Abstract
Studies in children have shown that the genetic influence on cognition is positively correlated with socioeconomic status. COMT Val158Met, a common, functional polymorphism, has been implicated in executive cognition and working memory. Imaging studies have shown that the variant Met allele is associated with more efficient pre-frontal cortical processing and better attention but also emotional vulnerability to stress. We hypothesized that COMT Val158Met genotype would interact with years of education (yrs ed), one indicator of socioeconomic adversity, to predict cognitive task performance. We therefore administered the WAIS-R to 328 community-derived, genotyped, Plains American Indians (mean yrs ed = 12; range = 5 to 18). We found significant genotypic effects on WAIS-R measures of long-term memory, working memory and attention. The Met allele was associated with improved performance in the Information and Picture Completion subscales; Met/Met homozygotes performed the best. COMT genotype interacted with yrs ed to influence Information and Block Design scores: Met allele carriers' scores improved markedly with increasing yrs ed whereas the scores of Val/Val individuals were only marginally influenced by yrs ed. There was a crossover of effects at 11-12 yrs ed: in the less educated group, Met allele carriers actually performed worse than Val/Val individuals perhaps due to emotional vulnerability to educational adversity, but in the better educated group, Met allele carriers excelled. Our study in Plains American Indians has shown that COMT Val158Met influences several aspects of cognition and some of its effects are moderated by educational adversity.
Keywords: working memory, long-term memory, attention, WAIS-R, COMT Val158Met, education, resilience, anxiety
INTRODUCTION
Convergent evidence from animal studies and human neuroimaging studies indicate that dopamine (DA) plays a major role in executive processing and enhances the cortical physiological signal to noise ratio (reviewed in Tunbridge et al. 2006). Catechol-O-methyltransferase (COMT) is largely responsible for the metabolism of DA and norepinephrine in the pre-frontal cortex (PFC). Studies in COMT deficient mice have shown that half of the DA decline in the PFC results from COMT-mediated enzymatic degradation (Yavich et al. 2007). A functional polymorphism, COMT Val158Met, responsible for a fourfold difference in enzyme activity in humans (Chen et al. 2004; Weinshilboum et al. 1999) is present at high frequencies in all ethnic groups (Palmatier et al. 1999).
COMT Val158Met has so far only been found in humans and it is therefore of interest that this polymorphism has been implicated in cognition, a behavioral domain in which humans differ from non-human primates. COMT Val158Met modulates PFC function; individuals with the ancestral high activity Val allele perform less well on tests of working memory and executive cognition than individuals with the low activity Met allele, and Val allele load predicts a less efficient physiological response in the PFC (Barnett et al. 2007; Egan et al. 2001; Hariri & Weinberger, 2003; Tunbridge et al. 2006). COMT inhibitors have been shown to improve working memory and attention in animals (Tunbridge et al. 2004) and also in humans (Apud et al. 2007). The Met allele has been associated with a more anxious, cautious personality (Enoch et al. 2003; Hettema et al. 2008; Olsson et al. 2005) and with greater activation in response to negative emotional stimuli in the limbic system and connected prefrontal areas (Smolka et al. 2005).
Several studies in young children and adolescents have shown that the genetic influence on cognition is positively correlated with socioeconomic status; i.e. the shared environment accounts for more of the variance in cognitive skills in disadvantaged children whereas the genetic contribution is greater in children from affluent families, and the influence of heritability on childhood cognitive skills increases with parental education (Harden et al. 2006; Rowe et al. 1999; Turkheimer et al. 2003). We were interested to see whether COMT Val158Met genotype would interact with years of education, one possible indicator of socioeconomic status, to predict cognitive skills in adults. We conducted a study using subscales from the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (Wechsler, 1981) that are indicators of various aspects of cognition including working memory, long-term memory, numerical reasoning, attention and concept formation. The participants in our study were Plains American Indians from rural Oklahoma with a high prevalence of lifetime DSM-III-R alcoholism who came from a deprived socioeconomic environment that included limited educational opportunities. We hypothesized that the COMT Val158Met polymorphism was likely to have an effect on cognitive skills assessed by the WAIS-R and, based on the previously described studies, that the effect might be moderated by years of education. Moreover, the effects might be modified by alcoholism.
MATERIALS AND METHODS
Participants
The study was reviewed and approved by the Plains Indian Tribal Council. Volunteers (328 total: 195 women, 133 men) were recruited from a Plains Indian tribe living in rural Oklahoma. The mean (S.D.) ages of participants were; women: 44.0 (14.9) yrs; men: 41.7 (12.9) yrs. Probands were initially ascertained at random from the tribal register, and the families of alcoholic probands were extended. Exclusion criteria included a history of brain trauma and neurological diseases, together with current use of psychotropic medications and evidence of alcohol intoxication or withdrawal at the time of testing. The study was carefully explained to participants and written informed consent was obtained according to a human research protocol approved by the human research committee of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), NIH. The protocol and consent forms were also formally approved by the Plains Indian Tribal Council.
Blind-rated DSM-III-R lifetime psychiatric diagnoses (American Psychiatric Association, 1987) were derived from the Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS-L). A clinical social worker (B.A.) knowledgeable in the tribe's customs and culture conducted the SADS-L interviews. The prevalence of lifetime alcohol use disorders (AUD) (nearly all dependence) in this sample was 73% in men and 47% in women (Enoch et al. 2006) however 63% of the alcoholics had been abstinent for at least one year.
Tests of Cognitive Ability
The WAIS-R is a standard instrument for measuring cognitive abilities. A truncated version was administered to the Plains Indians. Four tests that are considered to be culturally biased in favor of Caucasians were omitted: Vocabulary, Comprehension, Picture Arrangement and Object Assembly (Sattler, 1988). The remaining seven tests that were administered in this study were: verbal tests: Information (Inf), Digit Span (DSp), Arithmetic (Ar), Similarities (Sim) and performance tests: Picture Completion (PC), Block Design (BD) and Digit Symbol (DSym). Dsp, DSym and BD are tests of working memory. Inf tests longterm memory; Ar tests numerical reasoning; Sim tests concept formation and PC tests attention to fine detail. Inf is correlated with Ar, DSym is correlated with BD and DSp is correlated with Ar (Wechsler, 1981). The WAIS-R tests load on to three factors: Freedom from Distractibility (FFD); Verbal Comprehension (VC); Perceptual Organization (PO) (Wechsler, 1981).
Measure of Socioeconomic Status
A measure of socioeconomic status based on occupation was derived from the Hollingshead Social Index that was completed by 283 individuals (Hollingshead, 1957). Scores ranged from: 1 = long-term unemployed, 2 = unskilled workers, up to 8 = teachers / social workers etc and 9 = major professionals / higher executives.
Genotyping
Genomic DNA was extracted from lymphoblast cell lines and diluted to 10 ng/μl. Genotyping was performed by the 5' exonuclease genotyping assay (TaqMan®) that combines polymerase chain reaction amplification and detection into a single step using fluorogenic allele-specific probes. The genotyping method has been fully described elsewhere (Enoch et al. 2006). Duplicate genotyping was performed on almost all (93%) of the DNA samples. All genotype frequencies conformed to Hardy-Weinberg equilibrium. The frequency of the Met allele was 0.29 in the total sample.
Population Stratification
The Plains Indian sample was also genotyped for 186 ancestry markers (AIMS) that were included on an Illumina array (Hodgkinson et al. 2008). The AIMs were also genotyped in 1051 individuals from the 51 worldwide populations represented in the HGDP-CEPH Human Genome Diversity Cell Line Panel (http://www.cephb.fr/HGDP-CEPH-Panel). PHASE Structure 2.2 (http://pritch.bsd.uchicago.edu/software.html) was run simultaneously using the AIMS genotypes from our sample and the 51 CEPH populations to identify population substructure and to compute individual ethnic factor scores. This ancestry assessment showed that within the Plains Indian sample there was on average 0.05 admixture with Caucasian ancestry (median value = 0.014) and only 0.009 (median value = 0.001) with African ancestry. Ethnic factor scores were included as covariates in the analyses. Since the ethnic factor scores were highly skewed (due to very low admixture) we used a median split in analyses. Ethnic factor scores had no influence on any of the analyses.
Statistical Analyses
The proportion of genetic identity shared between any two individuals through common descent was calculated for all possible pairs (related and unrelated) in each population using S.A.G.E. (Case Western Reserve University). Although most Plains Indians derived from one large, multigenerational pedigree the average sharing of descent was only 0.003. This is less than the degree of relationship between third cousins, and indicates that most pairs of individuals in this population have a very low degree of relationship. Thus the Plains Indians in this sample were regarded as essentially independent for the purposes of the analyses.
The WAIS-R subscale scores approximated normal distributions. We performed ANOVA with years of education and age as covariates and sex and alcoholism status as between subject factors. Alcoholism status was included because alcoholism has been associated in some (including in the Plains Indian dataset) but not all studies with deficits in WAIS-R tests (reviewed in Harris et al. 2003). Some studies have shown that COMT has sexually dimorphic effects (Harrison & Tunbridge, 2007). We tested for interactions between COMT and sex but found no significant results. Main effects are given as mean (S.D.).
After the aforementioned primary analyses on the total group of 328 Plains Indians we divided the sample into those individuals with ≥ 13 yrs ed who had completed a high school education (N = 96) and those individuals with < 13 yrs ed (N = 231). (Yrs ed was not known for one individual). The analyses that had been conducted in the total group (Table 1) were also conducted in these two sub-groups (Table 2). We chose this split at 13 yrs ed because in the USA many jobs and life opportunities are dependent on completing high school which generally occurs after 13 yrs ed. Therefore individuals with less than 13 yrs ed were likely to be disadvantaged.
TABLE 1.
Total Sample: Main Effects of Years of Education, Age, Sex, Alcohol Use Disorders and COMT Val158Met on WAIS-R Subscale Scores
| WAIS-R subscale | Yrs Ed | Age | Sex | AUD | COMT Val158Met | G × Yrs Ed | Df | Total Var |
|---|---|---|---|---|---|---|---|---|
| Inf | F = 30 | F = 36 | F = 30 | F = 4.3 | F = 5.0 | |||
| P < 0.0001 | P < 0.0001 | P < 0.0001 | - | P = 0.014 | P = 0.007 | 7 | 0.35 | |
| *F = 9.1 | *F = 10.7 | |||||||
| *P = 0.003 | *P = 0.001 | 5 | ||||||
|
| ||||||||
| Dsp | F = 7 | F = 6 | F = 4.6 | |||||
| P = 0.009 | P = 0.014 | - | - | - | P = 0.011 | 6 | 0.14 | |
| *F = 7.3 | ||||||||
| *P = 0.008 | 4 | |||||||
|
| ||||||||
| Ar | F = 44 | |||||||
| P < 0.0001 | - | - | - | - | *F = 3.6 | 3 | 0.15 | |
| *P = 0.059 | ||||||||
|
| ||||||||
| Sim | F = 15 | F = 3.0 | ||||||
| P = 0.0001 | - | - | - | - | P = 0.047 | 5 | 0.18 | |
| *F = 6.5 | ||||||||
| *P = 0.011 | 3 | |||||||
|
| ||||||||
| PC | F = 18 | F = 5.0 | F = 5.0 | |||||
| P < 0.0001 | - | - | P = 0.023 | P = 0.006 | - | 4 | 0.10 | |
| *F = 9.6 | ||||||||
| *P = 0.002 | 3 | |||||||
|
| ||||||||
| DSym | F = 27 | F = 55 | F = 2.6 | |||||
| P < 0.0001 | - | P < 0.0001 | - | P = 0.074 | - | 4 | 0.22 | |
| *F = 2.8 | ||||||||
| *P = 0.098 | 3 | |||||||
|
| ||||||||
| BD | F = 8.6 | F = 3.3 | F = 3.3 | F = 5.5 | ||||
| P = 0.0036 | - | P = 0.071 | P = 0.069 | - | P = 0.005 | 7 | ||
| *F = 11.1 | 0.13 | |||||||
| *P = 0.001 | 5 | |||||||
ANOVA P values ≤ 0.10 are given here. Val/Val, n = 168; Val/Met, n = 134; Met/Met, n = 26, total N = 328.
G × Yrs Ed =interaction effects of COMT Val158Met × Yrs Ed on test scores. Df = degrees of freedom; Var = variance.
Results are given for main and G × Yrs Ed effects for the 3 COMT Val158Met genotypes.
Inf = Information; Dsp = Digit Span; Ar = Arithmetic; Sim = Similarities; PC = Picture Completion; DSym = Digit Symbol; BD = Block Design
Results are for COMT Val/Val vs [Val/Met + Met/Met].
TABLE 2.
Main Effects of COMT Val158Met Genotype on WAIS-R Subscale Scores
| WAIS-R subscale | < 13 years of education. N = 230 | ≥ 13 years of education. N = 96 | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Val/Val 123 | Val/Met 94 | Met/Met 14 | F,(df) P value | Val/Val 44 | Val/Met 40 | Met/Met 12 | F, (df) P value |
| Inf | 6.9 | 7.4 | 8.1 | 8.0 | 9.0 | 9.3 | F = 5.9, (5) | |
| (1.9) | (1.8) | (1.5) | - | (2.1) | (2.0) | (1.9) | P = 0.004 | |
| *F = 11.6, (4) | ||||||||
| *P = 0.001 | ||||||||
| Dsp | 7.7 | 7.8 | 8.9 | 9.1 | 9.8 | 8.2 | F = 2.5 (3) | |
| (2.3) | (2.5) | (2.2) | - | (2.2) | (2.4) | (2.9) | P = 0.083 | |
| PC | 8.3 | 9.4 | 9.3 | F = 4.7, (3) | 9.3 | 9.5 | 10.6 | |
| (2.1) | (2.2) | (1.9) | P = 0.010 | (2.3) | (2.4) | (3.0) | - | |
| *F = 9.4 (2) | ||||||||
| *P = 0.003 | ||||||||
| BD | 10.0 | 9.4 | 10.0 | F = 2.3 (6) | 10.6 | 11.4 | 11.2 | |
| (3.0) | (2.3) | (2.4) | P = 0.10 | (3.1) | (3.0) | (3.4) | - | |
ANOVA P values ≤ 0.10 are given here.
F and p values are for COMT genotype effects
Inf = Information; Dsp = Digit Span; Ar = Arithmetic; PC = Picture Completion; BD = Block Design. Digit Symbol, Similarities and Arithmetic were not included since there were no significant or even trend effects.
Results are for COMT Val/Val vs [Val/Met + Met/Met]
There were only 26 individuals with the Met/Met genotype; 12 Met/Met individuals with ≥ 13 yrs ed and 14 Met/Met individuals with < 13 yrs ed. Therefore because of the low ‘N’ of Met/Met individuals, analyses were first conducted across all genotypes and then across Val/Val and Val/Met + Met/Met combined.
Analyses were performed using JMP, version 6.0.
RESULTS
Years of education: main effect
Years of education (yrs ed) were approximately normally distributed: the mean (S.D.) was 11.9 (1.8) years, the median and mode was 12 years. The range was 5 to 18 years. Only 29% of individuals had completed their high school education (13 yrs ed). Years of education predicted occupation, a measure of socioeconomic status (F (1,281) = 26.0, p < 0.0001) but accounted for only 8.5% of the variance.
Yrs ed correlated with age in women but not men; older women were less educated than younger women: r = 0.2, F (1,211) = 7.0, p = 0.009. There was no correlation between socioeconomic status (occupation) and age in women or men. Men and women did not differ in yrs ed. As expected, there was a significant main effect of yrs ed on all WAIS-R test scores (Table 1).
Main Effects of AUD, sex and age (Table 1)
Sex had a significant effect on DSym, Inf and BD scores contributing to 14%, 6% and 1% respectively of the variance. Women performed better than men on the DSym test whereas the reverse was true for the Inf and BD tests. Age had an effect on Inf and DSp contributing to about 2 - 5% of the variance. Predictably, Inf scores improved with age but DSp scores diminished with age. AUD had a significant effect on PC and a trend effect on BD.
Effects of COMT genotype
As can be seen from Table 1 there was a significant main effect of COMT genotype on Inf and PC as follows: Inf: Val/Val 7.2 (2.0); Val/Met 7.9 (2.0); Met/Met 8.7 (1.8); F(2,324) = 4.3, p = 0.014. PC: Val/Val 8.6 (2.2); Val/Met 9.4 (2.3); Met/Met 9.9 (2.5); F(2,324) = 5.0, p = 0.006. These main effects of COMT genotype contributed to 5 - 3% of the variance in scores. In addition, there was a trend effect for Dsym: Val/Val 10.4 (2.5); Val/Met 10.8 (2.6); Met/Met 12.0 (2.3); F(2,324) = 2.6, p = 0.074.
Table 2 shows the main effects of COMT Val158Met on WAIS-R tests stratified by yrs ed. There were significant main gene effects on PC for the group with < 13 yrs ed and on Inf for the group with ≥ 13 yrs ed.
Correlation between COMT genotype and yrs ed (rGE)
Genotype predicted yrs ed in both men and women and accounted for 4% of the variance (F(2,322) = 5.9, p = 0.003): Val/Val (n=167) 11.6 (2.1); Val/Met (n=134) 12.0 (1.6); Met/Met (n=26) 12.8 (1.1).
Interaction between COMT genotype and years of education (G×E)
There were significant interactions between COMT genotype and yrs ed on four tests: Inf, BD, Dsp and Sim (Table 1, Figure 1). From Figure 1a and 1b it can be seen that Inf and BD scores for individuals with at least one Met allele (Met/Met and Val/Met genotypes) improved considerably with increasing years of education. In contrast, Inf and BD scores in individuals with the Val/Val genotype were far less dependent on yrs ed. For Sim and Dsp (Figure 1c and 1d) the Val/Met heterozygotes showed the greatest beneficial response to increased yrs ed.
FIGURE 1.
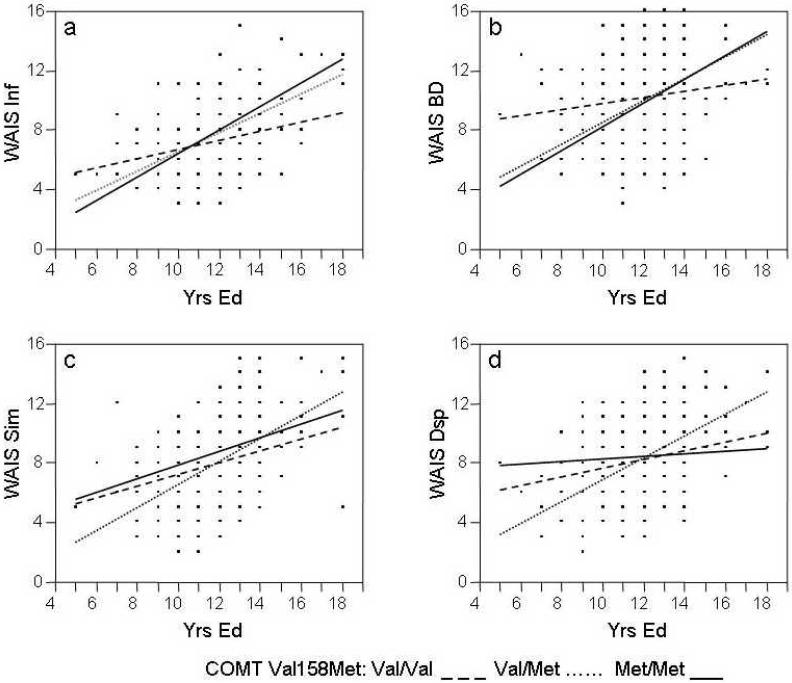
Effects of Interaction between COMT Val158Met Genotype and Years of Education on WAIS-R Tests
Wechsler Adult Intelligence Scale-Revised (WAIS-R).
a: Inf = Information; b: BD = Block Design; C: Sim = Similarities; D: Dsp = Digit Span.
Influence of rGE on G×E
Since it was possible that our G×E results might have been influenced by rGE we conducted secondary analyses to test for this possibility. The proportion of Met alleles in individuals who had the following yrs ed was as follows: 5 - 9 yrs ed (n = 33) 0.14; 10 - 11 yrs ed (n = 81) = 0.23; 12 yrs ed (n = 124) = 0.31; 13 - 18 yrs ed (n = 97) = 0.33. When the 33 individuals with the lowest yrs ed (5 - 9) who had the lowest proportion of Met alleles were eliminated there was no longer a correlation between genotype and yrs ed: Val/Val = 12.2 (1.6); Val/Met = 12.2 (1.3); Met/Met = 12.8 (1.1) (F(2,291) = 2.2, p = 0.11). G×E analyses were therefore re-done in this dataset that only included individuals with ≥ 10 yrs ed. The following results for G×E interactions were obtained (for COMT Val/Val vs Val/Met + Met/Met): Inf: F(5,288) = 5.7, p = 0.018; BD: F(4,289) = 8.0, p = 0.005; Dsp: F(3,290) = 0.2, p = 0.66; Sim: F(3,290) = 0.9, p = 0.35.
Interaction between COMT genotype and age
There was also a significant interaction between COMT genotype and age on BD scores (F(2,322) = 3.7, p = 0.027; for Val/Val vs Val/Met + Met/Met, F = 7.0, p = 0.009). BD scores increased with age for Met/ Met individuals, decreased with age for Val/Val individuals and did not vary with age for Val/Met heterozygotes. The was no COMT × age × yrs ed interactive effect on BD.
DISCUSSION
Our study sample consisted of 328 Plains American Indian men and women, 71% of whom had not completed high school and were therefore considered educationally deprived. We found significant main effects of COMT and interactive effects of COMT and yrs ed on cognition accessed by three of the seven WAIS-R subscales: Inf, PC and BD. COMT Val158Met genotype had a significant effect on Inf and PC. The Met allele was associated with higher scores for both tests in a dose-dependent fashion. Secondary analyses revealed that this significant COMT main effect derived from the more educated group (≥ 13 yrs ed) for Inf but from the less educated group (< 13 yrs ed) for PC. From Table 2 it can be seen that scores for Inf, an indicator of verbal comprehension and long-term memory, improved for all genotypes with increasing education, but more markedly for Met allele carriers. In contrast, Val/Met individuals in both the high and low education groups had essentially the same scores for PC, an indicator of attention to fine detail and perceptual organization, whereas Val/Val and Met/Met individuals benefited from increased yrs ed. Our results indicate that the effects of COMT Val158Met on cognition may differ, depending on the domains that are being tested.
Figure 1a shows that Inf scores for Met/Met and Val/Met individuals improved markedly with increasing education whereas Val/Val individuals were less influenced by yrs ed. Also, Figure 1 shows a crossover effect at approximately 11 yrs ed: Met/Met and Val/Met individuals with less than 11 yrs ed actually performed worse than Val/Val individuals but the reverse was the case in individuals with more than 11 yrs ed. There was a similar interaction effect on BD (working memory) scores with a crossover of effect at approximately 12.5 yrs ed (Figure 1b). There were also apparent interactive COMT × yrs ed effects on the Sim (concept formation) and Dsp (working memory) tests (Figure 1c, 1d) but these were more modest (p = 0.047 - 0.011) than the effects on Inf and BD (p = 0.007 - 0.005) and may have been due to gene-environment correlation (see below). For Sim and Dsp the Val/Met heterozygotes were more sensitive to the influence of education than either homozygote. The Val/Met heterozygotes' scores increased with yrs ed at the same rate for all four tests. Although the Val/Val homozygotes' scores also increased with yrs ed at the same rate for all four tests this rate was much lower than that of the heterozygotes (Figure 1c, 1d). The crossover point between Val/Val and Val/Met individuals in all four tests was at 11 - 12 yrs ed. In contrast, the rate of increase for the Met/Met homozygotes differed between tests: it was in the expected direction for Inf, BD and Sim scores but for Dsp, Met/Met homozygotes unexpectedly derived no benefit from increased yrs ed. One explanation for this result may be the small sample size of Met/Met individuals (N = 26). Indeed, the significance of all G×E interactions improved considerably when Met/Met and Val/Met individuals were counted as one group. Another possibility may be the influence of gene-environment correlation, discussed below. Finally, it is also plausible that not all tasks are sensitive to the same variables.
We found evidence of a gene-environment correlation (rGE). Met allele dosage was significantly associated with increased yrs ed although this only accounted for 4% of the variance. Possible explanations for this rGE might be that if Met carriers are cognitively more gifted they may be more motivated to stay on in education. Perhaps the more cautious, anxious, inhibited personality of a Met carrier may also contribute to perseverance in education. It is known that G×xE interactions are difficult to assess in the presence of rGE (Rutter & Silberg, 2002). When we repeated the analyses after excluding the 33 individuals with 5 - 9 yrs ed who had the lowest proportion of Met alleles, rGE was no longer significant however the G×E interactions remained significant for the Inf and BD tests but not for the Sim and Dsp tests. This suggests that these are true G×E interactions influencing Inf and BD scores but the G×E results for Sim and Dsp might have been influenced by rGE.
As discussed in the introduction, a large body of literature has demonstrated that COMT activity is an important modulator of dorsolateral PFC (DLPFC) -dependent cognitive tasks (Apud et al. 2007; Egan et al. 2001; Hariri & Weinberger, 2003; Malhotra et al. 2002; Tunbridge et al. 2006). We found that one (BD) of the three WAIS-R tests that access working memory was influenced by COMT genotype interacting with yrs ed, a marker for educational adversity. Moreover, another working memory test, DSym, showed a trend main effect for COMT in the predicted direction: the Met allele was associated with higher test scores. COMT Val158Met has also been shown to modulate attention via the cingulate cortex, located in the PFC. The Val allele has been linked with poorer attentional control coupled with greater activity in the cingulate cortex and the effects of Val allele load increase as attentional demands increase (Blasi et al. 2005). Attentional demands may be greater in less educated individuals and our finding that COMT genotype had the strongest impact on PC scores in the less educated group appears to fit in here.
A network of brain regions is known to be involved in the encoding and retrieval of memory including two linked regions, the hippocampus and the ventrolateral PFC (VLPFC). Dopamine is an important modulator of their interactions (Schacter & Wagner, 1999). COMT is also widely expressed in the hippocampus. Carriers of the Val158 allele perform less well on tests of long-term memory (de Frias et al. 2004). During encoding and retrieval of a recognition memory task, the COMT Val158 allele has been associated with poorer performance, decreased fMRI hippocampal activation together with less efficient VLPFC activation and impaired functional coupling between these two regions (Bertolino et al. 2006). Our results for the WAIS-R Inf test are supported by these earlier studies.
There are few published studies that have looked at the influence of COMT genotype on WAIS subscales. In a study of psychotic individuals, the COMT Val158 allele was associated with greater deterioration over time in Inf, DSym and Vocabulary scores (Mata et al. 2006). In healthy, elderly individuals the Val/Val genotype has been linked with diminished cognition, particularly for the DSym test (for which we found a trend main effect) (Starr et al. 2007) and the Met/Met genotype has been linked with better performance on a measure of attention (Liu et al. 2008). Our results agree with these earlier findings.
The COMT Met158 allele has been associated with greater activation to negative emotional stimuli in the limbic system, hippocampus and VLPFC (Drabant et al. 2006; Smolka et al. 2005). Met/Met homozygotes show increased frontal coupling of the limbic and prefrontal regions (Drabant et al. 2006). The Met allele association with heightened reactivity and connectivity in corticolimbic circuits may reflect a predisposition for inflexible processing of affective stimuli and a tendency for rumination that may account for the Met allele association with increased anxiety and emotional dysregulation noted in earlier studies (Drabant et al. 2006; Hettema et al. 2008; Olsson et al. 2005), including in our sample (Enoch et al. 2003). Thus the crossover effect that we have noted in Figure 1 may indicate a balance between emotional resilience / poorer cognitive skills (Val allele) and stress-vulnerability / superior cognition (Met allele). One possible interpretation of our G × E interaction results for the Inf and BD subtests is that in good educational environments Met allele carriers (Met/Met and / or Val/Met individuals) may be able to develop their full potential for cognitive skills that are superior to those of Val/Val homozygotes. However, when Met allele carriers are exposed to significant educational adversity their emotional vulnerability may overwhelm their ability to develop cognitive skills. In contrast, Val/Val homozygotes may be more resilient to educational adversity.
Our sample had a high prevalence of lifetime AUD (73% in men and 47% in women) and therefore AUD was included as a between subject factor. AUD had a modest effect on PC scores in the total sample (p = 0.02) and on BD scores in the less educated group (p = 0.02). However there was no interactive effect with COMT genotype.
There are a few limitations to our study. We did not correct for multiple testing. We analyzed seven WAIS-R subscales with no a priori hypothesis as to which test might have the strongest association therefore a Bonferroni corrected p value would be p = 0.007. Several, but not all, of our association results achieved that degree of statistical significance. Moreover, when analyses were conducted with Met/Met and Val/Met individuals included as one group to allow for the small Met/Met sample size, the significance level improved considerably. Nevertheless the results from our study should be considered preliminary until reproduced in other datasets. The effects of the COMT Val158Met polymorphism on the WAIS-R tests were not huge: COMT genotype accounted at best for 5% of the variance in test scores in the Plains Indians. This is similar to the variance (4%) previously found for COMT influence on perseverative errors in executive cognition tests (Egan et al. 2001). Numerous other factors also influence cognition, such as the quality of education, parental, socioeconomic and cultural factors. Other genes, for example brain-derived neurotrophic factor, may also influence cognitive function. In this study of American Indians we used the WAIS-R subtests that are considered to be culturally appropriate. Nevertheless our results should be interpreted with caution until they have been replicated in other ethnic groups with similar educational adversity.
In conclusion, our study in Plains American Indians has shown that COMT genotype has an effect on measures of cognition that access several brain regions including the dorso- and ventrolateral PFC, the cingulate cortex and the hippocampus. These effects were detected within the broad range of cognitive tests that form the WAIS-R. Met allele carriers generally performed better on tests of cognition, particularly when raised in better learning environments, but Val/Val individuals appeared to be more resilient when exposed to educational adversity.
ACKNOWLEDGEMENTS
We would like to thank Pei-Hong Shen and Longina Akhtar for technical assistance. This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH, and in part by the Office of Research on Minority Health.
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised American Psychiatric Association Press; Washington, DC: 1987. [Google Scholar]
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32:1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson LG. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women; a role for a functional COMT polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Enoch M-A, Waheed J, Harris CR, Albaugh B, Goldman D. Sex Differences in the Influence of COMT Val158Met on Alcoholism and Smoking in Plains American Indians. Alcohol Clin Exp Res. 2006;30:399–406. doi: 10.1111/j.1530-0277.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents' cognitive aptitude. Behav Genet. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Harris CR, Albaugh B, Goldman D, Enoch M-A. Neurocognitive impairment due to chronic alcohol consumption in an American Indian Community. J Stud Alcohol. 2003;64:458–466. doi: 10.15288/jsa.2003.64.458. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-Methyltransferase (COMT): A Gene Contributing to Sex Differences in Brain Function, and to Sexual Dimorphism in the Predisposition to Psychiatric Disorders. Neuropsychopharmacology. 2007 Sep 5; doi: 10.1038/sj.npp.1301543. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hettema JM, An SS, Bukszar J, van den Oord EJ, Neale MC, Kendler KS, Chen X. Catechol-O-Methyltransferase Contributes to Genetic Susceptibility Shared Among Anxiety Spectrum Phenotypes. Biol Psychiatry. 2008 Apr 22; doi: 10.1016/j.biopsych.2008.03.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Xu K, Yuan Q, Shen P-H, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions Biology: Haplotype Based Analysis for 130 Candidate Genes on a Single Array. Alcohol Alcohol. 2008 May 12; doi: 10.1093/alcalc/agn032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A,B. Two Factor Index of Social Position. New Haven, CT: 1957. [Google Scholar]
- Liu ME, Hong CJ, Liou YJ, Tsai YL, Hsieh CH, Tsai SJ. Association study of a functional catechol-O-methyltransferase polymorphism and executive function in elderly males without dementia. Neurosci Lett. 2008;436:193–195. doi: 10.1016/j.neulet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mata I, Arranz MJ, Staddon S, Lopez-Ilundain JM, Tabares-Seisdedos R, Murray RM. The high-activity Val allele of the catechol-O-methyltransferase gene predicts greater cognitive deterioration in patients with psychosis. Psychiatr Genet. 2006;16:213–216. doi: 10.1097/01.ypg.0000218626.26622.a2. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet. 2005;15:109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Jacobson KC, Van den Oord EJ. Genetic and environmental influences on vocabulary IQ: parental education level as moderator. Child Dev. 1999;70:1151–1162. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- Rutter M, Silberg J. Gene-environment interplay in relation to emotional and behavioral disturbance. Annu Rev Psychol. 2002;53:463–490. doi: 10.1146/annurev.psych.53.100901.135223. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children. 3rd Edition J.M. Sattler; San Diego, CA: 1988. [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr JM, Fox H, Harris SE, Deary IJ, Whalley LJ. COMT genotype and cognitive ability: a longitudinal aging study. Neurosci Lett. 2007;421:57–61. doi: 10.1016/j.neulet.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol-O-methyltransferase, thiopurine methyltransferase, and histamine N- methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale - Revised. Psychological Corp; New York: 1981. [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


