Abstract
Ionizing Radiation (IR) therapy is one of the most commonly used treatments for cancer patients. The responses of tumor cells to IR are often tissue specific and depend on pathway aberrations present in the tumor. Identifying molecules and mechanisms that sensitize tumor cells to IR provides new potential therapeutic strategies for cancer treatment. In this study, we used two genetically engineered mouse (GEM) carcinoma models, brain choroid plexus (CPC) and prostate to test the impact of inactivating gadd45a, a DNA damage response p53 target gene, on tumor responses to IR We show that gadd45a deficiency significantly increases tumor cell death after radiation. Impact on survival was assessed in the CPC model and was extended in IR-treated mice with gadd45a deficiency compared to those expressing wild type gadd45a. These studies demonstrate a significant effect of gadd45a inactivation in sensitizing tumor cells to IR, implicating gadd45a as a potential drug target in radiotherapy management.
Introduction
Ionizing Radiation (IR) is one of the most commonly used therapies in oncology. Tumor cell responses to IR are tissue specific and depend greatly on the pathway defects present within tumors. Therefore, understanding the molecular mechanisms of the cellular responses to IR is essential for managing and improving this mode of cancer treatment.
The tumor suppressor gene Trp(p53) is a key player in the cell's response to stress signals, including IR. For example, following IR treatment murine thymocytes undergo rapid p53-dependent apoptosis, fibroblasts enter irreversible p53-dependent cell cycle arrest, while epithelial cells usually go through reversible cell cycle arrest (1). Stress signals, including DNA damage and oncogenic events, induce p53 activity eliciting differential expression of p53 target genes. These downstream genes can be divided into major groups categorized by established p53 roles in a given biological response. The best characterized of these include cell cycle arrest genes (e.g p21(Cdkn1), gadd45a and 14-3-3σ and apoptosis genes (e.g. bax, Apaf1, puma, p53AIP1 and noxa et al.)(2, 3). Among p53 regulated cell cycle control genes, gadd45a has been shown to play an important role in DNA damage-induced cell responses. For example, gadd45a deficiency causes defective UV-induced nucleotide excision repair (4). Gadd45a participates in the proper control of the G2-M checkpoint in response to UV radiation and of the S-phase checkpoint under multiple conditions of nutrient deprivation (5-7). Gadd45a null MEF cells exhibit increased aneuploidy accompanied with abnormal centromere amplification; when exposed to IR, gadd45a knockout mice also show increased lymphomagenesis compared to control mice (8). Interestingly, in vivo studies have shown that gadd45a inactivation also causes abnormal p38 MAPK phosphorylation, T cell hyperproliferation and a lupus-like autoimmune disease in mice (9, 10). In addition to p53, BRCA1 and FOXO3a have also been shown to activate gadd45a gene expression (11, 12).
In addition to cell cycle control, there is evidence that gadd45a is also involved in DNA damage induced apoptosis. For example, gadd45a prevents UV-induced skin tumors and promotes keratinocyte apoptosis in mice via the p38 and p53 pathways (13). Similarly, gadd45a suppresses Ras-induced mammary tumorigenesis by p38-mediated cell cycle arrest and apoptosis (14). Overexpression of gadd45a in Hela cells induces apoptosis through translocation of Bim to mitochondria (15). However, little is known about gadd45a's role in control of apoptosis in the cellular response to IR in vivo.
In the current study, we utilized in genetically engineered mouse (GEM) models of spontaneous brain and prostate carcinoma to investigate the role of gadd45a role in epithelial tumor responses to IR treatment. We found that gadd45a inactivation increased the in vivo sensitivity of carcinoma cells to IR resulting in significantly delayed tumor progression.
Materials and Methods
Mice
The transgenic TgT121 brain tumor mouse model(16, 17), TgAP-T121 prostate carcinoma mouse models (18)and mice harboring a homozygous deletion of the gadd45a gene (8) or of the p21 gene (19) were previously described. TgT121;gadd45a−/− and TgT121,gadd45a+/− were generated by crossing hemizygous TgT121 mice with gadd45a−/− mice, and TgT121,p21−/− and TgT121;p21+/− were generated by crossing hemizygous TgT121 mice with p21−/− mice. TgAPT121;gadd45a−/− mice were generated by crossing TgT121 mice with gadd45a−/− mice. To produce homozygous null backgrounds transgenic mice that were heterozygous at the desired locus were crossed to respective homozygous null animals. In every case the oncogenic transgene was maintained in the hemizygous state.
Radiation treatment
To assess brain tumor cell responses, two-month old mice (male and female) were treated with one 10 Gy dose whole-body radiation, and euthanized 4.5 hours after treatment for TUNEL assay. A different group of mice were treated with the same dose of irradiation and were injected with BrdU (30 μg/gram bodyweight) 4.5 hours after treatment, the mice were euthanized 1 hour after the injection and brain tissues were fixed for immunohistochemical assay. For analysis of prostate tumor cells, 2 month old male mice were treated with one 10 Gy whole-body dose. For survival analysis, 2 month old TgT121;gadd45a−/− and TgT121;gadd45a+/+ mice were irradiated (heads only) at a dose of 2 Gy per day for a total of 10 Gy with a one day interval after receiving treatment for 2 days. Mice were anesthetized with 2.5% Avertin (0.3 ml /20g bodyweight) before irradiation. Mice were euthanized when signs of illness were present (e.g. domed head, lethargy).
TUNEL and proliferation assays
Brain and prostate tissues were fixed, embedded and sectioned as described (20). Apoptotic cells were detected in sections using the TdT-mediated dUTP nick end labeling (TUNEL) assay (17, 20). For each mouse, 8 to 10 different fields were counted under microscope. At least 3 mice of each genotype were analyzed, and the counts of apoptotic indexes were averaged and the standard deviations within each genotype group were calculated (represented by error bars). Proliferation rate of tumor cells were measured by BrdU immnostaining as described previously (20).
Statistics
T-tests were used to evaluate the difference in apoptosis level between different groups of mice. Log rank tests were used for survival analysis.
Results
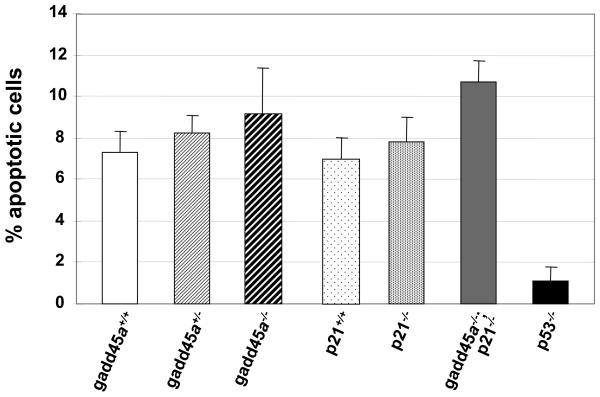
We previously established a mouse brain epithelial (choroid plexus epithelium, CPE) tumor model, TgT121, in which CP carcinoma (CPC) development is initiated by cell-specific transgenic expression of T121, an N-terminal fragment of SV40 T antigen that inactivates pRb and related proteins, p107 and p130 (21). T121 acutely induces aberrant CPE cell proliferation accompanied by p53-mediated apoptosis and predisposes to aggressive tumor growth which occurs upon p53 inactivation. Tumors are histologically indistinguishable from human CPCs (17). To evaluate the contribution of p53 downstream genes to p53′s tumor suppression function in TgT121 mice, we generated TgT121; gadd45a−/− mice, and found that, unlike p53 deficiency, gadd45a deficiency does not affect the apoptosis level induced by pRb function loss (Figure 1). To determine whether the response to irradiation was impacted by gadd45a deficiency, we treated TgT121;gadd45a−/−, TgT121;gadd45a+/− and TgT121;gadd45a+/+ mice with a single dose of IR to the head (10 gray) and examined acute effects within the tumor 4.5 hours after the treatment. Apoptosis, measured by the TUNEL assay was significantly increased in TgT121;gadd45a−/− tumors (16.5% ± 3.6%, n=5) compared to the TgT121;gadd45a+/+ controls (8.2% ± 0.8%, n=5) (p<0.05). TgT121; gadd45a+/− tumors yielded an intermediate apoptosis index (12.4% ± 2.7%, n=4) (Figure 2). The CPE of non-transgenic mice, both gadd45a+/+ and gadd45a−/−, contained a very low level of IR-induced apoptosis(<1%) (data not shown).
Figure1.
Gadd45a and p21 are not effectors of the p53-dependent apopotic response to oncogenic stress. Brain sections from 3 or more mice of each genotype were analyzed by TUNEL. Average apoptotic indexes (AI) and standard deviations were calculated as described in Materials and Methods. Inactivation of either gadd45a or p21 does not significantly affect p53-dependent apoptosis; deficiency in both effectors causes a small but statistically significant increase of apoptosis, potentially due to relaxation of feedback regulation (P<0.05).
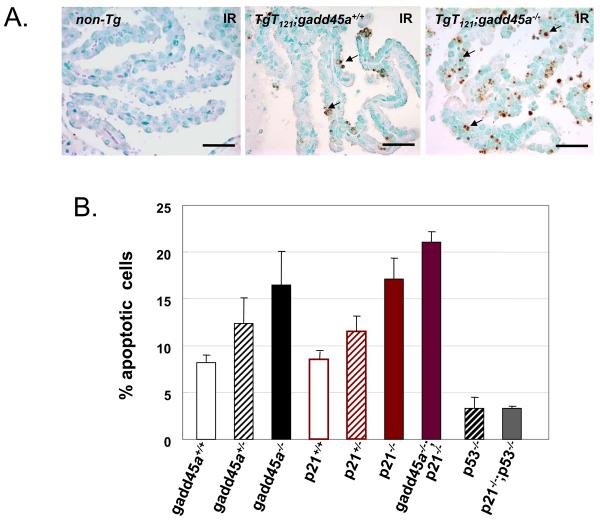
Figure 2.
Gadd45a or p21 deficiency sensitizes brain epithelial cancer cells to ionizing irradiation (IR) in vivo. (A) Apoptosis of CPE tumor cells after IR treatment. 6 to 8 week-old mice were treated with one dose 10 Gy radiation, and euthanized 4.5 hours after IR treatment. Representative apoptotic cells detected by TUNEL are indicated by arrows. TgT121;gadd45a−/− CPE contained significantly more apoptotic cells than the TgT121,gadd45a+/+ CPE. Non-transgenic CPE had a minimal level of apoptosis. Bar = 50μm. (B) Quantitative analysis of apoptosis. All mice were TgT121 positive with indicated genotypes (3 or more mice of each genotype were analyzed as described in Materials and Methods). Gadd45a or p21 deficiency increased the sensitivity to IR-induced apoptosis. Loss of both Gadd45a and p21 increased the effect but not additively. Increased IR-induced apoptosis was p53 dependent as evidenced by its reduction in p53-null backgrounds.
Another p53 downstream cell cycle control gene, p21, also plays an important role in the cellular response to DNA damage signals, eliciting G1 or G2-M cell cycle arrest (19, 22, 23). Thus, we also examined the IR-induced apoptosis in CPE tumors of TgT121;p21−/− mice. Similar to that of TgT121;gadd45a−/− mice, without IR treatment the average CPE tumor cell apoptosis index (AI) of TgT121;p21−/− mice was about the same as that of TgT121;p21+/+ mice. However, with IR treatment the average apoptosis index (AI) in tumors of TgT121;p21−/− mice (17.1% ± 2.3%, n=3) was about two fold greater than that of the TgT121;p21+/+ mice (8.5% ± 0.8%, n=5) (p < 0.05), with an intermediate level of apoptosis in the tumors of TgT121;p21+/− mice (11.5% ± 1.7% ,n=4) (Figure2.). Inactivating both gadd45a and p21 genes caused an even higher level of IR induced apoptosis (21.1% ± 1.1%, n=5) compared to inactivating either gadd45a or p21 alone (Figure2). Although the apoptosis level was significantly increased, there was no significant change in the tumor cell proliferation rates in TgT121;gadd45a−/− and TgT121;p21−/− mice compared with TgT121 control mice as determined by BrdU incorporation (data not shown).
These data indicate that inactivation of p53 downstream cell cycle arrest genes gadd45a or p21 sensitizes epithelial tumor cells to DNA damage in vivo. To determine whether these effects were mediated by p53, we measured the IR induced apoptosis levels of the TgT121;p53−/− and TgT121;p21−/−;p53−/− mice, which were 3.3% + 1.2% (n=4) and 3.3% + 0.2% (n=4) respectively, implying that the increased IR induced cell death in TgT121;p21−/− mice, like the oncogene-induced death was dependent on p53 function (Figure 2).
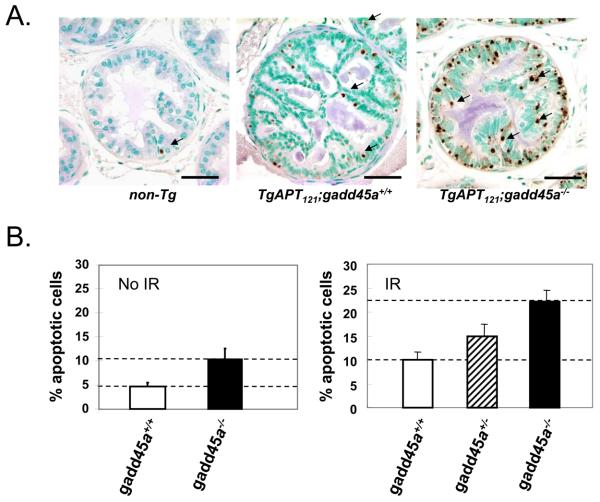
To determine whether IR induced tumor cell death enhancement by gadd45 or p21 deficiency was specific to CPE tumors, or might be more broadly applicable, we examined IR-induced apoptosis in a prostate cancer mouse model TgAPT121. In this model, tumors were initiated by prostate epithelial expression of T121 using the probasin promoter. Aberrant proliferation and abundant apoptosis occurs in prostate luminal epithelial cells, causing the development of mPIN(mouse prostatic intraepithelial neoplasia) and establishing the selective pressure for tumor progression. However, unlike the CPE model and a T121-induced mammary gland tumor model (17, 24) , the apoptosis is not mediated by p53 but rather by Pten (18). TgAPT121 male mice display slow progression to well-differentiated prostate adenocarcinoma (18). We generated TgAPT121;gadd45a+/+, TgAPT121;gadd45a+/− and TgAPT121;gadd45a−/− mice. Male mice at 2 to 3 months of age were treated with one dose of IR (10 Gray; whole body) and prostate apoptosis was measured by TUNEL. Non-transgenic prostate apoptosis was very low (<1%) (Figure 3A). TgAPT121;gadd45a+/+ prostate apoptosis increased to 10.0% ± 1.7%, (n=6; p < 0.05)(Figure 3B), while TgAPT121;gadd45a+/− prostates showed intermediate levels of apoptosis (14.9% ± 2.6%). Once again, gadd45a deficiency caused a high level of apoptosis in response to IR (22.1% ± 2.4%, n=6). Therefore, as in the brain epithelial tumor model, inactivating gadd45a sensitizes prostate cancer cells to IR in vivo. It is worth to note that in the absence of IR, gadd45 deficiency also caused increased apoptosis level without IR.
Figure 3.
Gadd45a deficiency sensitizes prostate tumor cells to ionizing irradiation (IR) in vivo. (A) Apoptosis of mouse prostate cancer cells after IR. Male mice (2-3 mo old) were treated with a single whole body dose (10 Gy) radiation, and euthanized 4.5 hours after the IR. Representative apoptotic cells detected by TUNEL are indicated by arrows. Gadd45a deficiency increased the sensitivity of these cells to IR. (B) Quantitative assessment of data as described in Materials and Methods. Gadd45a deficiency caused about a 2-fold increase in apoptosis in the absence (left panel) or presence of IR treatment (right panel).
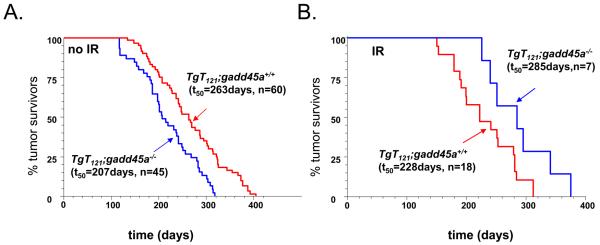
Because apoptosis levels are a critical factor in over all tumor growth rates and animal survival, we further examined the effect of gadd45a inactivation on the survival of IR-treated mice. Brain carcinomas of TgT121 mice develop life-threatening tumors with consistent timing, while prostate adenocarcinomas of TgAPT121 mice do not reproducibly affect survival (18, 25). Therefore, the brain tumor model was utilized for survival studies. In the absence of IR, TgT121;gadd45a−/− mice had a shorter survival time (t50 = 207days, n= 45) than TgT121;gadd45a+/+ mice (t50= 263days, n=60) (Figure 4A.) (P<0.05), indicating a tumor suppression function of gadd45a gene. In the IR treatment study, 2 month old TgT121;gadd45a+/+ and TgT121,gadd45a−/− mice were irradiated by a 137Cs irradiator using a modified clinical protocol. Consistent with an increased apoptosis index, TgT121;gadd45a−/− mice(t50= 285days, n=7) lived significantly longer than TgT121;gadd45a+/+ mice (t50= 228 days, n=18) (p<0.05) (Figure 4B). In addition, the survival time of IR-treated TgT121;gadd45a+/+ mice was shortened by approximately 30 percent after IR treatment compared to untreated mice. Also noteworthy, inactivating both gadd45a and p21 genes increased IR-induced apoptosis more than inactivation of either gene alone (Figure 2B). However, the effect of inactivating gadd45a or p21 was not additive, suggesting either that a maximum detectable level was reached or that there is overlap in IR-mediated DNA damage checkpoints. Interpretation of survival studies in mice with the compound deficiency (Supplementary Figure 1) was confounded by the observation that all TgT121 mice with a p21 deficiency developed severe hydrocephalus independent of IR treatment.
Figure 4.
Gadd45a inactivation extended survival from brain tumors in IR treated TgT121 mice. (A) Survival of TgT121;gadd45a+/+ mice and TgT121;gadd45a−/− mice without IR treatment. Kaplan Meier curves showed a shorter survival of TgT121;gadd45a−/− mice (t50 =207 days, n=45) than TgT121;gadd45a+/+ mice (t50=263 days n=60). (p<0.05, log-rank test). (B) After sub-lethal IR treatment to the head (see Methods), TgT121; gadd45a−/− mice had a better survival (t50=285 days, n=7) than TgT121; gadd45a+/+ mice (t50=223days, n=18) (log rank test, p<0.05).
Discussion
These studies demonstrate that gadd45a inactivation sensitizes both brain and prostate epithelial cancer cells to IR treatment. Tumor progression is slowed, and survival extended in the brain carcinoma mouse model. Interestingly, a previous clinical report showed that gadd45a expression levels correlated with radiotherapy prognosis in a group of cervical cancer patients (26). Patients with relatively low gadd45a expression induction showed better prognosis following radiotherapy than the patients with high gadd45a expression levels (26). Our data provide a possible explanation for this observation. Together, these data suggest that gadd45a may serve as a radiotherapy prognosis indicator and that inactivating gadd45a, possibly through small molecule inhibitors, could be used in conjunction with radiation to improve response to treatment.
Enhanced apoptotic response to IR in the absence of Gadd45a or p21 appears to depend on p53 function. While CPC tumor cell apoptosis was increased after IR treatment in TgT121;p21−/− mice compared to TgT121;p21+/+ mice, the effect was negated upon further deficiency in p53 (Figure 2B). Hence, this combined therapeutic approach is predicted to be effective only for tumors that retain p53 function. Interestingly, in the clinical study mentioned above, tumors of all patients included in the study were genotypically wild type for p53 (26). In the brain tumor system, inactivation of p21 was associated with adverse “side effects”; hydrocephalus was induced with high frequency by an undefined mechanism. However, inactivation of Gadd45a did not cause adverse effects and thus, based on the preclinical studies described here, would constitute a valid target for enhancement of radiation therapy. These observations underscore the need for target validation in specific tumor types using appropriate preclinical models. Finally, in the prostate cancer model Gadd45a inactivation caused increased apoptosis in the absence of IR (Figure 3B), although the oncogene-induced cell death in this tissue is p53-independent (18). This unanticipated result suggests that inhibition of Gadd45a alone in some tumor types may have significant anti-tumor activity. In future experiments, it will be important to test whether gadd45a inactivation-mediated sensitization is also effective in other cancer types, especially in those cancers for which surgery or chemotherapy have only modest effects.
Supplementary Material
Acknowledgements
We thank Li, Lin and Dominic, Moore statistical analyses; Karl.Simin for critical reading of the manuscript and for discussions; P. Anne Wolthusen and Drew Fogarty for animal care and genotyping; UNC Division of Lab Animal Medicine for animal care and the UNC histopathology core facility for tissue processing ; The work was supported by NIH grant 5-RO1CA46283.
References
- 1.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–29. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 4.Smith ML, Ford JM, Hollander MC, et al. p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol. 2000;20:3705–14. doi: 10.1128/mcb.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XW, Zhan Q, Coursen JD, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A. 1999;96:3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin S, Antinore MJ, Lung FD, et al. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275(22):16602–8. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 7.Hollander MC, Philburn RT, Patterson AD, Wyatt MA, Fornace AJ., Jr Genomic instability in Gadd45a−/− cells is coupled with S-phase checkpoint defects. Cell Cycle. 2005;4:704–9. doi: 10.4161/cc.4.5.1675. [DOI] [PubMed] [Google Scholar]
- 8.Hollander MC, Sheikh MS, Bulavin DV, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–84. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 9.Salvador JM, Hollander MC, Nguyen AT, et al. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- 10.Salvador JM, Mittelstadt PR, Belova GI, Fornace AJ, Jr., Ashwell JD. The autoimmune suppressor Gadd45alpha inhibits the T cell alternative p38 activation pathway. Nat Immunol. 2005;6:396–402. doi: 10.1038/ni1176. [DOI] [PubMed] [Google Scholar]
- 11.Harkin DP, Bean JM, Miklos D, et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–86. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 12.Tran H, Brunet A, Grenier JM, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 13.Hildesheim J, Bulavin DV, Anver MR, et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62:7305–15. [PubMed] [Google Scholar]
- 14.Tront JS, Hoffman B, Liebermann DA. Gadd45a suppresses Ras-driven mammary tumorigenesis by activation of c-Jun NH2-terminal kinase and p38 stress signaling resulting in apoptosis and senescence. Cancer Res. 2006;66:8448–54. doi: 10.1158/0008-5472.CAN-06-2013. [DOI] [PubMed] [Google Scholar]
- 15.Tong T, Ji J, Jin S, et al. Gadd45a expression induces Bim dissociation from the cytoskeleton and translocation to mitochondria. Mol Cell Biol. 2005;25:4488–500. doi: 10.1128/MCB.25.11.4488-4500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saenz Robles MT, Symonds H, Chen J, Van Dyke T. Induction versus progression of brain tumor development: differential functions for the pRB- and p53-targeting domains of simian virus 40 T antigen. Mol Cell Biol. 1994;14:2686–98. doi: 10.1128/mcb.14.4.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symonds H, Krall L, Remington L, et al. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994;78:703–11. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 18.Hill R, Song Y, Cardiff RD, Van Dyke T. Heterogeneous tumor evolution initiated by loss of pRb function in a preclinical prostate cancer model. Cancer Res. 2005;65:10243–54. doi: 10.1158/0008-5472.CAN-05-1579. [DOI] [PubMed] [Google Scholar]
- 19.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–7. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 20.Pan H, Yin C, Dyson NJ, Harlow E, Yamasaki L, Van Dyke T. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol Cell. 1998;2:283–92. doi: 10.1016/s1097-2765(00)80273-7. [DOI] [PubMed] [Google Scholar]
- 21.Chen JD, Van Dyke T. Uniform cell-autonomous tumorigenesis of the choroid plexus by papovavirus large T antigens. Mol Cell Biol. 1991;11:5968–76. doi: 10.1128/mcb.11.12.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci U S A. 1997;94:14590–5. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000;14:1584–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Simin K, Wu H, Lu L, et al. pRb inactivation in mammary cells reveals common mechanisms for tumor initiation and progression in divergent epithelia. PLoS Biol. 2004;2:E22. doi: 10.1371/journal.pbio.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Magrane G, Yin C, Louis DN, Gray J, Van Dyke T. Selective inactivation of p53 facilitates mouse epithelial tumor progression without chromosomal instability. Mol Cell Biol. 2001;21:6017–30. doi: 10.1128/MCB.21.17.6017-6030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santucci MA, Barbieri E, Frezza G, et al. Radiation-induced gadd45 expression correlates with clinical response to radiotherapy of cervical carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:411–6. doi: 10.1016/s0360-3016(99)00459-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.