Abstract
Cell surface coating is a methodology wherein specific molecules are transiently anchored onto cell membrane to modulate cell behavior. Cell surface coating was tested as a method to deliver mesenchymal stem cells (MSC) to endothelial cells via binding to intercellular cell adhesion molecule-1 (ICAM-1). MSCs coated with palmitated protein G (PPG) followed by antibodies to ICAM-1 (AbICAM-1), and incubated on ICAM-I coated coverslips showed a 40-fold increase in cell binding over PPG-only controls. AbICAM-1-coated MSCs incubated with human vascular endothelial cells (HUVECs), with and without exposure to TNFα (to upregulate ICAM-1 expression), showed 2.6-fold increased binding to control HUVECs over PPG-only controls, and a 16-fold increase in binding to TNFα-treated HUVECs. Pretreatment of HUVECs with ICAM-1 antibody promoted the attachment of PPG-only MSCs while reducing the attachment of AbICAM-1-MSCs by approximately 50%. In flow chamber studies on TNFα-stimulated HUVECs, PPG-only, and MSC-only lost 80–90% of their initial binding at 4 dynes/cm2, while AbICAM-1-MSCs maintained 100% binding at 4 dynes/cm2 and 40% binding at 25 dynes/cm2. These results demonstrate that cell surface coating promotes the attachment of MSCs to endothelial cells, and provides a methodology for the delivery of stem cells to sites of inflammation.
Keywords: Cell adhesion, Endothelial cell, Inflammation, Mesenchymal stem cell
Introduction
Advances in stem cell biology and tissue engineering are being applied to the repair of complex tissues with progenitor cells. The precise delivery of repair cells to appropriate sites is an essential component of any progenitor cell-based tissue engineering strategy. The efficiency of local delivery of stem cells, such as satellite cells of muscle [1], can be very low, compromising the utility of some cell-based therapies. An effective systemic delivery of repair cells to specific sites of injury or any target organ or tissue of therapeutic interest could have a high impact on the therapeutic utility of those repair cells. A recent study outlined the use of a cell surface modification strategy to target cells to bone marrow [2]. In this study, the endogenous CD44 found on MSCs was enzymatically modified by converting the native CD44 glycoform to confer potent E-selectin/L-selectin-like binding affinity. While this method proved successful, there remain four major limitations to a more broad application of this technology: (i) finite numbers of glycoconjugate ‘acceptors’ within cells of interest; (ii) difficulty in finding appropriate enzymatic reagents and conditions to produce stereo-specific carbohydrate substitution without compromising cell viability or producing unwanted phenotypic effects; (iii) limited number of identified specific carbohydrate receptors at tissue of interest; and (iv) uncertainty as to the enzymatic specificity. Another cell targeting strategy is the use of an avidin-biotin system [3] wherein the targeting cell is first biotinylated and then coated with an avidin-tagged protein. This method has significant limitations, including its dependence on covalent modifications that could perturb multiple proteins on cell surfaces. Finally, the use of genetic introduction of cell surface proteins, such as CXCR4 receptor, to promote homing has also been tested [4], but has several disadvantages, such as ensuring high transfection levels, unintended alteration of other genes, and potential problems of random integration.
The present study investigates the use of a two-step cell surface antibody coating method to promote the adhesion of MSCs to activated endothelial cells (ECs) as a systemic cell delivery system. The first studies of this type were based on the use of recombinant GPI-modified co-stimulator and MHC protein derivatives, termed “protein paints”, for delivery to cell membranes [5]. The primary disadvantage of the GPI protein transfer strategy is the time, cost and technical difficulties of scaling up production and purification. A more simplified cell surface coating method was developed based on the use of palmitated protein A is inserted into the cell membrane which could then bind antibody [6]. This methodology was then modified and used with a fusion protein containing an Fc region and co-stimulator molecule [7]. Cell surface coating was first used for targeting cells locally to cartilage defects using antibodies to cartilage matrix molecules with the use of palmitated protein G (PPG) [8]. The unique advantage of this targeting technique is that virtually any cell can be coated with either PPG or PPA which can then be used as a docking point for any number of antibodies, or antibody fusion proteins. In addition, cell surface coating methodologies are able to modify poorly transfectable cells such as hematopoietic cells and immune cells [9].
For systemic delivery of stem cells, it is the endothelium of the target organ or tissue that holds the molecular signature, addressins [10], that help determine the specificity of local immune responses through binding to homing receptors, such as VCAM-1 [11], MAdCAM-1 [12] and ICAM-1 [13]. These endothelial addressins direct leukocyte migration to specific organs via their cognate receptors, such as α4β1 integrin (VLA-4) that binds VCAM-1 or fibronectin [14], α4β7 that binds MAdCAM-1 [15] and CD11a/CD18, that bind ICAM-1 [16]. Addressins have also been shown to be upregulated by inflammatory cytokines such as TNFα and IL-1 [17]. Since these adressins are used naturally to direct the migration of leukocytes to endothelium, and ICAM-1 expression is regulated by TNFα, HUVEC cells were used as an in vitro model of endothelial homing, and MSCs were chosen based on their potential use as repair cells or as cells with immunoregulatory properties [18].
In addition to providing a testable model for cell binding, there are practical reasons for choosing ICAM-1 as a target molecule for cell surface coating and targeting. At sites of inflammation, ICAM-1 is up-regulated on endothelial cells where it mediates the adhesion and paracellular migration of leukocytes expressing activated LFA-1 and Mac-1 [19], and is up-regulated in response to TNFα in HUVECs [20]. Another advantage to choosing ICAM-1 as a target molecule is that several additional antibodies against ICAM-1 are being developed as therapeutics and affinity carriers in animal model experiments, and early clinical studies [21].
The role of MSC for tissue regeneration has been well studied [2,22] and include preclinical studies to improve myocardial function after myocardial infarction [4], cerebral function after cerebral infarction [23], and to repair liver [24] and joint damage [25]. However, the amount of homing of reparative MSCs to damaged tissues and organs is fairly low [26]. The goal of this study is to determine if a cell coating method, using a well-defined in vitro model, to direct MSCs to bind more efficiently to inflamed endothelium. To test this, palmitated protein G (PPG) was mixed with MSCs and intercalated into cell membranes by lipid-lipid interaction [27], and, in the second coating step, ICAM-1 antibodies were added to the PPG-coated MSCs such that the antigen binding domain extends outward from the cell surface (Fig. 1a). The targeting of ICAM-1 antibody-coated MSCs was then tested on activated endothelial cells (Fig. 1b) under static and flow conditions.
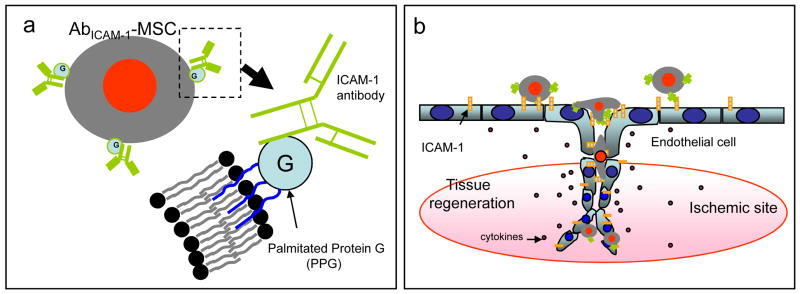
Fig. 1.
Diagrammatic representation of endothelial cell (EC) targeting using antibody-coated mesenchymal stem cells (Ab-MSC) for tissue regeneration. (a) A schematic representation of the membrane structure of AbICAM-1-MSC. (b) Targeting activated ECs expressing ICAM-1 abundantly in ischemic sites with AbICAM-1-MSCs.
Materials and Methods
Cell culture
Mouse mesenchymal stem cell line (mMSC, BMC-9 line) [28] immortalized using SV-40 large T-antigen in this lab were cultured at 33°C, 5% CO2 in low glucose DMEM supplemented with 10% FBS, penicillin and streptomycin. HUVEC (Lonza Group, LTd., Walkerville, MD) were cultured in the EGM BulletKit medium containing bovine brain extract (BBE), hEGF, hydrocortisone, 2% FBS and GA-1000. Fourth through seventh passage HUVEC were used for cell binding experiments.
Cell coating
Recombinant protein G (Pierce, Rockford, IL) was derivatized with N-hydroxysuccinimide ester of palmitic acid (Sigma, St. Louis, MO), as previously described [8] except the palmitation reaction was conducted at 4°C for 1.5 hr instead of at 37°C. The lipid-derivatized protein G was purified on a 10 ml Sephadex G-25 (Pharmacia, Piscataway, NJ) column as described previously [29].
Cell coating was conducted as previously described [8] with minor modifications. In brief, mMSC were incubated with 0.25% trypsin, 0.25 mM EDTA for 5 min at 37°C, 5% CO2, collected, washed with serum free DMEM, and re-suspended at a density of 3.0 × 106 cells/ml in DMEM, incubated in 10 μM Vybrant™ (Molecular Probes, Eugene, OR) in DMEM for 15 min at 37°C in a 5% CO2 incubator, and washed once with fresh DMEM. Vybrant™ staining was verified by fluorescent microscopy. Palmitated protein G (PPG) was added to the cell suspension at 50 μg/ml in DMEM, and the mixture was incubated at 37°C for 10 min with constant gentle mixing, followed by washing with DMEM. PPG-coated cells (PPG-MSCs) were incubated in 100 μg/ml sheep anti-human ICAM-1 antibody (R&D systems, Minneapolis, MN) in DMEM on ice for 1 hr, washed with DMEM, and viability confirmed by trypan blue exclusion. ICAM-1 antibody-coated cells were termed AbICAM-1-MSCs. To examine the effect of PPG concentration on the antibody coating on cell membrane, MSCs were treated with PPG at 0, 10, 20, 50, 100, and 150 μg/ml and then incubated with FITC-human IgG antibody at 100 μg/ml. 10,000 cells of each PPG concentration were analyzed by flow cytometry (Coulter EPICS XL-MCL) with a 488 nm argon ion laser.
In vitro cell adhesion on ICAM-1 molecules
For cell-substrate binding assay, recombinant human ICAM-1 (ADP4; R&D Systems, Inc., Minneapolis, MN) was dissolved in PBS (−), diluted to 10 μg/ml, and 100 μl of ICAM-1 solution added to 48 well microplates and incubated at room temperature for 2 hr. After incubation, the ICAM-1 solution was discarded and unbound ICAM-1 was washed with PBS (−) three times.
Prior to cell seeding, microplate wells were blocked with 1% BSA in PBS (−) at a 37°C incubator for 30 min, and then 0.1 ml of 5 × 105 cells/ml of MSCs only, PPG-MSCs, and AbICAM-1-MSCs were added to the wells, incubated for 30 min in a 37°C in a 5% CO2 incubator, washed with DMEM with 5 times, and visualized with fluorescent microscope. Fluorescent cells were quantified using NIH ImageJ software (particle counting: minimum size (10 pixels), Bins (256)).
Flow cytometry
HUVECs were cultured on 60 mm tissue culture dishes in EGM BulletKit medium containing BBE, hEGF, hydrocortisone, 2% FBS and GA-1000 and incubated with recombinant human TNFα (Peprotech, Inc., Rocky Hill, NJ) with a 10 ng/ml concentration for 4 and 16 hr [30]. The TNFα-treated HUVEC were detached with 2% EDTA in PBS (−) solution, incubated with 1% BSA in DMEM for 30 min at room temperature, followed by 2 μg/ml ICAM-1 antibody on ice, and washed in DMEM twice. Cells were then incubated in 0.25 μg/ml biotinylated anti-sheep antibody (R&D Systems, Inc., Minneapolis, MN), washed twice in DMEM, and incubated with 20 μg/ml of R-phycoerythrin (PE)-conjugated streptavidin (Invitrogen, Inc., Carlsbad, CA). 10,000 events were analyzed by flow cytometry (Coulter EPICS XL-MCL) with a 488 nm argon ion laser.
Western blotting
ICAM-1 molecules from HUVEC were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (100 V, 1 h) under reducing conditions, and electro-transferred to nitrocellulose membrane at 100 V for 1 h (Powerpac, Bio-Rad Laboratories, Hercules, CA). ICAM-1 antibody was diluted to 0.1 μg/ml in tris buffered saline (TBS) with 3% BSA and alkaline phosphatase (AP)-conjugated anti-sheep IgG was diluted 1:10,000 (Sigma, Inc., St. Louis, MO). After washing 4 times in TBS AP activity was visualized with NBT-BCIP AP substrate (Promega).
Cell adhesion on HUVEC under static conditions
For cell attachment studies, 0.2 ml of 5 × 105 HUVEC were seeded onto cover glasses (1.8 mm × 1.8 mm, Surgipath Medical Industries, Richmond, IL) pre-sterilized by incubation in 70% ethanol for 2 hr, transferred into a 6-well microplate, and cultured for 1 day prior to the adherence assays.
HUVEC-seeded cover glasses were blocked with 1% BSA in DMEM in a 37°C, 5% CO2 incubator for 30 min, and then incubated for 30 with 0.2 ml of 5 × 105 cells/ml of MSCs-only, PPG-MSCs, and AbICAM-1-MSCs. The cell suspension was discarded and the cover glasses washed with DMEM three times. The attached cells were visualized with fluorescent microscope (DMIRB, Leica microscope), the images captured, and fluorescent cells were counted using ImageJ software (NIH). The results were plotted as relative cell attachment by normalizing the number of cells in the MSCs-only group attached to un-stimulated HUVECs.
Cell binding under shear flow
To assess the cell binding strength of AbICAM-1-MSCs on HUVECs, MSC detachment from HUVECs was determined using a custom-built laminar flow chamber system (Raghavachari M, PhD thesis 2000, Case Western Reserve University), which is designed to allow samples to be subjected to different surface wall shear stress up to 40 dynes/cm2. Briefly, A 25 mm round coverglass (Fisherbrand, Pittsburg, PA) covered with HUVECs (as described above) was placed in a flow chamber. The HUVECs-coverglass was placed on an O-ring (13/16 in inner diameter, 1/16 in width) located in a recess within the lower plate. The upper and lower plate was sealed with 10 wing-nut screws and mounted on the stage of an inverted phase contrast microscope (Nikon Diapot) equipped with digital camera (SPOT RT slider, Diagnostic Instrument Inc.), and controlled by MetaMorph® (Universal Imaging Corp). Images were recorded using screen capturing software (SCREEN MOVIE STUDIO™, Mandsoft).
A 10 ml BD syringe mounted on a calibrated syringe pump (Harvard Apparatus, Holliston, MA) was attached to the inlet port of the chamber and flow started with cell solutions in 1% BSA in DMEM at 0.5 dynes/cm2. To allow MSCs to attach to the HUVECs, the flow was stopped for 10 min and then re-initiated 0.5, 1, 2, and 4 dynes/cm2 of shear stress for 1 min at each flow rate. At the end of each increment of flow, bright-field images were recorded and the remaining cells counted manually. In some flow chamber experiments using AbICAM-1-MSCs on TNFα-stimulated HUVECs the flow was increased at 10, 20, and 25 dynes/cm2.
For one-dimensional laminar flow, the wall shear stress τw (dynes/cm2) is related to volumetric flow rate Q (cm3/s) [31]:
where μ is the media viscosity, w is the width of the flow channel, and H (x) is the height of the flow chamber as a function of position along the microscope slide. The applied τw ranged from 0.5 dynes/cm2 to 25 dynes/cm2.
Results
Cell coating parameters
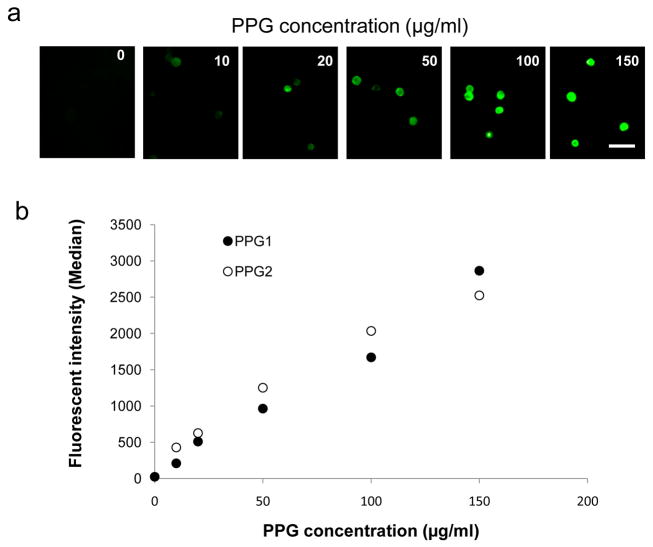
Initial experiments were performed to determine coating parameters that maximize PPG integration into or onto cell surfaces without cell death. MSCs were incubated in a range of PPG concentrations, buffer only, or with non-palmitated protein G. There was no fluorescence detected in the samples using buffers only (0 μg/ml of PPG) or non-palmitated protein G (data not shown). With increased PPG concentration, MSCs showed enhanced fluorescent brightness and above 100 μg/ml of PPG concentration, strong fluorescence was observed on the surface and inside of cells (Fig. 2a). Flow cytometric results showed a linear increase of mean fluorescence intensity in samples incubated in 10–150 μg/ml of PPG (Fig. 2b). The PPG coating was tested up to a concentration nearly double that tested for palmitated protein A [9], but the intensity curve had not reached a plateau, indicating that the coating had not yet reached saturation. Incorporation of antibodies on MSCs was examined using various concentrations of antibodies. As shown in Fig. S1, enhanced antibody incorporation was observed with the increase of antibody concentration.
Fig. 2.
Effect of PPG concentration and FITC-human IgG antibody concentration on intercalation of PPG and antibody into cell membranes. (a) The images show representative fluorescent micrographs of cells at each test PPG concentration. Scale bars indicate 100 μm. (b) Effect of PPG concentrations on PPG incorporation into cell membranes (n=2) and each error bar represents the mean ± SD..
MSC binding to Immobilized ICAM-1
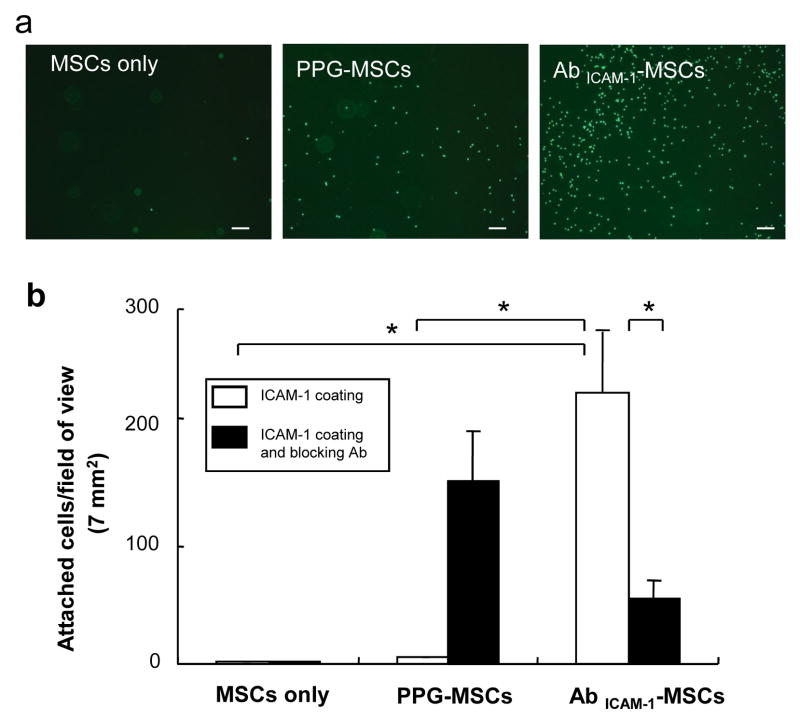
To examine the functional binding of exogenously incorporated antibody to ICAM-1 on MSC surfaces to ICAM-1 molecules, cell adhesion of AbICAM-1-MSCs was tested on ICAM-1-coated microplates. AbICAM-1-MSCs showed the highest cell number on ICAM-1 coated surfaces among MSCs only and PPG-MSCs (Fig. 3a). No MSCs attached to uncoated microplates blocked by BSA molecules (data not shown). Quantification of Vybrant-labeled MSCs showed the number of AbICAM-1-MSCs to be 200 times higher than MSCs only and PPG-MSCs (Fig. 3b). There was no significant difference between MSCs only and PPG-MSCs, indicating that ICAM-1 antibody incorporated onto the cell membrane via PPG binding retains functional affinity to ICAM-1 molecules.
Fig. 3.
Specific binding of AbICAM-1-MSC on ICAM-1-coated surfaces. (a) Representative fluorescent microscopic pictures of MSCs-only, PPG-MSCs, and AbICAM-1-MSC on ICAM-1-coated microplates. Scale bar indicates 200 μm. The fluorescent cells were counted using ImageJ software and plotted (b) (n=4). Open bars show the number of cell attached on ICAM-1 coated surfaces and black bars show attachment to ICAM-1-surfaces pretreated with antibody to ICAM-1, respectively. * indicates a significant difference at p< 0.01 (Student’s t-test).
To determine whether AbICAM-1-MSC binding is specific, excess ICAM-1 antibody was added to the ICAM-1-coated microplates prior to the cell binding assay. ICAM-1 antibody pre-treatment reduced the binding of AbICAM-1-MSCs to ICAM-1 molecules by approximately 80%, indicating the specific binding of incorporated antibodies on MSCs to ICAM-1 molecules. Interestingly, there were an increased number of PPG-MSCs attached to the surface pretreated with free ICAM-1 antibody, which indicates that palmitated protein G on the MSCs surface was able to bind with the ICAM-1 antibodies already bound to ICAM-1 molecules. No significant difference in the number of attached MSCs-only was observed in samples with and without ICAM-1 Ab pre-treatment.
ICAM-1 expression on HUVECs
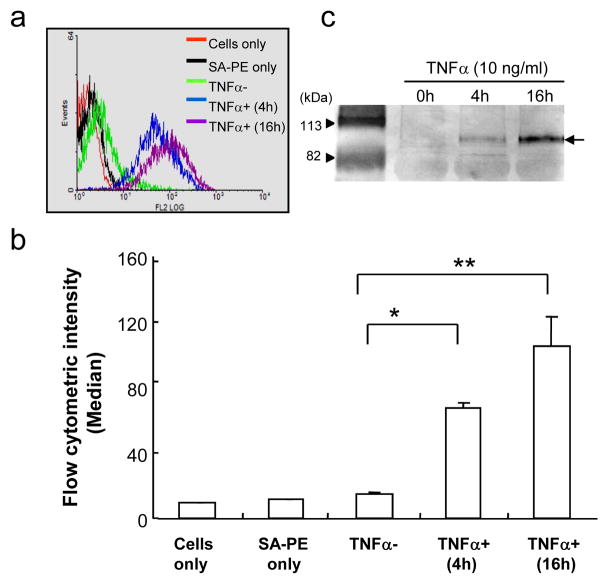
To investigate whether AbICAM-1-MSCs can recognize ICAM-1 molecules exposed on ECs, control and AbICAM-1-MSCs were tested on cultured HUVECs with and without pretreatment with TNFα. First, the expression and cell-surface ICAM-1 on EC was confirmed by analyzing antibody binding by flow cytometry and western blotting. Fig. 4a shows a representative result from a single flow cytometric experiment where strong binding of ICAM-1 antibody to HUVECs is observed in EC incubated in TNFα for 4 hr, and signal level of ICAM-1 increased with TNFα treatment for 16 hr. However, EC with no TNFα treatment, were indistinguishable from cells only and isotype-matched antibody controls. The fluorescent intensity of 3 separate flow cytometry assays was plotted against the median fluorescent intensity in Fig. 4b. TNFα treatment for 4 hr dramatically increased the amount of ICAM-1-positive cells from 10% to 95% (data not shown), as defined by the percentage of fluorescent cells against cells treated with only PE-streptavidin. After 16 h of TNFα exposure, 99% of the EC were ICAM-1-positive and the ICAM-1 fluorescence intensity level per cell was a 1.5 times higher than EC incubated in TNFα for 4 h. The ICAM-1 expression level was confirmed by western blotting as shown in Fig. 4c. The most intense band (95 kDa) was observed in HUVECs stimulated with TNFα for 16 hrs while no ICAM-1 expression was detected in untreated ECs at 20 μg protein per lane.
Fig. 4.
Effect of TNFα treatment time on ICAM-1 expression on HUVECs. (a) Flow cytometric results and the fluorescent intensity for each sample is plotted as the median fluorescence intensity (n=3) (b) with the statistical significance. * p<0.0005, ** p<0.01 (Student’s t-test). Each error bar represents the mean ± SEM. (c) Western blotting for ICAM-1 expression on HUVECs treated with TNFα for different time periods. The data is a representative of three experiments.
MSC Binding to HUVECs
The cell binding capability of MSCs, PPG-MSCs and AbICAM-1-MSCs was tested on HUVECs expressing ICAM-1 molecules. The effect of PPG concentration on MSC binding to HUVECs was also examined. Preliminary testing showed that 50 μg/ml of PPG concentration was the optimum concentrations (see supplementary data, Figure S2).
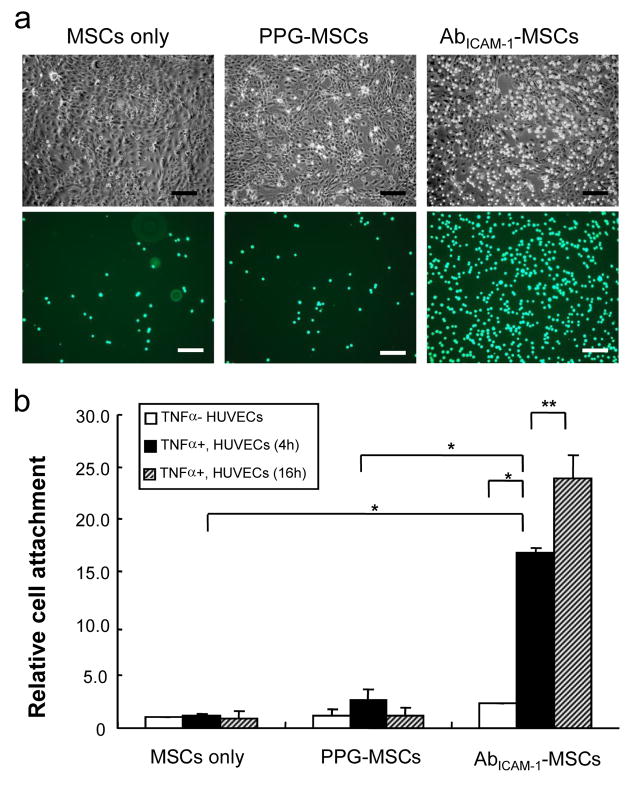
To examine whether AbICAM-1-MSC binding to HUVECs is dependent on ICAM-1 expression on HUVECs, HUVECs were exposed to TNFα for different treatment times based on the results in Fig. 4, then challenged with Vybrant labeled: MSCs-only, PPG-MSCs and AbICAM-1-MSCs; and their binding quantified. Fig. 5a shows a representative example of the phase and fluorescence microscopy results from one of these experiments. Fig. 5b shows the quantified results from 3 independent experiments, showing a 5-fold and 7-fold increase in AbICAM-1-MSCs binding to TNFα-treated HUVECs compared to untreated HUVECS, at 4h and 16h respectively. Notably, the 5-fold cell binding difference between un-stimulated and stimulated HUVECs for 4 hr is statistically significant (P<0.005; Student’s t-test). However, MSCs-only and PPG-MSCs attachment did not show any significant difference in affinity for, HUVECs whether exposed to TNFα or not.
Fig. 5.
The binding of AbICAM-1-MSCs to HUVECs. (a) The morphology of MSCs only, PPG-MSCs, and AbICAM-1-MSCs on HUVECs. Upper panels show the phase contrast and the lower panels the matching fluorescent images. Scale bars indicate 200 μm. (b) AbICAM-1-MSCs binding to HUVECs as a function of TNFα treatment. Open, black, and shaded bars represent cell binding to untreated, TNFα treated for 4 hr, and 16 h, respectively. The *, ** indicates a significant difference at * p<0.005, ** p<0.05 (Student’s t-test).
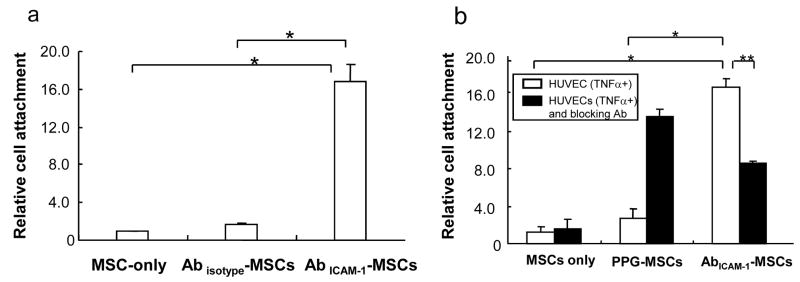
To test whether the binding of AbICAM-1-MSCs to HUVECs was ICAM-1 antibody specific, isotype control instead of ICAM-1 antibody was incorporated onto MSCs (Abisotype-MSCs) and assayed on TNFα-treated HUVECs. As shown in Fig. 6a, Abisotype-MSCs showed a low level cell binding not significantly different from MSCs-only (relative cell attachment equal to 1.0) to HUVECs stimulated with TNFα, indicating that the attachment of AbICAM-1-MSCs to HUVECs is specifically a result of ICAM-1 antibody affinity. Furthermore, the pre-incubation of HUVECs with ICAM-1 antibody studies showed that PPG-MSCs could specifically bind to ICAM-1 antibody located on HUVECs, but bound ICAM-1 antibody competed with AbICAM-1-MSCs resulting in a 50% decrease in attachment (Fig. 6b).
Fig. 6.
Specificity of binding of AbICAM-1-MSCs on HUVECs. (a) MSC-only, Abisotype-MSCs, AbICAM-1-MSCs incubated with TNFα pre-treated HUVECs (n=3); MSC-only set at 1.0. * p<0.001. (b) Binding to TNFα pre-treated HUVECs with (black bars) and without (open bars) pre-incubation with ICAM-1 antibody. (n=4–6). * p<0.001, ** p<0.01 (Student’s t-test).
MSC binding under flow conditions
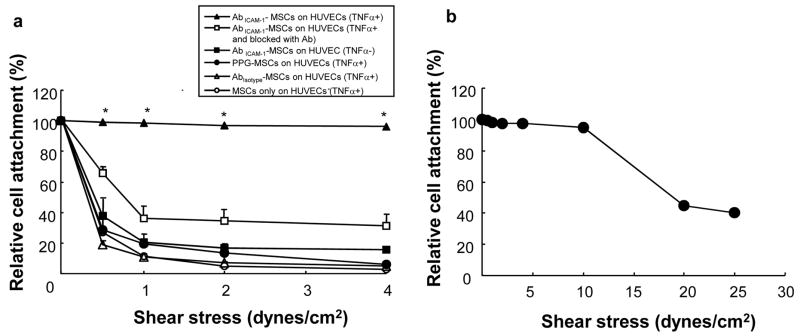
To investigate the effect of ICAM-1 antibody coating on MSC attachment under shear stress, parallel-plate flow chamber studies were performed on HUVECs exposed to MSCs that had undergone various cell surface coating treatments. AbICAM-1-MSCs showed marked binding affinity to ICAM-1 molecules on HUVECs (TNFα+) showing that 100% of AbICAM-1-MSCs remained adherent on HUVECs even at 4 dynes/cm2 of shear stress (Fig. 7). By blocking ICAM-1 molecules on HUVECs (TNFα+) with free ICAM-1 antibody, the adherent AbICAM-1-MSCs decreased with the increase of shear stress and showed less than 40% relative cell attachment at 4 dynes/cm2. Also, the remaining cell percentage of AbICAM-1-MSCs from HUVECs (TNFα−) dramatically decreased to 20% at 4 dynes/cm2 of shear stress, indicating that HUVEC expression of ICAM-1 was needed for increased resistance to flow loss of AbICAM-1-MSCs. Abisotype-MSCs, PPG-MSCs, and MSCs-only on HUVEC (TNFα+) showed 20% cell attachment at 0.5 dynes/cm2 and less than 10% relative cell attachment at 2 dynes/cm2. Interestingly, during a single experimental run to determine what shear stress level was necessary to remove AbICAM-1-MSCs, most AbICAM-1-MSCs remained attached to HUVECs (TNFα+) even at 10 dynes/cm2 and 40% were still present at 25 dynes/cm2 (Fig. 7b). Altogether, these data strongly support that there is strong and specific binding between ICAM-1 antibody-coated MSCs and ICAM-1 molecules on HUVECs which helps maintain cell-cell interaction under shear stress.
Fig. 7.
AbICAM-1-MSCs binding to HUVECs under shear flow. (a) After attachment of cells for 10 min, the chambers were subjected to flow at 0.5, 1, 2, and 4 dynes/cm2. During the flow, the attached cells were counted from recorded video images and plotted as a relative cell attachment with T-0 equal to 100%. Values are displayed as a mean ± SEM (n= 5 for AbICAM-1-MSCs on HUVECs (TNFα+), n=3 for all other groups). *p<0.0001 for comparisons of AbICAM-1-MSCs binding to stimulated HUVECs to all other groups (ANOVA), (b) AbICAM-1-MSCs binding to TNFα-stimulated HUVECs at prolonged shear stresses (n=1).
Discussion
This study examined the functional contributions of exogenously incorporated antibodies on MSC membranes to promote attachment and increase resistance to detachment when seeded onto activated endothelial cells. In this model, membrane-bound antibodies to ICAM-1 on MSCs specifically promoted adherence to HUVEC stimulated to produce ICAM-1 by exposure to TNFα. In addition, the data showed that MSCs coated with ICAM-1 antibody were able to maintain the cell-cell binding between MSCs and EC under high flow conditions. While this study used MSCs as a model for coating cells, these results have broad implications for the specific delivery of any of a number of repair cells to organs and tissues, since the cell surface coating methodology applies to all types of cells.
Cell surface coating Mimics Attachment via Selectins
E- and L-selectins mediate lymphoid trafficking via their binding to carbohydrate moities such as P-selectin glycoprotein ligand-1 (PSGL-1) and HCELL, a sialofucosylated glycoform of CD44. The molecular basis of cell trafficking features are being used as a method to artificially direct stem or repair cells to bind and engraft to specific organs and tissues. For example, Sackstein et al. [2] demonstrated that MSCs that were enzymatically modified to produce the HCELL glycoform of CD44 were able to home more efficiently to bone marrow, and in another study it was shown that transduction of MSCs with α4 integrin, which leads to heterodimerization with β1 integrin, resulted in increased bone marrow engraftment via adhesion to VCAM-1 [32]. These methods capitalize on the inherent mechanisms of cell trafficking and use the underlying homing/signaling principals and apply them to cells that do not normally express those homing molecules. The goal of this study was to test whether antibodies tethered to cell surfaces could also be used to mimic the specific homing-like attachment of MSCs to endothelial cells via the binding to ICAM-1 antibodies. These results provide a new paradigm for guiding cell trafficking by the transient coating of stem cells with antibodies. These results demonstrate the feasibility of applying this methodology using other vascular-specific antibodies, such as HECA452 and CSLEX1 [2] that recognize a Lewis X (NeuAcα2-3Falβ1-4FlcNAcβ1-R) E-selectin epitope [33], or any set of a number of other antibodies to integrins involved in bone marrow trafficking, such as α4β1 (CD49d/CD29), αEβ7 (ligand to MAdCAM-1), α5β1(VLA-5), α4β7 (LPAM-1) or αLβ2 (LFA-1) [12]. Other potential target sites include the endothelium in Peyer’s patches expressing MAdCAM-1 (mucosal addressin cell adhesion molecule-1; [12]), that has also been shown to be up-regulated by TNFα.
The use of TNFα as a stimulatory molecule for ICAM-1 expression in this in vitro model in this study has strong implications for application of this technology in vivo. VCAM-1 and ICAM-1 expression on lymphatic endothelial cells is also up-regulated by TNFα expression [17], which is a potential mechanism by which cells could be targeted to lymph nodes, and fractalkine, another molecule expressed on endothelial cells that promotes leukocyte attachment, is also upregulated by TNFα [34]. In addition, ICAM-1-mediated lymphocyte homing in liver is responsive to in vivo production of TNFα [35].
Antibody subtype is an important aspect of this two-step cell surface coating methodology. Constitutively, protein G has various antibody affinity depending on antibody subclass (product information from Pierce). In this experiment, we tested two different types of antibody; IgG and IgG1. Flow cytometry data showed that FITC-IgG exhibited 3-fold higher binding affinity to protein G than FITC-IgG1 (Fig. S3-a). Thus, AbICAM-1(IgG)-MSCs showed 16 fold binding and Ab ICAM-1(IgG1)-MSCs showed 5 fold binding compared to MSC-only (p<0.005), indicating that more membrane-bound antibody induce enhanced cell binding to ICAM-1 molecules (Fig. S3-b).
It should be noted that lymphocyte targeting is just one step in the process of cell homing, and that additional steps are required for cells to engraft. Selectins provide the initial mechanism for cell adhesion, rolling and tethering, which is then followed by firm attachment via integrins, after which cells react to various chemokine signals to initiate diapedesis [36]. Therefore, this cell surface coating methodology may effectively mimic the attachment process, but it must be recognized that the cells that have targeted and attached to specific endothelium will still require appropriate integrin-binding and/or chemokine receptor(s) to complete the process of engraftment. Another important aspect of this cell surface coating methodology is the lack of any signaling mechanism. For example, fractalkine not only promotes attachment, but also provide an activation signal via the ligand-receptors of the target leukocytes, in this case via G protein-coupled receptor CX3CR1 [37]. This signaling capability like that of fractalkine is not something that this cell surface coating methodology is able to recapitulate, which is a clear limitation of this technique.
Mechanism of adhesion to activated ECs
MSCs have been transplanted intravenously and shown to distribute to spleen, skin, bone, lung, and cartilage in several rodent models [26,38]. Even in these models where MSCs have been detected, the efficiency of delivery is very low. For example, Barbash et al [39], found less than 1% of infused MSCs in heart tissue after systemic injection of 99MTc-labeled MSCs. The goal of this study was to develop a method to increase the efficiency of cell delivery. It is poorly understood to what degree MSCs use specific adhesion mechanism(s) for egress from the bloodstream and whether they home in a tissue-specific manner. MSCs have been reported to express adhesion molecules for homing to specific sites. For example, MSC express CD44, which has been shown to mediate binding to E-selectin of hematopoietic progenitors [40], yet when HUVECs are pre-treated with function-blocking anti-E-selectin antibody, the binding of MSCs to ECs was unchanged, indicating that MSC binding to ECs is not E-selectin mediated [41]. Very recently, the study by Sackstein [2] verified that only E-selectin ligand, which is an ex vivo glycan modified form of CD44 (HCELL), can provide E-selectin binding affinity.
Ruster B et al [41] reported that MSCs interact in a coordinated fashion with HUVECs under shear flow by engaging P-selectin and VCAM-1, which could be blocked by the addition of antibodies to P-selectin or VCAM-1; the VCAM-1 binding could also be blocked by the addition of VLA-4 antibody to the MSCs. Their data suggest that MSCs already have an endogenous mechanism for binding to ECs. However, our study showed that the efficiency of binding of MSCs to HUVECs was greatly increased with coating with ICAM-1 antibody, indicating that the attachment effect of coating with ICAM-1 antibody is much greater than any endogenous binding affinity present within the system. Again, it is strongly implied from our data that more binding ligands on MSCs to receptors in targeting sites is closely involved with an optimal targeting strategy.
Conclusions
The cell surface coating technique presented herein can be used as method to more efficiently deliver reparative cells to specific tissue sites. These results demonstrate that cell surface coating promotes attachment to specific molecules on endothelial cells and that attached cells resist detachment under physiologic flow conditions. Importantly, the cell surface coating methodology is applicable to all cells, does not require genetic modification, and has no effect on cell viability or, for MSCs, differentiation potential.
Supplementary Material
Acknowledgments
We thank Dr. Dave Carrino for AP conjugated anti-sheep antibody, Paul Lin, Jeff Beamish in Dr. Marchant for their kind help during flow chamber experiments. The project described was supported by Grant Number 1R01AR49785 from NIH (NIAMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–22. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–7. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 3.Darling D, Galea-Lauri J, Gaken J, Towner P, Kuiper M, Hollingsworth S, et al. In vitro immune modulation by antibodies coupled to tumour cells. Gene Ther. 1997;4:1350–60. doi: 10.1038/sj.gt.3300534. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 5.Brunschwig EB, Levine E, Trefzer U, Tykocinski ML. Glycosylphosphatidylinositol-modified murine B7-1 and B7-2 retain costimulator function. J Immunol. 1995;155:5498–505. [PubMed] [Google Scholar]
- 6.Kim SA, Peacock JS. The use of palmitate-conjugated protein A for coating cells with artificial receptors which facilitate intercellular interactions. J Immunol Methods. 1993;158:57–65. doi: 10.1016/0022-1759(93)90258-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Zheng G, Tykocinski ML. Hierarchical costimulator thresholds for distinct immune responses: application of a novel two-step Fc fusion protein transfer method. J Immunol. 2000;164:705–11. doi: 10.4049/jimmunol.164.2.705. [DOI] [PubMed] [Google Scholar]
- 8.Dennis JE, Cohen N, Goldberg VM, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res. 2004;22:735–41. doi: 10.1016/j.orthres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Medof ME, Nagarajan S, Tykocinski ML. Cell-surface engineering with GPI-anchored proteins. FASEB J. 1996;10:574–86. doi: 10.1096/fasebj.10.5.8621057. [DOI] [PubMed] [Google Scholar]
- 10.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–91. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 11.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, et al. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res. 1999;84:1237–44. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 12.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–6. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 13.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–58. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 14.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–53. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–112. [PubMed] [Google Scholar]
- 17.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–77. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 19.Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 20.Zapolska-Downar D, Siennicka A, Kaczmarczyk M, Kolodziej B, Naruszewicz M. Simvastatin modulates TNFalpha-induced adhesion molecules expression in human endothelial cells. Life Sci. 2004;75:1287–302. doi: 10.1016/j.lfs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, et al. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101:3977–84. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 22.Dennis JE, Esterly K, Awadallah A, Parrish CR, Poynter GM, Goltry KL. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells. 2007;25:2575–82. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 23.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 24.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–8. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 25.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 27.Teramura Y, Kaneda Y, Totani T, Iwata H. Behavior of synthetic polymers immobilized on a cell membrane. Biomaterials. 2008;29:1345–55. doi: 10.1016/j.biomaterials.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–9. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 29.Huang A, Huang L, Kennel SJ. Monoclonal antibody covalently coupled with fatty acid. A reagent for in vitro liposome targeting. J Biol Chem. 1980;255:8015–8. [PubMed] [Google Scholar]
- 30.Tan PH, Chan C, Xue SA, Dong R, Ananthesayanan B, Manunta M, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173:171–83. doi: 10.1016/j.atherosclerosis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Bhat VD, Truskey GA, Reichert WM. Using avidin-mediated binding to enhance initial endothelial cell attachment and spreading. J Biomed Mater Res. 1998;40:57–65. doi: 10.1002/(sici)1097-4636(199804)40:1<57::aid-jbm7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–27. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 33.Polley MJ, Phillips ML, Wayner E, Nudelman E, Singhal AK, Hakomori S, et al. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991;88:6224–8. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–9. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick AL, Rullo J, Beaudin S, Liaw P, Fox-Robichaud AE. Hepatic leukocyte recruitment in response to time-limited expression of TNF-alpha and IL-1beta. Am J Physiol Gastrointest Liver Physiol. 2007;293:G663–72. doi: 10.1152/ajpgi.00070.2007. [DOI] [PubMed] [Google Scholar]
- 36.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–73. [PubMed] [Google Scholar]
- 37.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 38.Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–61. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 40.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–86. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–44. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.