Abstract
All type II topoisomerases require divalent metal ions in order to cleave and ligate DNA. In order to further elucidate the mechanistic basis for these critical enzyme-mediated events, the role of the metal ion in the DNA cleavage reaction of human topoisomerase IIβ was characterized and compared to that of topoisomerase IIα. The present study utilized divalent metal ions with varying thiophilicities in conjunction with DNA cleavage substrates that substituted a sulfur atom for the 3′-bridging oxygen or the non-bridging oxygens of the scissile phosphate. Based on time courses of DNA cleavage, cation titrations, and metal ion mixing experiments, we propose the following model for the use of divalent metal ions by human type II topoisomerases. First, both enzymes employ a two-metal-ion mechanism to support DNA cleavage. Second, an interaction between one divalent metal ion and the 3′-bridging atom of the scissile phosphate greatly enhances enzyme-mediated DNA cleavage, most likely by stabilizing the leaving 3′-oxygen. Third, there is an important interaction between a divalent second metal ion and a non-bridging atom of the scissile phosphate that stimulates DNA cleavage mediated by topoisomerase IIβ. If this interaction exists in topoisomerase IIα, its effects on DNA cleavage are equivocal. This last aspect of the model highlights a difference in metal ion utilization during DNA cleavage mediated by human topoisomerase IIα and IIβ.
A number of essential nuclear processes, such as DNA recombination and replication, generate knots and tangles in the genetic material (1–3). DNA knots make it impossible to separate the two strands of the double helix, and tangled chromosomes cannot be segregated during mitosis or meiosis (2–5). Consequently, these detrimental DNA linkages must be resolved to maintain chromosomal integrity. The enzymes that remove knots and tangles from the genome are type II DNA topoisomerases (2, 4, 6–10).
Type II topoisomerases act by passing an intact double helix through a transient double-stranded break that they generate in a separate segment of DNA (4, 7, 11–13). Humans encode two isoforms of the enzyme, topoisomerase IIα and IIβ (4, 7, 9, 10, 13). These isoforms differ in their protomer molecular masses (170 vs. 180 kDa, respectively) and are encoded by separate genes (2, 4, 9, 10). The physiology of topoisomerase IIα and IIβ differs greatly. Topoisomerase IIα is essential for the survival of proliferating cells (14). Enzyme levels are low in quiescent cells and increase dramatically during periods of growth (15–17). Furthermore, topoisomerase IIα is regulated over the cell cycle, with protein concentrations peaking in G2/M (17–19). In contrast, topoisomerase IIβ is dispensable at the cellular level, and its expression is independent of proliferative status and cell cycle (20, 21). Finally, while topoisomerase IIα is associated with replication forks and remains tightly bound to chromosomes during mitosis (6, 14, 22–24), the β isoform dissociates from chromosomes during mitosis (9, 22, 25).
Despite the distinct expression patterns and cellular roles of the two isoforms, topoisomerase IIα and IIβ display a high degree (~70%) of amino acid sequence identity and often show similar enzymological characteristics (2, 9, 10). However, numerous studies of enzyme-mediated DNA cleavage and ligation suggest that there are significant (but subtle) differences in the active sites of topoisomerase IIα and IIβ.
First, the DNA cleavage specificities of the two isoforms are similar, but not identical (26–28). Second, some anticancer drugs that enhance topoisomerase II-mediated DNA cleavage display preferential effects on one isoform or the other (28–30). Third, covalent topoisomerase IIα-cleaved DNA complexes (i.e., cleavage complexes) that are formed during scission generally persist longer than equivalent complexes with topoisomerase IIβ (29, 31). Fourth, topoisomerase IIβ is more sensitive than topoisomerase IIα to alterations in the scissile bond of DNA. For example, topoisomerase IIβ is less able to cleave a DNA substrate that contains a 3′-bridging sulfur atom (i.e., phosphorothiolate) in place of the normal oxygen atom (32). It also ligates a nick that has been activated by the addition of a 5′-pnitrophenyl group more slowly (33).
All type II topoisomerases require a divalent metal ion in order to cleave and ligate DNA. Recently, it was shown that human topoisomerase IIα utilized a two-metal-ion mechanism similar to that used by primases, some DNA polymerases, and bacterial DNA gyrase (34–38). Given the differences in the active sites of topoisomerase IIα and IIβ, we wanted to determine whether the two isoforms used divalent metal ions in a similar manner. Results indicate that topoisomerase IIβ also utilizes a two-metal-ion mechanism for DNA cleavage. Furthermore, they provide the first kinetic evidence for an important interaction between a divalent metal ion and the non-bridging atom of the scissile phosphate in the DNA cleavage reaction of type II topoisomerases.
EXPERIMENTAL PROCEDURES
Enzymes
Human topoisomerase IIα and IIβ were expressed in Saccharomyces cerevisiae and purified as described previously (39–41).
Preparation of Oligonucleotides
A 50 bp duplex oligonucleotide was designed using a previously identified topoisomerase II cleavage site from pBR322 (42). Wild-type oligonucleotide sequences were generated using an Applied Biosystems DNA synthesizer. The 50-mer top and bottom sequences were 5′-TTGGTATCTGCGCTCTGCTGAAGCC↓AGTTACCTTCGGAAAAAGAGTTGGT-3′ and 5′-ACCAACTCTTTTTCCGAAGGT↓AACTGGCTTCAGCAGAGCGCAGATACCAA-3′, respectively. The arrow denotes the point of scission by topoisomerase II. The top strand was composed of two shorter sequences that produced a nick at the location of the scissile bond.
DNA containing a single 3′-bridging phosphorothiolate linkage was synthesized as described previously (32). The location of the phosphorothiolate was at the normal scissile bond on the bottom strand. Substrates containing a racemic phosphorothioate in place of the non-bridging scissile bond oxygens of the bottom strand were synthesized by Operon.
Radioactive Labeling of Oligonucleotides
[γ-32P]ATP (~5000 Ci/mmol) was obtained from ICN. Single-stranded oligonucleotides were labeled on their 5′-termini using T4 polynucleotide kinase (New England Biolabs). Following labeling and gel purification, complementary oligonucleotides were annealed by incubation at 70 °C for 10 min and cooling to 25 °C.
DNA Cleavage
DNA cleavage assays were carried out by the procedure of Deweese et al. (32). Unless stated otherwise, oligonucleotide substrates were always 5′-end-labeled. DNA cleavage reactions with human topoisomerase IIα or IIβ contained 200 nM enzyme and 100 nM double-stranded oligonucleotide in a total of 20 μL of 10 mM Tris-HCl, pH 7.9, 135 mM KCl, 0.1 mM EDTA, and 2.5% glycerol. Unless otherwise noted, the concentration of the divalent cation was 5 mM. In some cases, the concentration of divalent cation (MgCl2, MnCl2, or CaCl2) was varied and/or combinations of the cations were used. Experiments that monitored DNA cleavage over a range that included divalent cation concentrations below 1 mM utilized cleavage buffer that lacked EDTA. Reactions were initiated by the addition of enzyme and were incubated for 0 to 30 min at 37 °C. DNA cleavage products were trapped by the addition of 2 μL of 10% SDS followed by 2 μL of 250 mM NaEDTA, pH 8.0. Proteinase K (2 μL of 0.8 mg/mL) was added to digest the enzyme. Cleavage products were resolved by electrophoresis in a 14% denaturing polyacrylamide gel. To inhibit oxidation of cleaved oligonucleotides containing 3′-terminal –SH moieties and the formation of multimers in the gel, 100 mM DTT was added to the sample loading buffer. DNA cleavage products were visualized and quantified using a Bio-Rad Molecular Imager.
Pre-equilibrium DNA cleavage reactions were monitored for 0.5 s to 3 s using a KinTek (Austin, TX) model RQF-3 chemical quench flow apparatus. Cleavage was initiated by rapidly mixing equal volumes of two independent solutions. The first contained a noncovalent complex formed between human topoisomerase IIβ and 32P-labeled oligonucleotide in cleavage buffer that lacked divalent cation. The second solution contained cleavage buffer in which the divalent cation concentration was 2 times higher than normal (10 mM). The two solutions were mixed at 37 °C, and DNA cleavage was quenched with 1% SDS (v/v final concentration). Products were processed and analyzed as described above.
RESULTS AND DISCUSSION
Interactions Between Divalent Metal Ions and Scissile Phosphate Atoms During DNA Cleavage Mediated by Topoisomerase IIβ
As a first step towards defining the requirement for a divalent metal ion in the DNA cleavage reaction of human topoisomerase IIβ, interactions between the cation and the scissile phosphate atoms were assessed. All DNA cleavage substrates utilized in the present study had the same sequence. However, experiments took advantage of three alterations in the scissile bonds of these oligonucleotides. First, all substrates contained a nick at the scissile bond on the strand that was not being monitored for cleavage. The presence of this nick on the opposite strand greatly enhances the sensitivity of the DNA cleavage reaction, stimulating both rates and levels of scission ~10–fold (32).
Second, a substrate was employed that substituted a sulfur for the 3′-bridging oxygen atom at the scissile phosphate (i.e., S–P scissile bond) (32). Topoisomerase IIβ cleaved these phosphorothiolate-containing oligonucleotides with all of the characteristics of wild-type substrates, with the following exception: since the resulting 3′-terminal –SH moiety is a poor nucleophile at phosphorous (32, 43, 44), the 3′-bridging phosphorothiolate did not support ligation (data not shown). As a result, S–P substrates isolate the forward DNA scission event from ligation, allowing high levels of cleavage complexes to accumulate (32). This is in contrast to wild-type oligonucleotides with 3′-bridging oxygen atoms (i.e., O–P scissile bond), which establish a rapid DNA cleavage-ligation equilibrium and maintain low levels of cleavage complexes (32).
Third, a substrate was utilized that substituted a sulfur atom for the non-bridging oxygen at the scissile phosphate. This phosphorothioate substrate was a racemic mixture that replaced either the RP or SP non-bridging oxygen atom with a sulfur atom. In contrast to the 3′-bridging phosphorothiolate oligonucleotide, the non-bridging phosphorothioate substrate maintained a DNA cleavage-ligation equilibrium and cleavage complexes did not accumulate over time (32).
Interactions between divalent cations and the scissile phosphate atoms were determined by comparing the ability of topoisomerase IIβ to cleave DNA substrates containing an oxygen atom or a sulfur atom at the 3′-bridging or non-bridging position in the presence of metal ions of varying “hardness” (i.e., thiophilicity) (38). The ions used for this study were Ca2+, Mg2+, and Mn2+. Within this series, Mn2+ is the “softest,” or most thiophilic metal, and Mg2+ and Ca2+ are harder, or less thiophilic (45–47). Soft metal ions often prefer sulfur over oxygen as an inner-sphere ligand, while hard metals usually coordinate more readily with oxygen (35, 45–50). If there is a direct interaction between the metal ion and a scissile phosphate atom that facilitates catalysis, relative rates (or levels) of scission with substrates containing a sulfur atom in place of the oxygen should increase in the presence of soft (thiophilic) metals (35, 38, 48–50). Conversely, less cleavage should be generated in reactions that contain hard metals (35, 38, 48–50).
The first set of experiments established the ability of divalent metal ions to support cleavage of a wild-type O–P substrate by topoisomerase IIβ. Consistent with previous work with topoisomerase IIα, Mn2+ and Ca2+ generated higher levels of DNA cleavage than Mg2+ (Figure 1, top left panel). Levels of cleavage complexes formed in the presence of Mn2+ or Ca2+ were ~5 or 2 times higher, respectively than those observed with Mg2+. Despite the low equilibrium levels of cleavage seen with Mg2+, initial rates of scission (determined by rapid chemical quench over the first seconds of the cleavage reaction) with the divalent cation were comparable to those generated in the presence of Mn2+ (top right panel).
Figure 1.
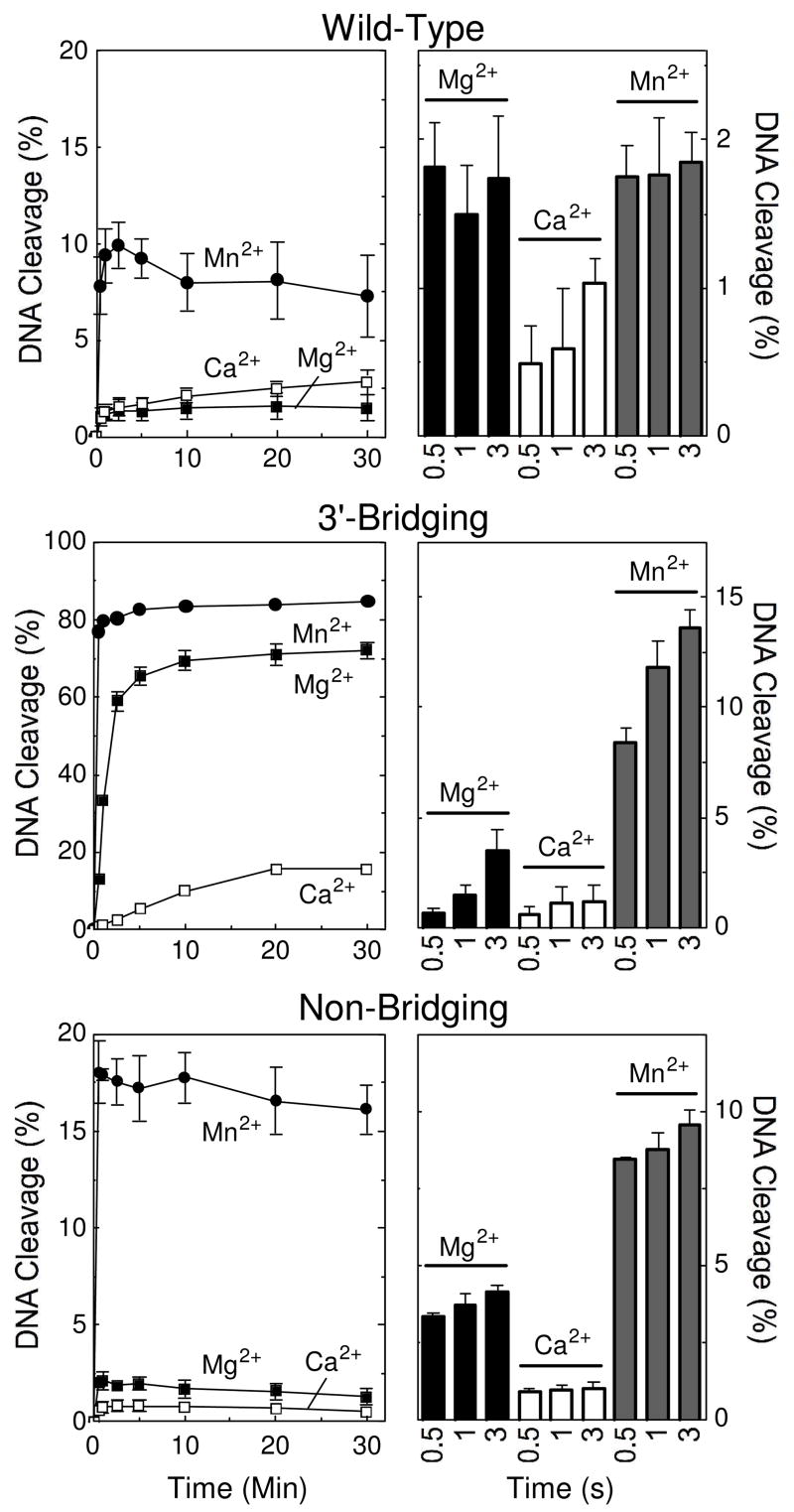
Cleavage of nicked oligonucleotide substrates by topoisomerase IIβ in the presence of different divalent metal ions. The nick is located at the scissile bond on the unlabeled strand. Thirty-minute time courses (left panels) and rapid quench time points (right panels) for the wild-type (top), 3′-bridging phosphorothiolate (middle), and non-bridging phosphorothioate (bottom) are shown. DNA cleavage was carried out in the presence of 5 mM Mg2+ (closed square), Ca2+ (open square), and Mn2+ (closed circle). Rapid quench results for 0.5, 1, and 3 seconds are shown in the presence of Mg2+ (black bars), Ca2+ (open bars), and Mn2+ (gray bars). Error bars represent the standard deviation of at least three independent experiments.
A previous study demonstrated that there is an important interaction between the metal ion and the 3′-bridging atom of the scissile bond in the active site of human topoisomerase IIα that significantly enhances the ability of the enzyme to cleave DNA (38). Therefore, a second set of experiments examined the divalent cation preference for cleavage of the 3′-bridging S–P (phosphorothiolate) oligonucleotide by topoisomerase IIβ (Figure 1, middle panels). Levels and rates of cleavage observed in the presence of the thiophilic metal ion, Mn2+, were considerably higher than those generated with the hard metal, Ca2+. Both the levels and rate of cleavage for reactions that contained Ca2+ fell significantly below those with Mg2+. The drop in Ca2+-supported scission of the S–P, as compared to the O–P DNA substrate, together with the relative rise in cleavage levels and rates with Mn2+, strongly suggest that the divalent cation contacts the 3′-bridging atom of the scissile bond in the active site of human topoisomerase IIβ. Furthermore, like topoisomerase IIα, this metal ion-DNA interaction mediated by topoisomerase IIβ stimulates rates of enzyme-mediated scission.
Models proposed for the active site geometry of Escherichia coli DNA gyrase (36) and human topoisomerase IIα (38) both postulate interactions between divalent metal ions and a non-bridging oxygen atom of the scissile phosphate. However, no direct evidence supporting this interaction has been reported. To this point, replacement of non-bridging oxygen atoms with sulfur had little effect on levels of DNA scission generated by either enzyme (38, 51). Thus, it was concluded that if these postulated interactions between metal ions and non-bridging oxygen atoms exist, their effects on DNA cleavage mediated by these enzymes were equivocal.
Based on mutagenesis studies, it has been proposed that topoisomerase IIβ utilizes divalent cations in a manner similar to that of DNA gyrase (52, 53). Nonetheless, a third set of experiments was carried out to determine whether interactions could be observed between the metal ion and a non-bridging atom of the scissile phosphate in the active site of human topoisomerase IIβ.
In marked contrast to the findings with DNA gyrase and topoisomerase IIα (38, 51), dramatic differences were seen with a substrate that contained a sulfur atom in the non-bridging position (Figure 1, bottom panels). Levels of cleavage generated by topoisomerase IIβ in the presence of Mn2+ were ~8–fold higher than those seen with Mg2+, and rates of scission were 2 to 3 times faster. Furthermore, levels and rates of cleavage for reactions that contained Ca2+ dropped below those observed with Mg2+. Finally, as compared to results with the wild-type substrate (top panel), substitution of sulfur at the non-bridging position increased cleavage ~2–fold in the presence of Mn2+ and decreased it ~3–fold in the presence of Ca2+. These results provide the first evidence for an interaction between the divalent metal ion and a non-bridging atom of the scissile phosphate for any type II topoisomerase and suggest that this interaction enhances the ability of topoisomerase IIβ to cleave DNA.
A Two-Metal-Ion Mechanism for DNA Cleavage Mediated by Human Topoisomerase IIβ
On the basis of metal ion mixing experiments, DNA gyrase and topoisomerase IIα were demonstrated to use a two-metal-ion mechanism for DNA cleavage (36, 38). Two approaches were utilized to determine whether human topoisomerase IIβ uses a similar mechanism to mediate DNA scission. In the first, DNA cleavage was monitored over a range of divalent cation concentrations. These experiments utilized Mn2+ (a soft cation) and Ca2+ (a hard cation). As seen in Figure 2, the concentration of Mn2+ that was required to promote DNA scission mediated by topoisomerase IIβ was considerably lower than that seen with Ca2+.
Figure 2.
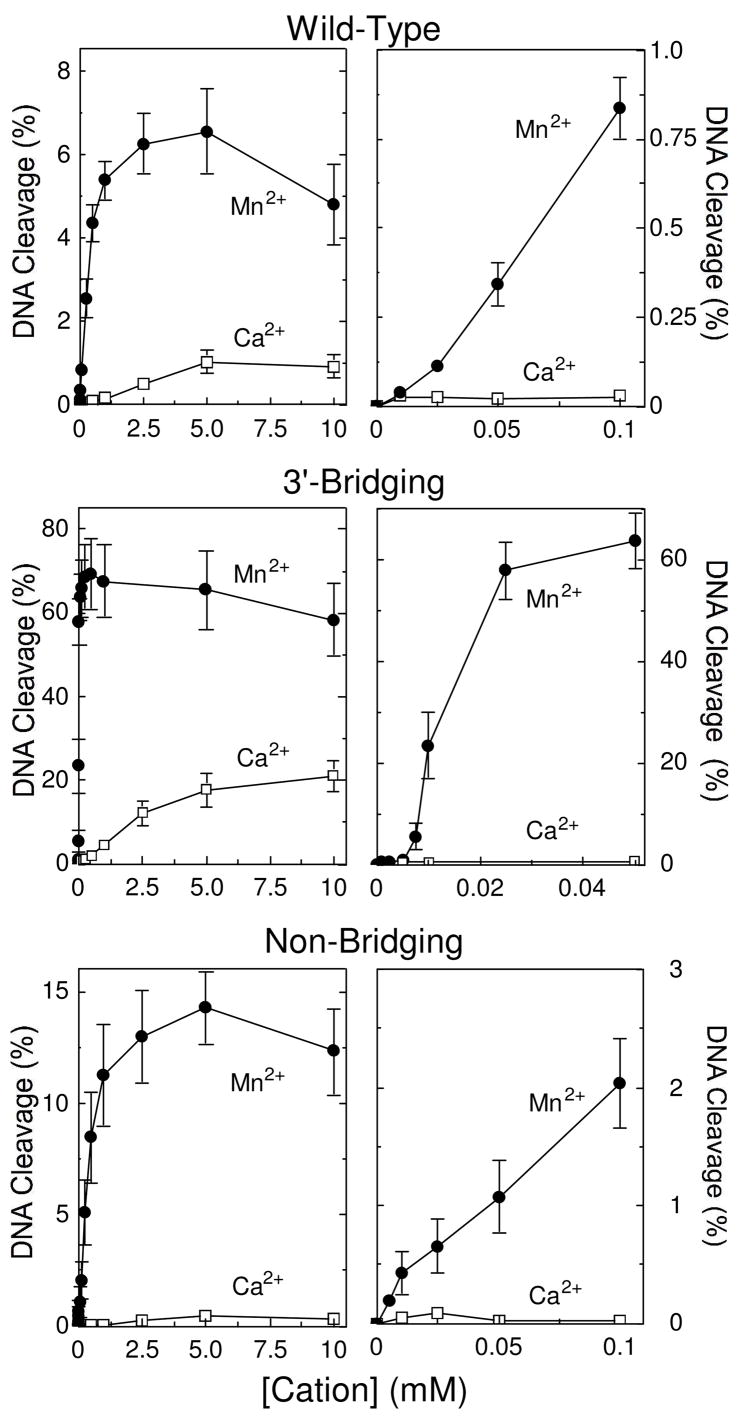
Metal ion concentration dependence for DNA cleavage of nicked wild-type, phosphorothiolate, and phosphorothioate substrates by topoisomerase IIβ. Results for the wild-type (top), 3′-bridging phosphorothiolate (middle), and non-bridging phosphorothioate (bottom) are shown. Left panels, Mn2+ (closed circles) or Ca2+ (open squares) were titrated from 10 μM to 10 mM for wild-type, 1 μM to 10 mM for 3′-bridging substrates, or 5 μM to 10 mM for non-bridging. Right panels, expanded DNA cleavage at concentrations up to 100 μM for wild-type and non-bridging substrates or 50 μM for the 3′-bridging substrate are shown. All reactions were incubated for 30 min prior to stopping the reaction. Error bars represent the standard error of the mean of two independent experiments or the standard deviation of at least three independent experiments.
When cleavage of the wild-type O–P oligonucleotide was monitored (Figure 2, top panels), both divalent cations displayed a mild biphasic concentration dependence. This finding suggests that topoisomerase IIβ utilizes more than one divalent metal ion to mediate DNA cleavage.
Introduction of a 3′-bridging phosphorothiolate had a significant effect on cation titrations, and resulted in a dramatically more pronounced initial phase with Mn2+ (Figure 2, middle panels). This biphasic metal ion concentration dependence suggests that there are two divalent cation sites in the DNA cleavage-ligation domain of topoisomerase IIβ and that both need to be filled in order to support scission. The finding that the initial (i.e., high affinity) phase with the 3′-bridging phosphorothiolate substrate became more prominent with the soft metal ion (Mn2+) implies that the first site that is filled by the divalent cation is the one that interacts with the bridging atom of the scissile bond.
Introduction of a sulfur at the non-bridging position resulted in an ~2–fold increase in levels of DNA cleavage in the presence of Mn2+ and an ~2–fold decrease in the presence of Ca2+ (Figure 2, bottom panels) as compared to the wild-type substrate. Although this finding provides further evidence for an important interaction between a metal ion and the non-bridging atom of the scissile phosphate, no prominent initial phase was observed.
Therefore, a second approach was utilized to confirm that two metal ions are required for DNA cleavage mediated by human topoisomerase IIβ. These studies took advantage of the enhanced scission observed for substrates that contained a sulfur atom in either the 3′-bridging or the non-bridging positions in the presence of Mn2+. Experiments compared levels of DNA cleavage monitored in the presence of Ca2+, Mn2+, or a combination of the two divalent cations. Near saturating concentrations of Ca2+ (5 mM) were paired with sub-saturating concentrations of Mn2+ (up to 100 μM depending on the cleavage substrate). These limiting concentrations of Mn2+ supported levels of enzyme-mediated DNA scission that were <15% of the observed maxima.
Figures 3–5 display results for topoisomerase IIβ-mediated DNA cleavage of the wild-type, 3′-bridging phosphorothiolate, or non-bridging phosphorothioate substrate, respectively. Results of parallel experiments that employed topoisomerase IIα are shown for comparison and are discussed following presentation of all of the data for topoisomerase IIβ.
Figure 3.
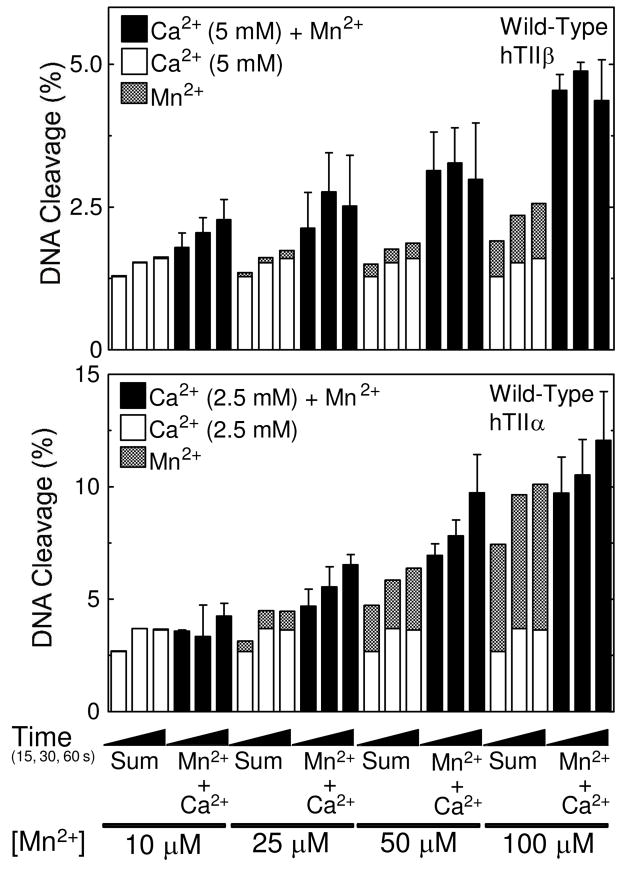
Cleavage of nicked wild-type oligonucleotide substrates by topoisomerase IIβ or IIα in the presence of divalent metal ion combinations. DNA cleavage reactions with topoisomerase IIβ (top) or IIα (bottom) were carried out for 15, 30 or 60 s in the presence of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ alone (open bars), 10–100 μM Mn2+ alone (stippled bars), or a mixture of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ and 10–100 μM Mn2+ (Ca2+ + Mn2+, closed bars). The calculated sum of the enzyme-mediated DNA cleavage from reactions containing either Ca2+ or Mn2+ alone is also shown (Sum, stacked open and stippled bars, respectively). All data represent the average of at least three independent experiments. Error bars for reactions carried out in the presence of Ca2+ + Mn2+ are shown. Error bars for reactions carried out in the presence of Ca2+ or Mn2+ alone are not shown for simplicity.
Figure 5.
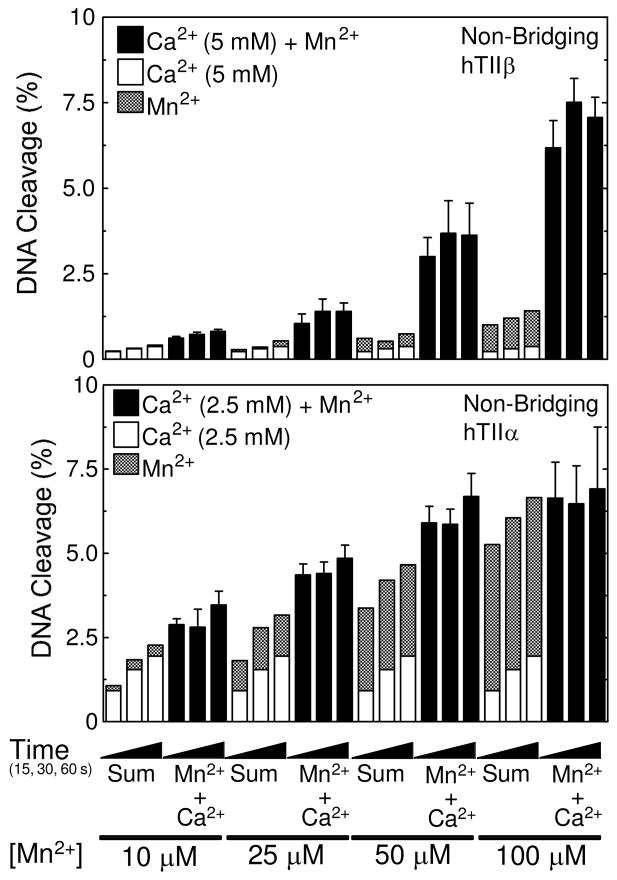
Cleavage of nicked non-bridging phosphorothioate oligonucleotide substrates by topoisomerase IIβ or IIα in the presence of divalent metal ion combinations. DNA cleavage reactions with topoisomerase IIβ (top) or IIα (bottom) were carried out for 15, 30 or 60 s in the presence of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ alone (open bars), 10–100 μM Mn2+ alone (stippled bars), or a mixture of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ and 10–100 μM Mn2+ (Ca2+ + Mn2+, closed bars). The calculated sum of the enzyme-mediated DNA cleavage from reactions containing either Ca2+ or Mn2+ alone is also shown (Sum, stacked open and stippled bars, respectively). All data represent the average of at least three independent experiments. Error bars for reactions carried out in the presence of Ca2+ + Mn2+ are shown. Error bars for reactions carried out in the presence of Ca2+ or Mn2+ alone are not shown for simplicity.
Combining metal ions in reactions that utilized topoisomerase IIβ and a wild-type substrate had a small but discernable effect (Figure 3, top panel). Levels of DNA cleavage in reactions that contained both divalent cations were slightly higher (~1.5– to 2–fold) than predicted by summing the amount of cleavage generated in reactions that contained either Mn2+ or Ca2+. This result supports a two-metal-ion mechanism for DNA cleavage mediated by the β isoform and indicates that minor differences between the two cation sites can be discerned even in the presence of an unmodified substrate.
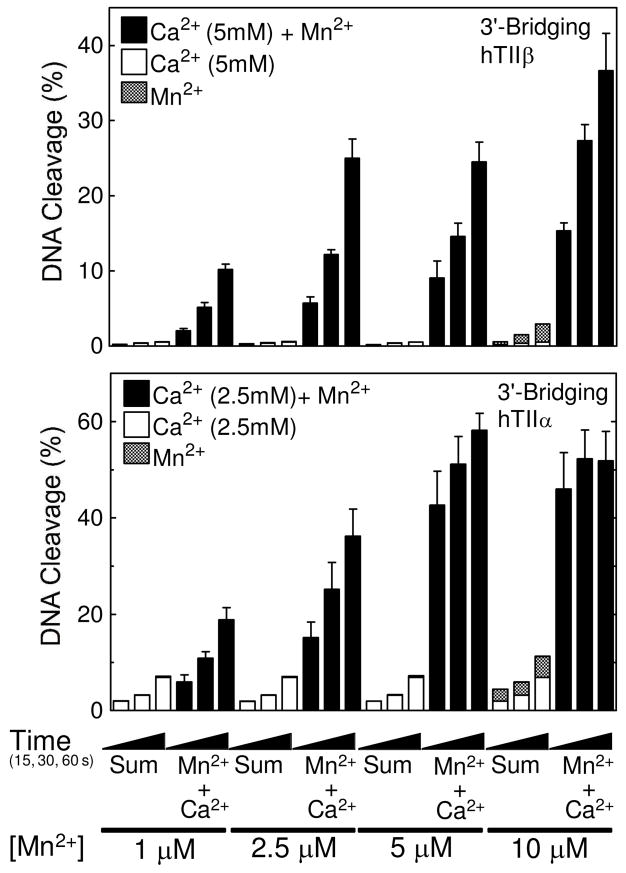
In contrast to the above results, a dramatic difference was seen in experiments that utilized the 3′-bridging S–P substrate (Figure 4, top panel). Levels of DNA scission in reactions that contained both Ca2+ and Mn2+ were ≥10 times greater than the calculated levels derived from the sum of cleavage observed in the presence of the individual metal ions. Furthermore, a large enhancement in the rate of DNA scission (compared to calculated sums) was observed when Ca2+ and Mn2+ were combined. It should be noted that these experiments used Mn2+ concentrations (1–10 μM) that partially filled or saturated the high affinity scissile bond site (see Figure 2, middle panel) without appreciably filling the second site. These data confirm the two-metal-ion mechanism as well as the importance of the metal ion interaction with the 3′-bridging atom of the scissile phosphate.
Figure 4.
Cleavage of nicked 3′-bridging phosphorothiolate oligonucleotide substrates by topoisomerase IIβ or IIα in the presence of divalent metal ion combinations. DNA cleavage reactions with topoisomerase IIβ (top) or IIα (bottom) were carried out for 15, 30 or 60 s in the presence of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ alone (open bars), 1–10 μM Mn2+ alone (stippled bars), or a mixture of 5 mM (IIβ) or 2.5 mM (IIα) Ca2+ and 1–10 μM Mn2+ (Ca2+ + Mn2+, closed bars). The calculated sum of the enzyme-mediated DNA cleavage from reactions containing either Ca2+ or Mn2+ alone is also shown (Sum, stacked open and stippled bars, respectively). Data for topoisomerase IIα are reproduced from ref. (38). All data represent the average of at least three independent experiments. Error bars for reactions carried out in the presence of Ca2+ + Mn2+ are shown. Error bars for reactions carried out in the presence of Ca2+ or Mn2+ alone are not shown for simplicity.
As seen in Figure 5 (top panel), the inclusion of a non-bridging sulfur at the scissile phosphate also stimulated DNA cleavage in reactions that contained a mixture of Ca2+ and Mn2+. Levels of cleavage in reactions that contained both metal ions were ~5 times higher than predicted by the calculated sums. Once again, these data confirm the two-metal-ion mechanism for DNA cleavage mediated by topoisomerase IIβ, and they also validate the significance of the metal ion interaction with the non-bridging atom of the scissile phosphate.
Trends similar to those seen with the β isoform were observed for mixing experiments that employed human topoisomerase IIα and the wild-type (Figure 3, bottom) or 3′-bridging phosphorothiolate substrate (38) (Figure 4, bottom). Experiments were identical to those with topoisomerase IIβ except that 2.5 mM Ca2+ [which is near saturating levels for the α isoform (38)] was used. While differences between predicted and actual results with the wild-type oligonucleotide were less apparent with topoisomerase IIα, there did appear to be a mild enhancement in cleavage when both Ca2+ and Mn2+ were included in reactions. As shown with topoisomerase IIβ, the ability of the α isoform to cleave a substrate with a 3′-bridging sulfur increased dramatically when both metal ions were present (38).
In contrast, a marked difference between the two enzyme isoforms was seen in reactions that utilized the non-bridging substrate. Scission levels generated by topoisomerase IIα in the presence of Ca2+ and Mn2+ were marginally higher compared to those predicted by the calculated sums (Figure 5, bottom) or by those observed in the presence of both divalent cations with the wild-type oligonucleotide (compare with Figure 3, bottom). Similar results were seen when 5 mM Ca2+ was used in reaction mixtures (data not shown). These findings indicate that human topoisomerase IIα and IIβ utilize divalent metal ions differently for some aspects of their DNA cleavage reactions. The interaction between a metal ion and the non-bridging atom of the scissile phosphate is important for the DNA cleavage reaction of topoisomerase IIβ and substantially raises levels of scission. However, if the parallel interaction in topoisomerase IIα exists, it has little effect on DNA scission.
A Model for the Use of Divalent Metal Ions for DNA Cleavage Mediated by Human Type II Topoisomerases
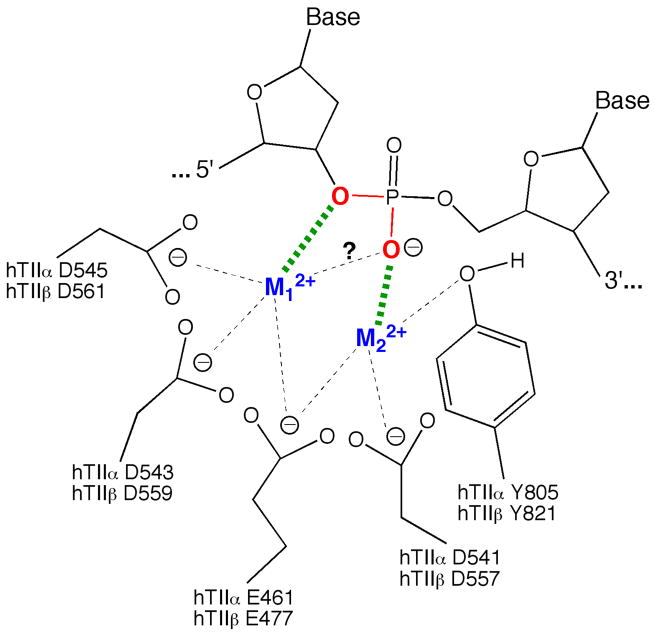
A model for the use of divalent metal ions in the DNA cleavage reaction of human type II topoisomerases is proposed in Figure 6. Amino acids that are postulated to interact with the metal ions in the active site of topoisomerase IIα and IIβ are assigned based on previous enzymological studies of mutated E. coli DNA gyrase and human topoisomerase IIβ proteins (36, 52, 53), as well as structural studies of bacterial topoisomerase III, yeast topoisomerase II, and other DNA enzymes that contain the toprim domain (54–57).
Figure 6.
A two-metal ion model for DNA cleavage by human topoisomerase IIα and IIβ. Details are described in the text. Amino acids that are postulated to interact with the metal ions in the active site of both isoforms are shown. The model postulates that the metal ions bind to topoisomerase II in an ordered fashion, in which the binding of metal ion 1 (M12+, shown in blue) is a prerequisite for the binding of metal ion 2 (M22+, shown in blue). M12+ makes a critical interaction with the 3′-bridging atom (shown in red) of the scissile phosphate (bond shown in green), which most likely is needed to stabilize the leaving 3′-oxygen (left, shown in red). M22+ is required for DNA scission and likely interacts with the non-bridging oxygen (shown in red) during scission. This divalent cation may stabilize the DNA transition state and/or help deprotonate the active site tyrosine. While interactions between M22+ and the non-bridging atom of the scissile phosphate significantly enhance DNA cleavage mediated by topoisomerase IIβ, effects on DNA scission mediated by topoisomerase IIα are equivocal. The model was adapted from Ref. (36).
Our model has several features. First, topoisomerase IIα and IIβ both employ a two-metal-ion mechanism to support DNA cleavage. Second, both enzyme isoforms utilize an interaction between a divalent metal ion (metal ion 1) and the 3′-bridging atom of the scissile phosphate to accelerate rates of enzyme-mediated DNA cleavage, most likely by stabilizing the leaving 3′-oxygen. At the present time, it is not known whether metal ion 1 also has interactions with the non-bridging oxygen. Third, a second metal ion (metal ion 2) appears to contact a non-bridging atom of the scissile phosphate in the active site of topoisomerase IIβ. This interaction plays a significant role in DNA cleavage mediated by the β isoform and greatly stimulates scission. As proposed previously, this metal ion is believed to stabilize the DNA transition state and/or help deprotonate the active site tyrosine (36, 38, 53). Although topoisomerase IIα has an absolute requirement for metal ion 2, the role of this divalent cation in its DNA cleavage reaction is unclear. Results of the present study cannot rule out an interaction between the metal ion and the non-bridging oxygen in the active site of topoisomerase IIα. However, if the interaction exists, it does not affect rates of DNA cleavage.
In conclusion, topoisomerase IIα and IIβ both employ a two-metal-ion mechanism to support DNA cleavage but appear to utilize one of the two metal ions differently. The present study provides the first evidence for the interaction between a divalent metal ion and the non-bridging atom of the scissile phosphate and a testable model to further investigate the role of metal ions in topoisomerase II-mediated processes.
Acknowledgments
We are grateful to Amanda C. Gentry for critical reading of the manuscript. We thank Dr. F. Peter Guengerich for use of the rapid quench apparatus and helpful discussions regarding rapid quench kinetics and Dr. Robert Eoff for instrument training.
Footnotes
This work was supported by National Institutes of Health research grants GM33944 and GM53960. JED was a trainee under grant T32 CA09592 from the National Institutes of Health. AMB was a participant in the Vanderbilt Summer Science Academy.
References
- 1.Kanaar R, Cozzarelli NR. Roles of supercoiled DNA structure in DNA transactions. Curr Opin Struct Biol. 1992;2:369–379. [Google Scholar]
- 2.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Bates AD, Maxwell A. DNA Topology. Oxford University Press; New York: 2005. [Google Scholar]
- 4.McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat Res. 2007;623:83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falaschi A, Abdurashidova G, Sandoval O, Radulescu S, Biamonti G, Riva S. Molecular and Structural Transactions at Human DNA Replication Origins. Cell Cycle. 2007;6:1705–1712. doi: 10.4161/cc.6.14.4495. [DOI] [PubMed] [Google Scholar]
- 6.Nitiss JL. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 7.Fortune JM, Osheroff N. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog Nucleic Acid Res Mol Biol. 2000;64:221–253. doi: 10.1016/s0079-6603(00)64006-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 9.Austin CA, Marsh KL. Eukaryotic DNA topoisomerase IIβ. BioEssays. 1998;20:215–226. doi: 10.1002/(SICI)1521-1878(199803)20:3<215::AID-BIES5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 10.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 11.Wang JC. Moving one DNA double helix through another by a type II DNA topoisomerase: the story of a simple molecular machine. Quart Rev Biophys. 1998;31:107–144. doi: 10.1017/s0033583598003424. [DOI] [PubMed] [Google Scholar]
- 12.Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 13.Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkn937. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grue P, Grasser A, Sehested M, Jensen PB, Uhse A, Straub T, Ness W, Boege F. Essential mitotic functions of DNA topoisomerase IIα are not adopted by topoisomerase IIβ in human H69 cells. J Biol Chem. 1998;273:33660–33666. doi: 10.1074/jbc.273.50.33660. [DOI] [PubMed] [Google Scholar]
- 15.Heck MM, Earnshaw WC. Topoisomerase II: A specific marker for cell proliferation. J Cell Biol. 1986;103:2569–2581. doi: 10.1083/jcb.103.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiang YH, Wu HY, Liu LF. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988;48:3230–3235. [PubMed] [Google Scholar]
- 17.Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991;2:209–214. [PubMed] [Google Scholar]
- 18.Heck MM, Hittelman WN, Earnshaw WC. Differential expression of DNA topoisomerases I and II during the eukaryotic cell cycle. Proc Natl Acad Sci USA. 1988;85:1086–1090. doi: 10.1073/pnas.85.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura K, Saijo M, Ui M, Enomoto T. Growth state- and cell cycle-dependent fluctuation in the expression of two forms of DNA topoisomerase II and possible specific modification of the higher molecular weight form in the M phase. J Biol Chem. 1994;269:1173–1176. [PubMed] [Google Scholar]
- 20.Lyu YL, Wang JC. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc Natl Acad Sci USA. 2003;100:7123–7128. doi: 10.1073/pnas.1232376100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyu YL, Lin CP, Azarova AM, Cai L, Wang JC, Liu LF. Role of topoisomerase IIβ in the expression of developmentally regulated genes. Mol Cell Biol. 2006;26:7929–7941. doi: 10.1128/MCB.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linka RM, Porter AC, Volkov A, Mielke C, Boege F, Christensen MO. C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 2007;35:3810–3822. doi: 10.1093/nar/gkm102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauman ME, Holden JA, Brown KA, Harker WG, Perkins SL. Differential immunohistochemical staining for DNA topoisomerase IIα and β in human tissues and for DNA topoisomerase IIβ in non-Hodgkin’s lymphomas. Mod Pathol. 1997;10:168–175. [PubMed] [Google Scholar]
- 24.Christensen MO, Larsen MK, Barthelmes HU, Hock R, Andersen CL, Kjeldsen E, Knudsen BR, Westergaard O, Boege F, Mielke C. Dynamics of human DNA topoisomerases IIα and IIβ in living cells. J Cell Biol. 2002;157:31–44. doi: 10.1083/jcb.200112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs RJ, Davies SL, Sandri MI, Redwood C, Wells NJ, Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 26.Cornarotti M, Tinelli S, Willmore E, Zunino F, Fisher LM, Austin CA, Capranico G. Drug Sensitivity and Sequence Specificity of Human Recombinant DNA Topoisomerases IIα (p170) and IIβ (p180) Mol Pharmacol. 1996;50:1463–1471. [PubMed] [Google Scholar]
- 27.Capranico G, Binaschi M. DNA sequence selectivity of topoisomerases and topoisomerase poisons. Biochim Biophys Acta. 1998;1400:185–194. doi: 10.1016/s0167-4781(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 28.Bandele OJ, Osheroff N. Bioflavonoids as poisons of human topoisomerase IIα and IIβ. Biochemistry. 2007;46:6097–6108. doi: 10.1021/bi7000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandele OJ, Osheroff N. The efficacy of topoisomerase II-targeted anticancer agents reflects the persistence of drug-induced cleavage complexes in cells. Biochemistry. 2008;47:11900–11908. doi: 10.1021/bi800981j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toyoda E, Kagaya S, Cowell IG, Kurosawa A, Kamoshita K, Nishikawa K, Iiizumi S, Koyama H, Austin CA, Adachi N. NK314, a topoisomerase II inhibitor that specifically targets the alpha isoform. J Biol Chem. 2008;283:23711–23720. doi: 10.1074/jbc.M803936200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willmore E, Frank AJ, Padget K, Tilby MJ, Austin CA. Etoposide targets topoisomerase IIα and IIβ in leukemic cells: isoform-specific cleavable complexes visualized and quantified in situ by a novel immunofluorescence technique. Mol Pharm. 1998;54:78–85. doi: 10.1124/mol.54.1.78. [DOI] [PubMed] [Google Scholar]
- 32.Deweese JE, Burgin AB, Osheroff N. Using 3′-bridging phosphorothiolates to isolate the forward DNA cleavage reaction of human topoisomerase IIa. Biochemistry. 2008;47:4129–4140. doi: 10.1021/bi702194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromberg KD, Velez-Cruz R, Burgin AB, Osheroff N. DNA ligation catalyzed by human topoisomerase IIα. Biochemistry. 2004;43:13416–13423. doi: 10.1021/bi049420h. [DOI] [PubMed] [Google Scholar]
- 34.Beese LS, Friedman JM, Steitz TA. Crystal structures of the Klenow fragment of DNA polymerase I complexed with deoxynucleoside triphosphate and pyrophosphate. Biochemistry. 1993;32:14095–14101. doi: 10.1021/bi00214a004. [DOI] [PubMed] [Google Scholar]
- 35.Curley JF, Joyce CM, Piccirilli JA. Functional evidence that the 3′-5′ exonuclease domain of Escheria coli DNA polymerase I employs a divalent metal ion in leaving group stabilization. J Am Chem Soc. 1997;119:12691–12692. [Google Scholar]
- 36.Noble CG, Maxwell A. The role of GyrB in the DNA cleavage-religation reaction of DNA gyrase: a proposed two metal-ion mechanism. J Mol Biol. 2002;318:361–371. doi: 10.1016/S0022-2836(02)00049-9. [DOI] [PubMed] [Google Scholar]
- 37.Kato M, Ito T, Wagner G, Richardson CC, Ellenberger T. Modular architecture of the bacteriophage T7 primase couples RNA primer synthesis to DNA synthesis. Mol Cell. 2003;11:1349–1360. doi: 10.1016/s1097-2765(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 38.Deweese JE, Burgin AB, Osheroff N. Human topoisomerase IIα uses a two-metal-ion mechanism for DNA cleavage. Nucleic Acids Res. 2008;36:4883–4893. doi: 10.1093/nar/gkn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worland ST, Wang JC. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989;264:4412–4416. [PubMed] [Google Scholar]
- 40.Elsea SH, Hsiung Y, Nitiss JL, Osheroff N. A yeast type II topoisomerase selected for resistance to quinolones. Mutation of histidine 1012 to tyrosine confers resistance to nonintercalative drugs but hypersensitivity to ellipticine. J Biol Chem. 1995;270:1913–1920. doi: 10.1074/jbc.270.4.1913. [DOI] [PubMed] [Google Scholar]
- 41.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 42.Fortune JM, Dickey JS, Lavrukhin OV, Van Etten JL, Lloyd RS, Osheroff N. Site-specific DNA cleavage by Chlorella virus topoisomerase II. Biochemistry. 2002;41:11761–11769. doi: 10.1021/bi025802g. [DOI] [PubMed] [Google Scholar]
- 43.Burgin AB, Jr, Huizenga BN, Nash HA. A novel suicide substrate for DNA topoisomerases and site-specific recombinases. Nucleic Acids Res. 1995;23:2973–2979. doi: 10.1093/nar/23.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henningfeld KA, Arsian T, Hecht SM. Alteration of DNA primary structure by DNA topoisomerase I. Isolation of the covalent topoisomerase I-DNA binary complex in enzymatically competent form. J Am Chem Soc. 1996;118:11701–11714. [Google Scholar]
- 45.Pearson RG. Acids and Bases. Science. 1966;151:172–177. doi: 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- 46.Pecoraro VL, Hermes JD, Cleland WW. Stability constants of Mg2+ and Cd2+ complexes of adenine nucleotides and thionucleotides and rate constants for formation and dissociation of MgATP and MgADP. Biochemistry. 1984;23:5262–5271. doi: 10.1021/bi00317a026. [DOI] [PubMed] [Google Scholar]
- 47.Sigel RKO, Song B, Sigel H. Stabilities and structures of metal ion complexes of adenosine 5′-O-thiomonophosphate (AMPS2−) in comparison with those of its parent nucleotide (AMP2−) in aqueous solution. J Am Chem Soc. 1997;119:744–755. [Google Scholar]
- 48.Basu S, Strobel SA. Thiophilic metal ion rescue of phosphorothioate interference within the Tetrahymena ribozyme P4–P6 domain. RNA. 1999;5:1399–1407. doi: 10.1017/s135583829999115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sontheimer EJ, Gordon PM, Piccirilli JA. Metal ion catalysis during group II intron self-splicing: parallels with the spliceosome. Genes Dev. 1999;13:1729–1741. doi: 10.1101/gad.13.13.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szewczak AA, Kosek AB, Piccirilli JA, Strobel SA. Identification of an active site ligand for a group I ribozyme catalytic metal ion. Biochemistry. 2002;41:2516–2525. doi: 10.1021/bi011973u. [DOI] [PubMed] [Google Scholar]
- 51.Dobbs ST, Cullis PM, Maxwell A. The cleavage of DNA at phosphorothioate internucleotidic linkages by DNA gyrase. Nucleic Acids Res. 1992;20:3567–3573. doi: 10.1093/nar/20.14.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West KL, Meczes EL, Thorn R, Turnbull RM, Marshall R, Austin CA. Mutagenesis of E477 or K505 in the B′ domain of human topoisomerase IIβ increases the requirement for magnesium ions during strand passage. Biochemistry. 2000;39:1223–1233. doi: 10.1021/bi991328b. [DOI] [PubMed] [Google Scholar]
- 53.Leontiou C, Lakey JH, Lightowlers R, Turnbull RM, Austin CA. Mutation P732L in human DNA topoisomerase IIβ abolishes DNA cleavage in the presence of calcium and confers drug resistance. Mol Pharmacol. 2006;69:130–139. doi: 10.1124/mol.105.015933. [DOI] [PubMed] [Google Scholar]
- 54.Aravind L, Leipe DD, Koonin EV. Toprim--a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998;26:4205–4213. doi: 10.1093/nar/26.18.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Changela A, DiGate RJ, Mondragon A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature. 2001;411:1077–1081. doi: 10.1038/35082615. [DOI] [PubMed] [Google Scholar]
- 56.Changela A, DiGate RJ, Mondragon A. Structural studies of E. coli topoisomerase III-DNA complexes reveal a novel type IA topoisomerase-DNA conformational intermediate. J Mol Biol. 2007;368:105–118. doi: 10.1016/j.jmb.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong KC, Berger JM. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature. 2007;450:1201–1205. doi: 10.1038/nature06396. [DOI] [PubMed] [Google Scholar]