the wet-surfaced phenotype in mucosal surfaces by providing hydrophilic carbohydrate chains.35 Our results confirm those by Imbert et al. that showed no differences in the mRNA expression of polypeptide GalNAc-transferases—enzymes responsible for the initiation of mucin O-glycosylation—between the conjunctival epithelium of dry eye patients and control groups.36 The analysis of mRNA expression levels alone could, however, provide a partial understanding of the role of mucin-specific glycosyltransferases in dry eye. Immunofluorescence analyses have shown an alteration in the distribution of polypeptide GalNAc-transferases as well as mucin-type O-glycans in the conjunctival epithelium of dry eye patients,13,15 which suggests a compensatory mechanism by the epithelial cells to produce mucin-type O-glycans on the cell surface.
The only glycogene upregulated in dry eye was interferon-induced transmembrane protein 1 (IFITM1). IFITM1, whose expression can be induced by interferon-gamma, encodes a cell surface protein known to influence cell differentiation.37 Interestingly, production of interferon-gamma at the ocular surface has been implicated in the progress of dry eye disease.38 Previous data in an experimental dry eye model suggested that interferon-gamma may affect conjunctival epithelial homeostasis and promote conjunctival squamous metaplasia.39 Therefore, it is possible to speculate that biosynthesis of interferon-gamma in the conjunctiva of patients with dry eye could enhance the expression of IFITM1, which could, in turn, then play a role in the abnormal terminal differentiation of the epithelium.
In conclusion, this study has identified the glycogene expression profile of normal human conjunctival epithelium and its alteration in patients with dry eye. Downregulation of members of the Notch signaling pathway—known to be involved in cell fate determination and differentiation—could compromise the integrity of the ocular surface. These findings may have relevance and therapeutic potential for the treatment of dry eye disease.
Acknowledgments
The authors are grateful to Dr. Ilene Gipson for providing human conjunctival biopsies and to Drs. Stefano Bonini, Audrey Chan, Magdalena Cortes, Pedram Hamrah, and Alessandra Micera for providing conjunctival impression cytology samples.
Financial support: Supported by National Eye Institute Grant EY014847 (PA). The resources and collaborative efforts provided by the Consortium for Functional Glycomics were funded by National Institute of General Medical Sciences Grant GM62116.
Footnotes
Commercial Relationship: N
References
- 1.Lee RT, Lauc G, Lee YC. Glycoproteomics: protein modifications for versatile functions. Meeting on glycoproteomics. EMBO Rep. 2005;6:1018–1022. doi: 10.1038/sj.embor.7400556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 3.Bode L, Salvestrini C, Park PW, et al. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal epithelial barrier function. J Clin Invest. 2008;118:229–238. doi: 10.1172/JCI32335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley P. Regulation of Notch signaling by glycosylation. Curr Opin Struct Biol. 2007;17:530–535. doi: 10.1016/j.sbi.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narimatsu H. Construction of a human glycogene library and comprehensive functional analysis. Glycoconj J. 2004;21:17–24. doi: 10.1023/B:GLYC.0000043742.99482.01. [DOI] [PubMed] [Google Scholar]
- 6.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zecchini V, Domaschenz R, Winton D, Jones P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691. doi: 10.1101/gad.341705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilmeau S, Flandez M, Bancroft L, et al. Intestinal deletion of Pofut1 in the mouse inactivates Notch signaling and causes Enterocolitis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The definition and classification of dry eye disease: report of the Definition and Classification subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 11.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 12.Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302. [PubMed] [Google Scholar]
- 13.Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 14.Versura P, Maltarello MC, Cellini M, Caramazza R, Laschi R. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. II. A quantitative study in dry-eye patients. Acta Ophthalmol (Copenh) 1986;64:451–455. doi: 10.1111/j.1755-3768.1986.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 15.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 16.Garcher C, Bara J, Bron A, Oriol R. Expression of mucin peptide and blood group ABH- and Lewis-related carbohydrate antigens in normal human conjunctiva. Invest Ophthalmol Vis Sci. 1994;35:1184–1191. [PubMed] [Google Scholar]
- 17.Watanabe H, Gipson IK. Detection of blood group differences in human corneal epithelium using a monoclonal antibody and lectins. Arch Ophthalmol. 1994;112:667–673. doi: 10.1001/archopht.1994.01090170111032. [DOI] [PubMed] [Google Scholar]
- 18.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 19.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung E, Tang SM, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 22.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 23.Ma A, Boulton M, Zhao B, Connon C, Cai J, Albon J. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Invest Ophthalmol Vis Sci. 2007;48:3576–3585. doi: 10.1167/iovs.06-1373. [DOI] [PubMed] [Google Scholar]
- 24.Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006 doi: 10.1126/stke.3642006cm7. 2006:cm7. [DOI] [PubMed] [Google Scholar]
- 25.Meller D. The fine structure of chromatin alterations in conjunctival epithelial cells in keratoconjunctivitis sicca. Cornea. 1999;18:225–232. doi: 10.1097/00003226-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Murube J, Rivas L. Biopsy of the conjunctiva in dry eye patients establishes a correlation between squamous metaplasia and dry eye clinical severity. Eur J Ophthalmol. 2003;13:246–256. doi: 10.1177/112067210301300302. [DOI] [PubMed] [Google Scholar]
- 27.Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13:242–253. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Ohtsuka T, Sekiyama E, et al. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cells. 2008:1549–4918. doi: 10.1634/stemcells.2007-1067. Electronic. [DOI] [PubMed] [Google Scholar]
- 29.Djalilian AR, Namavari A, Ito A, et al. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–1049. [PMC free article] [PubMed] [Google Scholar]
- 30.Riccio O, van Gijn M, Bezdek A, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Reports. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 32.Hayward P, Kalmar T, Arias AM. Wnt/Notch signalling and information processing during development. Development. 2008;135:411–424. doi: 10.1242/dev.000505. [DOI] [PubMed] [Google Scholar]
- 33.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 34.Kamimura K, Rhodes JM, Ueda R, et al. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J Cell Biol. 2004;166:1069–1079. doi: 10.1083/jcb.200403077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi L, Caldwell KD. Mucin adsorption to hydrophobic surfaces. J Colloid Interface Sci. 2000;224:372–381. doi: 10.1006/jcis.2000.6724. [DOI] [PubMed] [Google Scholar]
- 36.Imbert Y, Jumblatt MM, Foulks GN, Couzin EG, Steele PS, Young WW., Jr Expression in human ocular surface tissues of the GalNAc-transferases that initiate mucin-type O-glycosylation. Cornea. 2006;25:1193–1199. doi: 10.1097/01.ico.0000240099.16420.17. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP. IFITM/Mil/fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev Cell. 2005;9:745–756. doi: 10.1016/j.devcel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Stern ME, Siemasko KF, Gao J, Calonge M, Niederkorn JY, Pflugfelder SC. Evaluation of ocular surface inflammation in the presence of dry eye and allergic conjunctival disease. Ocul Surf. 2005;3:S161–164. doi: 10.1016/s1542-0124(12)70246-x. [DOI] [PubMed] [Google Scholar]
- 39.De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]


