Abstract
It is thought that the growth of uterine leiomyomas may be mediated by the interaction of estrogen receptor alpha (ERα) and growth factor pathways and that phosphorylation of ERα at serine 118 (ERα-phospho-Ser118) is important in this interaction. In this study, immunoblotting and immunohistochemistry were used to investigate the expression of ERα-phospho-Ser118, phosphorylated p44/42 mitogen-activated protein kinase (phospho-p44/42 MAPK), and proliferating cell nuclear antigen (PCNA) in human leiomyoma and myometrial tissues during the proliferative and secretory phases of the menstrual cycle. We found that tumors taken from the proliferative phase expressed significantly higher levels of ERα-phospho-Ser118, phospho-p44/42 MAPK, and PCNA compared to patient-matched myometria and had significantly higher ERα-phospho-Ser118 and PCNA expression compared to secretory phase tumors. Also, enhanced colocalization and association of phospho-p44/42 MAPK and ERα-phospho-Ser118 were observed in proliferative phase tumors by confocal microscopy and immunoprecipitation, respectively. These data suggest that ERα-phospho-Ser118 may be important in leiomyoma growth and is possibly phosphorylated by phospho-p44/42 MAPK.
Keywords: Uterine leiomyoma, Estrogen receptor alpha phosphorylated serine 118, Phosphorylated mitogen-activated protein kinase
Introduction
Uterine leiomyomas (fibroids; myomas) are one of the most common benign gynecologic tumors in women of reproductive age. These tumors represent a significant public health problem, since they are responsible for approximately 200,000 hysterectomies per year and are the leading cause of hysterectomy in the United States [2, 15, 39]. Fibroids have also been associated with clinical disease with moderate to high frequency in women in Europe [30], Africa [14], and Japan [33].
Uterine leiomyomas tend to grow during the reproductive years, but regress after menopause, suggesting that these tumors are hormonally regulated [10]. The ovarian steroid hormones, estrogen and progesterone, are believed to play an important role in the growth of uterine leiomyomas [10, 28, 31]; however, there is increasing evidence that sex steroids are not the only modulators of leiomyoma growth [28, 35]. Studies have shown that circulating steroid hormone levels are similar in women that have or do not have leiomyomas, indicating that specific local, tissue-regulated factors maybe involved in the pathogenesis of these tumors [5]. The heterogeneity of leiomyoma growth within a given uterus, despite identical exposure of these tumors to similar circulating sex steroid concentrations, suggests the involvement of local cytokines and/or growth factors [13, 28].
Estrogen exerts its physiological effects by binding to estrogen receptor alpha and beta (ERα and β). In addition to the traditional ERα activation (hormone binding), this receptor can be activated by growth factor signaling pathways via phosphorylation of the ERα at specific serines [11, 25]. In human breast cancer cells, it has been shown that in the presence or absence of estradiol binding, human ERα is predominately phosphorylated on Ser-118 and to a lesser extent on Ser-104 and Ser-106 [4, 7, 25]. Phosphorylation of ERα influences its activity and may lead to ERα-mediated transcription. To our knowledge, there are no studies that have reported the role of phosphorylation of ERα at Ser-118 in human or rodent uterine leiomyomas.
Progesterone has also been shown to be involved in fibroid growth, having both inhibitory and stimulatory effects on growth [8, 9, 26, 40]. Evidence shows that progesterone can induce leiomyoma cell proliferation and up-regulate the growth factor, epidermal growth factor [34]. Progesterone has also been shown to down-regulate insulin-like growth factor-I messenger RNA and protein expression in cultured leiomyoma cells [42]. There is also considerable biological evidence for “crosstalk” between the estrogen and progesterone hormone receptor signaling pathways [23]. In many cases, progestins suppress the stimulatory effects of estrogens in target tissues, although estrogen up-regulates the progesterone receptor [19, 24]. In a study using tissue from women with endometrial hyperplasia, which is also a hormonally regulated disease, ERα protein expression was down-regulated after use of the progestins, levonorgestrel, and medroxyprogesterone [38]. Progesterone has been shown to have a negative effect on ERα expression [16, 24], and ERα expression has been reported to be higher in myometrial and leiomyoma tissue from women in the proliferative versus secretory phase of the menstrual cycle based on immunohistochemisty and western blotting. The mechanisms involved in the “crosstalk” between the progesterone and estrogen hormone receptor pathways and how they interact with growth factor receptor pathways have not been fully elucidated in uterine leiomyomas.
Studies have indicated that hormones and growth factor signaling pathways are mediators of leiomyoma growth [28, 35, 37]. In uterine leiomyoma cell cultures, the mitogen-activated protein kinase (MAPK) pathway interacts with the estrogen system via the ER [1]. Constitutively activated MAPK (phospho-p44/42 MAPK) is highly expressed in leiomyoma and myometrial tissues and is increased in leiomyomas compared to normal myometrial tissues [6]. A study has indicated that MAPK activity increases in leiomyoma cells after treatment with estradiol and that an indirect interaction between the ER and growth factor pathways exists, because uterine leiomyoma cells treated with estradiol have increased secretion of platelet-derived growth factor and activation of the MAPK pathway [1]. In breast cancer cells, phosphorylation of ERα at Ser-118 occurs in response to activation of MAPK (phospho-p44/42) [1, 18, 25]. To date, there are no reports of whether ERα is phosphorylated in fibroids. Furthermore, if present, the molecular mechanism(s) by which phosphorylation occurs remains to be understood.
In this study, we evaluated the expression of ERα-phospho-Ser118, phospho-p44/42 MAPK, and proliferating cell nuclear antigen (PCNA) in uterine leiomyomas and patient-matched myometrial tissue and determined if there was differential expression during the menstrual cycle phases. We also examined colocalization and the interaction of ERα-phospho-Ser118 and phospho-p44/42 MAPK in tumors versus myometrial tissue.
Materials and methods
Patients
Uterine leiomyomas (n=26) and unaffected patient-matched myometrial tissue (n=16) samples were taken from 16 premenopausal women (six for western blot analysis×one tumor each and ten for immunohistochemistry×two tumors each) who underwent hysterectomy for symptomatic fibroids. The women had not taken hormonal medication at least 3 months prior to surgery. Informed consent was obtained, and the Institutional Review Board of the NIEHS NIH approved the study. All uterine leiomyoma and unaffected myometrial samples were confirmed by histological evaluation. The menstrual cycle phases were determined as either proliferative or secretory based on endometrial histology (case 11) or menstrual cycle history (cases 1–10 and 12–16) for each patient (Table 1). All patients had multiple fibroids that were typical in histomorphology and located in the intramural (60%), subserosal (25%), or submucosal (15%) regions of the myometrium. Tumor sizes were classified as <2 cm (n=10) or ≥2 cm (n=10) (Table 1), and the age of the patients ranged from 41 to 47 years, with a mean age of 43.5 years.
Table 1.
Tumor size and menstrual cycle phase of premenopausal women with fibroids
| Case # | Menstrual cycle phase | Tumor ID | Tumor size (cm) |
|---|---|---|---|
| 1 | Proliferative | 1a | <2 |
| 1b | ≥2 | ||
| 2 | Proliferative | 2a | <2 |
| 2b | ≥2 | ||
| 3 | Proliferative | 3a | <2 |
| 3b | ≥2 | ||
| 4 | Proliferative | 4a | <2 |
| 4b | ≥2 | ||
| 5 | Proliferative | 5a | <2 |
| 5b | ≥2 | ||
| 6 | Secretory | 6a | <2 |
| 6b | ≥2 | ||
| 7 | Secretory | 7a | <2 |
| 7b | ≥2 | ||
| 8 | Secretory | 8a | <2 |
| 8b | ≥2 | ||
| 9 | Secretory | 9a | <2 |
| 9b | ≥2 | ||
| 10 | Secretory | 10a | <2 |
| 10b | ≥2 | ||
| 11 | Proliferative | 11a | ≥2 |
| 12 | Proliferative | 12a | ≥2 |
| 13 | Proliferative | 13a | ≥2 |
| 14 | Secretory | 14a | ≥2 |
| 15 | Secretory | 15a | ≥2 |
| 16 | Secretory | 16a | ≥2 |
Western blotting
Western blotting was performed to detect ERα-phospho-Ser118 expression in the leiomyoma and patient-matched myometrial tissue lysates, which were taken from six women in the proliferative (three) and secretory (three) phases of the menstrual cycle. Aliquots of 30 μg of protein were electrophoresed on a 4–12% Bis–Tris Gel (Invitrogen, Carlsbad, CA, USA) under reducing conditions as previously described [37]. The proteins were electrotransferred onto 0.45 μm polyvinylidene fluoride membranes (Immobilon-P, Millipore, Bedford, MA, USA). Blots were incubated with a mouse monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA) against ERα-phospho-Ser118 diluted at 1:500. Antibody binding was detected with horseradish peroxidase-conjugated anti-mouse (Amersham Biosciences, Arlington Heights, IL, USA) diluted at 1:2,000. Antigen-antibody complexes were detected with the ECL western blot detection system (Amersham Biosciences). A densitometer (Fluor Chem™8900, Alpha Innotech, San Landro, CA, USA) was used for quantitation of ERα-phospho-Ser118 band densities.
Immunohistochemistry
ERα-phospho-Ser118, ERα, and phospho-p44/42 MAPK expression
Uterine leiomyoma (20) and myometrial (10) tissue samples were fixed overnight in 10% neutral-buffered formalin. Tissues were then processed through a graded series of alcohols, embedded in paraffin, sectioned at 6 μm, and mounted onto charged glass slides (ProbeOn Plus, Fisher Scientific, Pittsburgh, PA, USA). Tissues were deparaffinized, and endogenous peroxidase activation was blocked using 3% H2O2 for 15 min at room temperature. Antigen retrieval was performed by microwaving for 10 min (ERα and ERα-phospho-Ser118 staining) or by incubation for 20 min in a decloaker (phospho-p44/42 MAPK staining). Tissues were blocked using normal horse or goat serum at room temperature for 1 h. The tissues used for ERα and ERα-phospho-Ser118 were additionally blocked using an Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA, USA). Tissues were incubated with phospho-estrogen receptor α (Ser-118) monoclonal antibody (1:30 dilution), estrogen receptor monoclonal antibody (1:25 dilution; Immunotech Beckman Coulter, Fullerton, CA, USA), or phospho-p44/42 MAPK polyclonal rabbit IgG (1:50 dilution; Cell Signaling Technology) overnight at 4°C. Negative controls consisted of normal mouse or rabbit serum at a concentration the same as the respective primary antibody. The tissues were incubated in the secondary antibody (horse anti-mouse or goat anti-rabbit; Elite Vectastain ABC Kit, Vector Laboratories) for 30 min at room temperature. Immunoreactive complexes were detected by avidin–biotin affinity system (Elite Vectastain ABC Kit, Vector Laboratories) and visualized using 3,3′-diaminobenzidine tetrahydrachloride substrate chromogen system (Dako Cytometry, Carpinteria, CA, USA). Tissues were counterstained with Mayer's hematoxylin (Poly Scientific, Bay Shore, NY, USA), dehydrated, coverslipped, and observed by light microscopy.
A semiquantitative method described by Detre et al. [12], which incorporates the overall percent of positive staining and intensity of immunostaining, was used for assessing ERα-phospho-Ser118, ERα, and phospho-p44/42 MAPK protein expression in leiomyoma and myometrial tissue samples. Slides were evaluated blindly with a light microscope and a ×20 objective. Quickscores were assigned independently by two scorers and then averaged to obtain a mean quickscore. Sections were scanned at ×40 using an Aperio ScanScopeXT model (Aperio Technologies, Vista, CA, USA).
Proliferating cell nuclear antigen (PCNA) labeling
For PCNA staining, the deparaffinization and rehydration were done similarly to the immunohistochemical procedures described above. After inactivation of endogenous peroxidases, antigen retrieval was performed by microwaving the samples for 10 min in dH20. Samples were blocked using 0.5% milk for 20 min and then a primary antibody, monoclonal mouse anti-PCNA IgM (1:500 dilution; Chemicon, Temecula, CA, USA), was placed on the tissues for 1 h at room temperature. After rinsing, the tissues were incubated with a secondary antibody (Biotin-SP-conjugated AffiniPure Goat anti-mouse IgM, μ chain specific; Jackson Immunoresearch, West Grove, PA, USA) for 30 min at room temperature. Tissues were labeled with supersensitive conjugated streptavidin peroxidase (BioGenex, San Ramon, CA, USA). Immunoreactive complexes were visualized, and tissues were counterstained and coverslipped using the procedures mentioned in the previous section.
The percentage of PCNA labeling in the tissue samples was later determined using a light microscope, ×40 objective and an ocular grid. Approximately eight to 12 high power fields per tissue section were counted to reach a total cell count of 1,000 cells in uterine leiomyoma and myometrial tissues. Nuclei that stained intensely brown were counted as positive. Percent PCNA labeling was determined by the number of cells having positively stained nuclei divided by 1,000 cells (labeled and unlabeled) and multiplied by 100. A mean percent PCNA labeling was determined by averaging the numbers from the independent scorers.
Immunoflourescence
Frozen samples of uterine leiomyoma and patient-matched myometrial tissue were sectioned at 5 μm, thawed for 15 min at room temperature, and fixed in 4% paraformaldehyde at 4°C for 10 min followed by −20°C methanol for 20 min. The sections were then blocked for 1 h in 5% milk, 1% BSA, and 1.5% normal goat serum (Vectastain Kit) in 1× automation buffer. Tissues were incubated in primary antibodies for both ERα-phospho-Ser118 and phospho-p44/42 MAPK together overnight (4°C) at dilutions as stated above in the “Immunohistochemistry” section. The sections were then incubated with Alexa Fluor 488 goat anti-mouse IgG (green fluorescence) and Alexa Fluor 594 goat anti-rabbit IgG (red fluorescence; Molecular Probes, Carlsbad, CA, USA) for 45 min at room temperature in the dark. Tissues were counterstained using 4′, 6-diamidino-2-phenylindole (DAPI; Molecular Probes, Carlsbad, CA, USA) in the dark for 30 min at room temperature. Sections were coverslipped with aqueous anti-fade fluorescent mounting medium (Vector Laboratories). The tissue sections were observed with a laser scanning confocal microscope (LSM 510 UV mounted on Axiovert 100M microscope, Carl Zeiss) and analyzed using LSM Image Examiner v3.2 software.
Immunoprecipitation
To assess the association of ERα-phospho-Ser118 and phospho-p44/42 MAPK in myometrial and leiomyoma tissues, phospho-p44/42 MAPK was immunoprecipitated and immunoblotted for ERα-phospho-Ser118 and phospho-p44/42 MAPK (as a control). Aliquots of 500 μg of leiomyoma and myometrial tissue lysates were cleared by adding 50 μL of Protein A-Sepharose beads (Zymed Laboratories, San Francisco, CA, USA) and incubated for 30 min at 4°C with gentle rotation. The protein lysates were immunoprecipitated with 5 μg of phospho-p44/42 MAPK polyclonal rabbit antibody (Cell Signal Technology) overnight at 4°C with gentle rotation. Protein A-Sepharose beads (50 μL) were added to each tube, the mixtures were incubated overnight at 4°C, and the immune complexes were collected by centrifugation. The beads were washed with RIPA buffer (10 mL of 50 mM Tris HCL pH 7.4 with 150 mM NaCL, 1 mM EGTA, 1 mM NaF, and 1% triton X 100, 250 μL of 10% sodium deoxycholate, 50 μL of 200 mM activated sodium vanadate, 50 μL of 10 mM sodium molybdate, 20 μL of aprotinin and leupeptin, and 20 μL of 2 μg/mL of A-PMSF), and the supernatant was discarded. The sepharose beads were resuspended in 30 μL of Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) and then centrifuged. Amounts of 15 μL of the supernatant were used for western blotting as previously described (mouse monoclonal ERα-phospho-Ser118 antibody at 1:500 dilution and rabbit polyclonal phospho-p44/42 MAPK antibody at 1:1000 dilution (Cell Signaling Technology)). Antibody binding was detected with horseradish peroxidase-conjugated anti-mouse and anti-rabbit (Amersham Biosciences) diluted at 1:2000. Antigen-antibody complexes were detected with an ECL western blot detection system (Amersham Biosciences).
Statistics
Statistical comparisons were performed for mean of quickscores and intensities for western blot bands of uterine leiomyoma and patient-matched myometrial samples. Significant differences were determined using Wilcoxon signed ranks tests (p≤0.05) and Mann–Whitney tests (p≤0.05).
Results
ERα-phospho-Ser118 expression by western blot and densitometry
Western blot analysis showed significantly increased expression of ERα-phospho-Ser118 in uterine leiomyomas compared to myometrial samples from the proliferative phase (Fig. 1a and b). The overall mean expression of ERα-phospho-Ser118 was slightly higher in uterine leiomyomas from the secretory phase compared to myometrial samples, although this difference was not significant (Fig. 1a and b). Also, ERα-phospho-Ser118 expression was higher in both tumors and myometrial samples from the proliferative phase compared to those from the secretory phase (Fig. 1a and b).
Fig. 1.
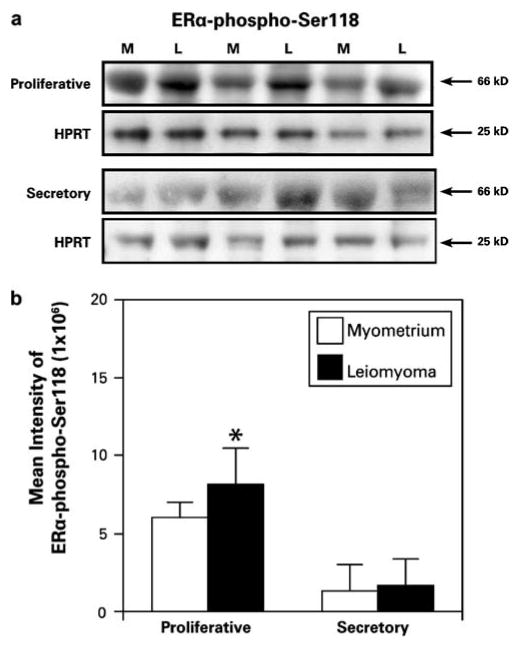
Immunoblot of ERα-phospho-Ser118 and HPRT (loading control) in uterine leiomyoma (L) and patient-matched myometrial (M) tissue lysates from women (n=6) in the proliferative or secretory phase of the menstrual cycle. a ERα-phospho-Ser118 is expressed in leiomyoma and myometrial tissue. b The bar graph represents the mean±SE intensity of western blot bands for ERα-phospho-Ser118 protein. Increased expression of ERα-phospho-Ser118 was observed in the leiomyomas compared to the myometrial tissue samples from the proliferative phase of the menstrual cycle. *p≤0.05; a significant difference versus myometrial samples (n=3)
ERα phospho-Ser118 and ERα expression by immunohistochemistry
Proliferative phase
Immunohistochemistry confirmed that leiomyoma samples from the proliferative phase had increased protein expression of ERα-phospho-Ser118 compared to matched myometrial tissue (Fig. 2). ERα-phospho-Ser118 was expressed in the nuclei of myometrial and leiomyoma cells (Fig. 2a and b). Also in this phase, ERα expression was increased in the tumors compared to myometrial tissue and was expressed predominantly in the nuclei of myometrial and leiomyoma cells with minimal staining in the cytoplasm (Fig. 2c and d). Scores of immunostaining intensity showed significant differences in ERα-phospho-Ser118 expression in myometrial versus leiomyoma samples with mean quickscores of 2.4±1.0 and 8.0±1.3, respectively (Fig. 2e). Mean quickscores for ERα immunoexpression in myometrial and leiomyoma samples were 7.7±2.0 and 16.6±0.6, respectively (Fig. 2e).
Fig. 2.
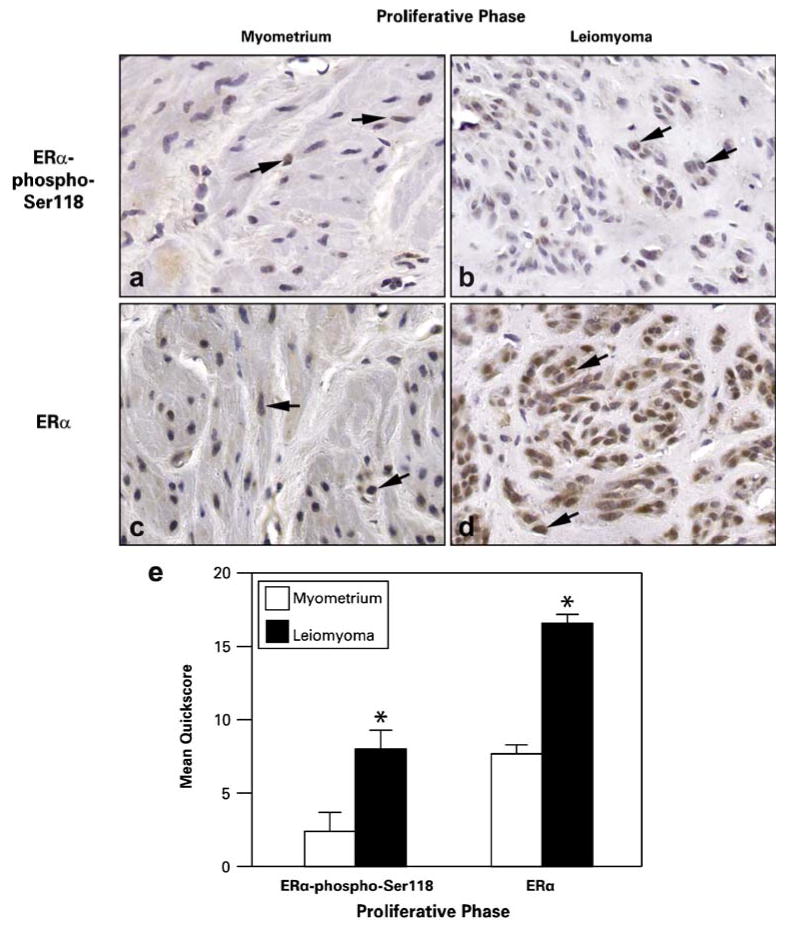
Representative immunohistochemical staining of ERα-phospho-Ser118 and ERα in patient-matched myometrium and uterine leiomyoma tissue samples from women in the proliferative phase of the menstrual cycle. a and b ERα-phospho-Ser118 expression in myometrium (a) and leiomyoma (b) tissue samples. c and d ERα expression in myometrium (c) and leiomyoma (d) tissue samples. Arrows show positively stained nuclei of cells. e The bar graph represents the mean±SE of quickscore values for ERα-phospho-Ser118 and ERα in myometrial tissue and tumors. *p≤0.05; a significant difference versus myometrial samples (n=10 for myometrial samples and n=20 for leiomyoma samples; original magnification, ×40)
Secretory phase
Both leiomyoma and myometrial tissues from the secretory phase showed expression of ERα-phospho-Ser118 (Fig. 3); however, ERα-phospho-Ser118 expression was significantly lower in the myometrium (0.1±0.1) compared to leiomyoma (3.6±0.5) tissues (Fig. 3a and b). ERα expression was also significantly lower in myometrial tissue compared to leiomyomas (myometrium=6.4±1.3; leiomyoma=12.7± 2.2; Fig. 3e).
Fig. 3.
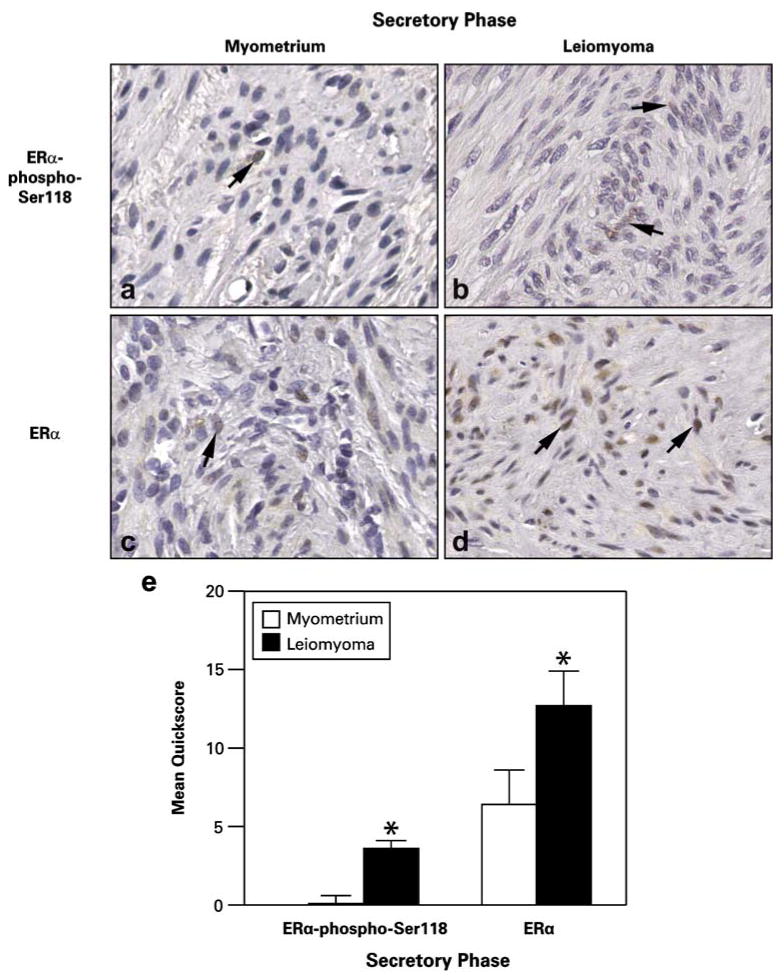
Representative immunohistochemical staining of ERα-phospho-Ser118 and ERα in patient-matched myometrium and uterine leiomyoma tissue samples from women in the secretory phase of the menstrual cycle. a and b ERα-phospho-Ser118 expression in myometrium (a) and leiomyoma (b) tissue. c and d ERα expression in myometrium (c) and leiomyoma (d) tissue samples. Arrows show positively stained nuclei of cells. e The bar graph represents the mean±SE of quickscore values for ERα-phospho-Ser118 and ERα in myometrial tissue and tumors. *p≤0.05; a significant difference versus myometrial samples (n=10 for myometrial samples and n=20 for leiomyoma samples; original magnification, ×40)
When ERα-phospho-Ser118 expression in leiomyomas from the secretory phase was compared to those from the proliferative phase, we found that ERα-phospho-Ser118 was expressed at significantly higher levels in the leiomyomas from the proliferative (8.0±1.3) versus secretory (3.6±0.5) phase (Figs. 2e and 3e). Also, no significant differences in ERα and ERα-phospho-Ser118 immunoexpression were observed between small leiomyomas (<2 cm) and large leiomyomas (≥2 cm) when tumors from both phases were combined or evaluated independently by phase (data not shown).
Expression of phospho-p44/42 MAPK by immunohistochemistry
Due to increased expression of ERα-phospho-Ser118 in leiomyomas, we evaluated phosphorylated p44/42 MAPK (phospho-p44/42 MAPK) expression in leiomyomas and myometrial tissue samples from the same ten patients used for the ERα and ERα-phospho-Ser118 studies (Fig. 4). Overall, phospho-p44/42 MAPK was expressed in the nuclei of myometrial and leiomyoma cells (Fig. 4a and b). In the proliferative phase, phospho-p44/42 MAPK was expressed at significantly higher levels in leiomyomas (9.2± 2.4) compared to myometrial (4.8±2.3) tissues (Fig. 4c). No significant difference was observed in the expression of phospho-p44/42 MAPK in myometrial (4.4±1.4) and leiomyoma (4.4±0.9) samples from women in the secretory phase. Although phospho-p44/42 MAPK was higher in leiomyomas from the proliferative phase, when mean expression data were compared between the two phases, there was no statistical difference in expression. Interestingly, a significant difference (p≤0.05) in phospho-p44/42 MAPK immunoexpression was observed between myometrial samples and small leiomyomas (≤2 cm) from the proliferative phase (4.8±2.3 and 11±3.1, respectively).
Fig. 4.
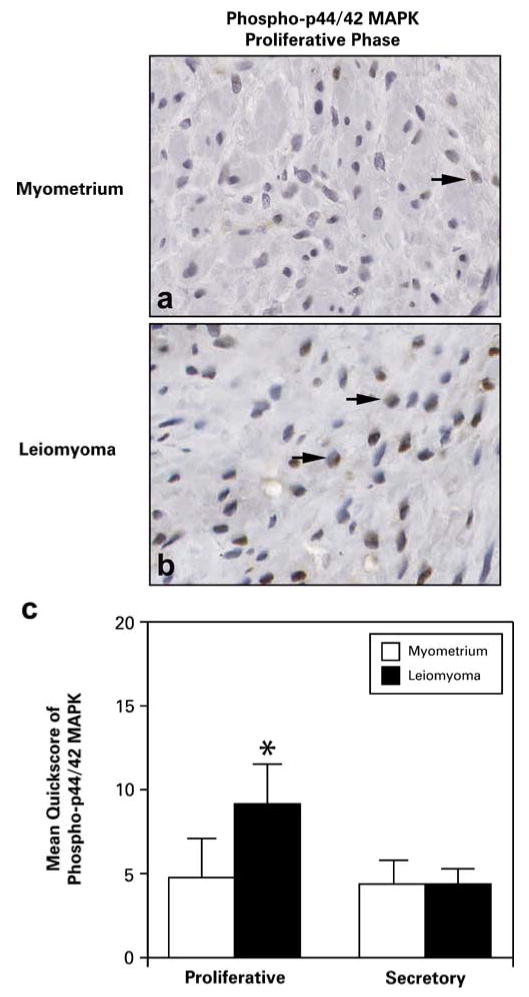
Representative immunohistochemical staining of phospho-p44/42 MAPK in patient-matched myometrial and uterine leiomyoma tissues from the proliferative phase of the menstrual cycle. a and b Phospho-p44/42 MAPK expression was expressed in the nuclei (arrows) of myometrial (a) and tumor (b) smooth muscle cells, although it was significantly increased in tumors from the proliferative phase. c The bar graph represents the mean±SE of quickscore values for phospho-p44/42 MAPK in myometrial tissue and leiomyomas from the proliferative and secretory phases of the menstrual cycle. *p≤0.05; a significant difference versus myometrial samples in the proliferative phase (n=10 for myometrial samples and n=20 for leiomyoma samples; original magnification, ×40)
Colocalization of ERα-phospho-Ser118 and phospho-p44/42 MAPK
To determine if significantly increased levels of ERα-phospho-Ser118 and phospho-p44/42 MAPK observed in the tumors from the proliferative phase were colocalized, we conducted immunoflourescence and confocal microscopy studies to evaluate the localization of ERα-phospho-Ser118 (Fig. 5a and b) and phospho-p44/42 MAPK (Fig. 5c and d) in tumor and myometrial tissues from the proliferative phase. Both ERα-phospho-Ser118 and phospho-p44/42 MAPK were present in the nuclei of myometrial and leiomyoma tissue samples (Fig. 5a,b,c,d). Colocalization was more apparent in the nuclei of leiomyoma cells compared to myometrial cells in tissue samples (Fig. 5e and f).
Fig. 5.
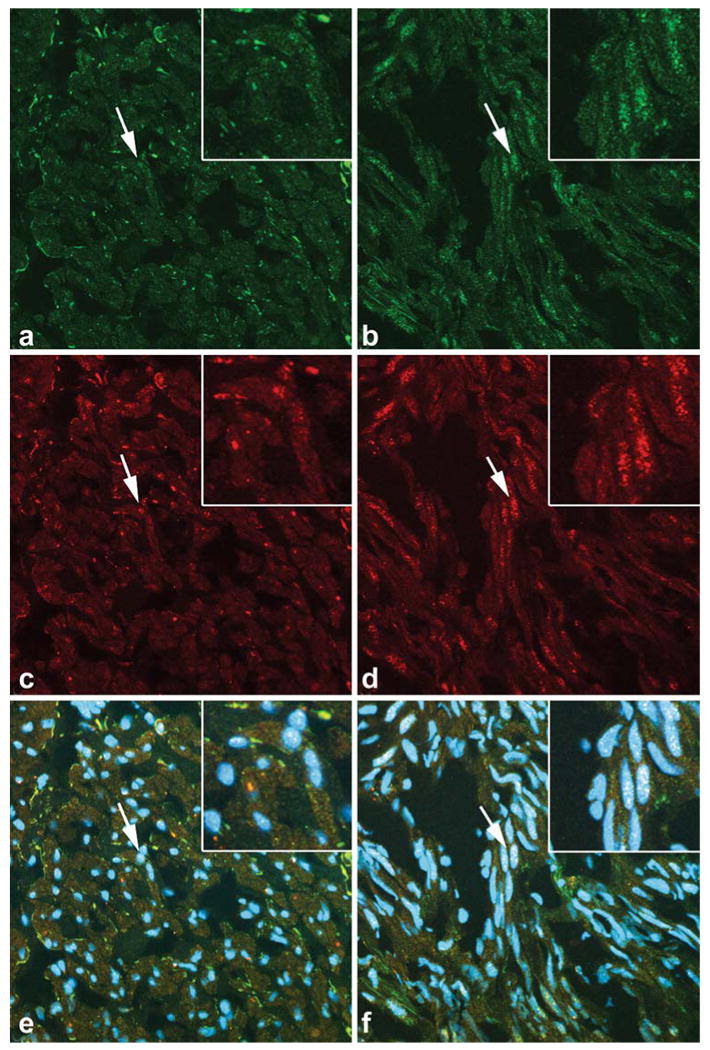
Colocalization of ERα-phospho-Ser118 and phospho-p44/42 MAPK in myometrium and uterine leiomyoma tissue. Arrows show nuclei in the inset (top right corner) that are positively stained for ERα-phospho-Ser118 (green fluorescence; a and b), phospho-p44/42 MAPK (red fluorescence; c and d), and both ERα-phospho-Ser118 and phospho-p44/42 MAPK (white/yellow fluorescence; e and f) in myometrial (a, c, and e) and leiomyoma (b, d, and f) tissues. DAPI was used to stain the nuclei (blue fluorescence; e and f; original magnification, ×40/zoom of 1)
Immunoprecipitation of ERα-phospho-Ser118 and phospho-p44/42 MAPK
To show the interaction of ER-phospho-Ser118 and phospho-p44/42 MAPK in myometrial and leiomyoma tissues from the proliferative phase, phospho-p44/42 MAPK was immunoprecipitated from myometrial and leiomyoma tissues and then immunoblotted for ER-phospho-Ser118 and phospho-p44/42 MAPK (control; Fig. 6). There was an increased association of ERα-phospho-Ser118 and phospho-p44/42 MAPK in leiomyomas compared to myometrial tissue.
Fig. 6.
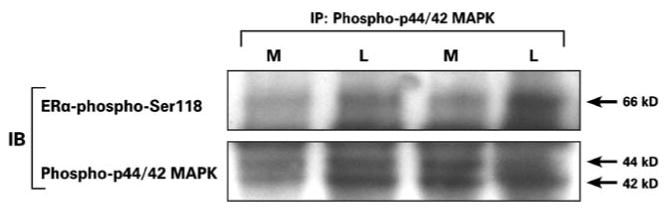
Immunoprecipitation of ERα-phospho-Ser118 and phospho-p44/42 MAPK in myometrial (M) and uterine leiomyoma (L) tissue lysates from the proliferative phase. Phospho-p44/42 MAPK was immunoprecipitated (IP) from leiomyoma and myometrial tissue and then immunoblotted (IB) with the anti-ERα-phospho-Ser118 (66 kDa) and phospho-p44/42 MAPK (44 and 42 kDa; control) antibodies
Proliferating cell nuclear antigen (PCNA) labeling
Based on our findings of increased ERα-phospho-Ser118 expression in leiomyomas and the abundant association and colocalization of phospho-p44/42 MAPK and ERα-phospho-Ser118 in tumors during the proliferative phase, we conducted PCNA labeling studies to assess whether there would be differential expression of this cell proliferation marker in tumors versus myometrial samples from the proliferative and secretory phases. PCNA was highly expressed in tumors compared to myometrial tissue in both the proliferative and secretory phases with more expression in the proliferative phase tumors compared to those from the secretory phase (Fig. 7a–d). We found that the mean PCNA labeling indices were significantly higher in tumors versus myometrial tissue in both the proliferative and secretory phases of the menstrual cycle (Fig. 7e). Also, PCNA labeling in tumors from women in the proliferative phase was significantly higher than in tumors from the secretory phase (28.60±3.20 and 18.39±3.20, respectively). No significant differences were observed in the PCNA labeling in small leiomyomas (<2 cm) compared to large (≥2 cm) leiomyomas when combined or evaluated independently by phase, although in both phases, the mean labeling indices were higher in the smaller leiomyomas.
Fig. 7.
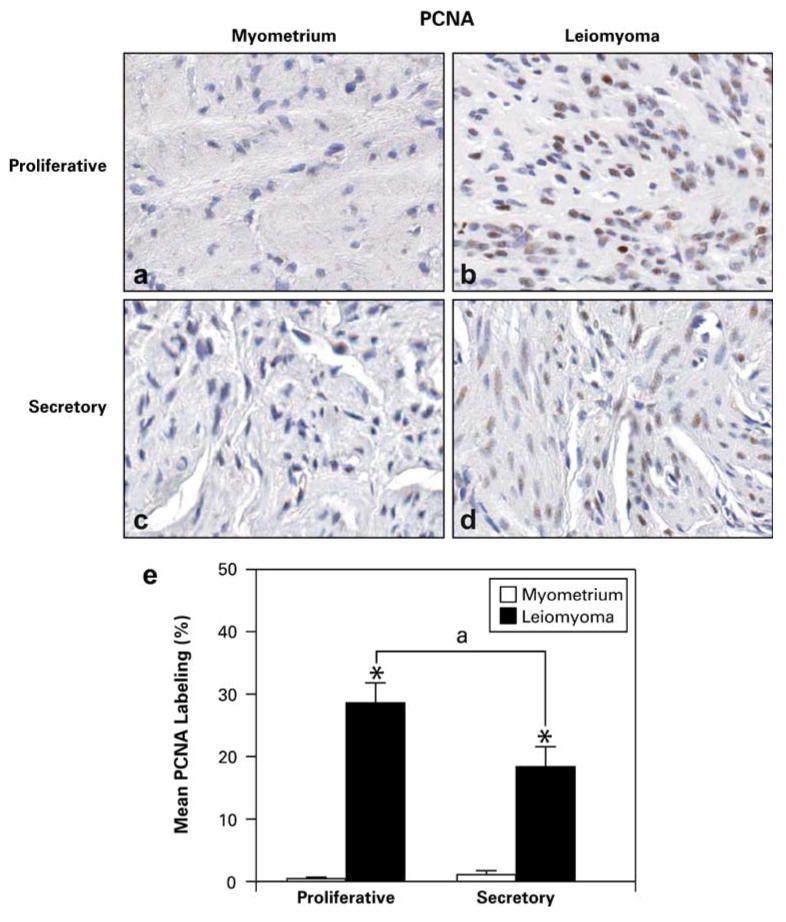
Representative PCNA immunohistochemical staining in patient-matched myometrium and uterine leiomyoma tissue samples from the proliferative (a and b) and secretory (c and d) phases (original magnification, ×40). e Bar graph represents the mean±SE of percent of cells labeled with PCNA in myometrial (white bar) and leiomyoma (black bar) tissues. *p≤0.05; a significant difference versus myometrial tissue, and “a” represents significance (p≤0.05) between leiomyomas of the proliferative versus secretory phase of the menstrual cycle (n=10 for myometrial samples and n=20 for leiomyoma samples)
Discussion
To our knowledge, this is the first study to evaluate ERα-phospho-Ser118 expression in uterine leiomyomas. These data show that ERα is a phosphoprotein that is phosphorylated at serine 118 residues in uterine leiomyomas. Phosphorylation of ERα at serine 118 has been extensively studied in breast cancer cells and is enhanced in response to estradiol binding and through the action of second messenger signaling pathways [25]. Previous in vitro and in vivo studies have demonstrated that ERα is phosphorylated at serine 118 in the presence or absence of estradiol and by growth factor peptides and their receptors through activation of the MAPK (ERK 1/2) pathway, which can lead to transcription [7, 11]. ERα from calf uterus is phosphorylated at serine residues in response to estradiol [11]. Also, phosphorylation of mouse uterine ERα at serine residues is enhanced in response to estrogen [41].
In our study, ERα-phospho-Ser118 immunoexpression was significantly higher in tumors from women in the proliferative phase of the menstrual cycle compared to those in the secretory phase, although expression was present in tumors and significantly higher than the myometrium in samples from both the secretory and proliferative phases. Increased phosphorylation of ERα at serine 118 observed in tumors from the proliferative (estrogen dominant) phase may indicate that phosphorylation may be regulated by higher concentrations of estrogen and lower concentrations of progesterone. There is evidence suggesting that estradiol can increase the expression of ERα-phospho-Ser118 in breast cancer cells [7]. Estrogen has also been shown to increase uterine leiomyoma cell proliferation and progesterone receptor mRNA and protein expression [1, 16, 19], whereas progesterone has been shown to have a dual effect on the growth of uterine leiomyomas by decreasing and increasing the size of some fibroids through regulation of the local growth factor milieu in women treated with the progestin, levonorgestrel [27]. In our study, the phosphorylation of ERα was lower in the secretory phase where progesterone is the dominant hormone. Also, other studies comparing estrogen receptor protein expression in leiomyomas from women in the proliferative and secretory phases have shown that ER was expressed at lower levels during the secretory phase. This supports the concept that progesterone may be decreasing the levels of ERα, thereby making less receptor available for phosphorylation [3, 22, 43].
In this study, we used immunohistochemistry and found nuclear expression of phospho-p44/42 MAPK to be significantly increased in leiomyomas compared to myometrial tissue from the proliferative phase of the cycle. Chegini et al. have demonstrated that phospho-p44/42 MAPK is expressed more in leiomyomas compared to myometrial tissue, but this difference was not significant, and no menstrual phase data were reported in their study. In our study, there was no significant difference in phospho-p44/42 MAPK expression in tumors in the secretory phase compared to patient-matched myometrial samples, and when tumors from both phases of the cycle were combined, this resulted in a lack of significance of expression of phospho-p44/42 MAPK in leiomyoma versus myometrial tissue. This is most likely due to significant phospho-p44/42 MAPK expression being phase specific and could be missed if data from the two phases are combined.
In breast cancer cells, phospho-p44/42 MAPK has been found to phosphorylate the ER at serine 118 in response to estrogen [7]. In this study, we found higher expression and colocalization of phospho-p44/42 MAPK and ERα-phospho-Ser118 in leiomyomas from the proliferative phase, which is the phase of the cycle where the estradiol/progesterone ratio is high. These data imply that in leiomyomas, phospho-p44/42 MAPK may be the kinase that phosphorylates the ER at serine 118, and this phosphorylation may be enhanced during the proliferative phase by higher concentrations of estradiol and lower concentrations of progesterone.
Previously, we found PCNA expression to be significantly higher in leiomyomas than in matched myometrial samples [13]. In this study, PCNA expression was also significantly higher in leiomyomas than in matched myometrial samples. On average, PCNA labeling was 0.77% in the myometrium and 23.50% in leiomyomas. PCNA labeling of cells in tumors from women in the proliferative phase (28.60%) was significantly higher compared to tumors from women of the secretory phase (18.39%). These data show that leiomyomas proliferate at a higher rate than normal myometrium and that proliferation is increased in tumors from the proliferative phase compared to those from the secretory phase of the menstrual cycle. The PCNA labeling indices in this study were higher compared to the values from a previous study, which was most likely due to the increased sensitivity of the primary antibody used in this study compared to our earlier studies. More importantly, however, the overall trend of increased PCNA labeling in leiomyomas versus myometria and increased labeling of proliferative phase tumors versus secretory phase are in agreement with our earlier findings and others, respectively [13, 20]. Also, although PCNA labeling was increased overall in the leiomyomas, there was variation of PCNA expression between tumors from individual women, which supports the importance of hormones in up- or down-regulating local cytokines and growth factors that in turn may control autonomous tumor growth. In this study, it appears that phosphorylation of ER at serine 118 is a predominant feature of the proliferative phase, and this may be an important factor in increased transcription, translation, and proliferation observed in uterine leiomyomas during this phase.
No significant differences in ERα-phospho-Ser118, ERα, and PCNA immunoexpression were observed between small leiomyomas and large leiomyomas when tumors from both phases were combined or evaluated independently by phase, which may be due to the abundant amount of extracellular matrix proteins that is involved in the expansion of fibroids [36]. Many uterine leiomyomas contain an abundant amount of fibrous connective tissue and extracellular matrix proteins (collagen, proteoglycans, and fibronectin) [36]. ERα-phospho-Ser118, ERα, and PCNA are expressed predominantly in the nuclei of smooth muscle tumor cells, which may not be the main contributor to size in larger leiomyomas containing an abundance of extracellular matrix proteins.
In summary, the mechanisms whereby ER and growth factor signaling pathways interact and promote leiomyoma growth are not known and have not been widely studied. In this study, we found that ERα-phospho-Ser118 and phospho-p44/42 MAPK protein expression levels were significantly increased independently and highly coexpressed in leiomyomas from women in the proliferative phase of the menstrual cycle compared to myometrial tissue samples. In addition, leiomyomas from the proliferative phase had significantly increased PCNA labeling compared to patient-matched myometrial tissue samples and tumors from the secretory phase.
Our data suggest that there are interactions between ER and growth factor signaling pathways in uterine leiomyomas. Also, these data show that ERα may be phosphorylated by phospho-p44/42 MAPK at Ser-118 and possibly play an important role in the growth of fibroids. Additionally, the expression of ERα-phospho-Ser118 may be, in part, regulated by high amounts of estrogen and low amounts of progesterone. To provide further evidence that ERα may be phosphorylated by phospho-p44/42 MAPK at Ser-118 in fibroids, immunoprecipitation assays were done and showed an increased association of ERα-phospho-Ser118 and phospho-p44/42 MAPK proteins in leiomyomas compared to myometrial tissue. The pathways involved in phosphorylation of ERα in uterine leiomyomas have not been studied. In Fig. 8, we propose a possible mechanism of interaction between the ERα and growth factor signaling pathways and the regulation of these pathways by estradiol (E2) and progesterone (P4), which is highly supported by the data reported in this paper. This schematic shows that activation of ERα via phosphorylation at serine 118 by phospho-p44/42 MAPK is regulated by E2 (positively) and P4 (positively or negatively) through the up- or down-regulation of local cytokines, growth factors, and growth factor receptors [17, 21, 29, 32]. Future studies are underway to test this hypothesis.
Fig. 8.
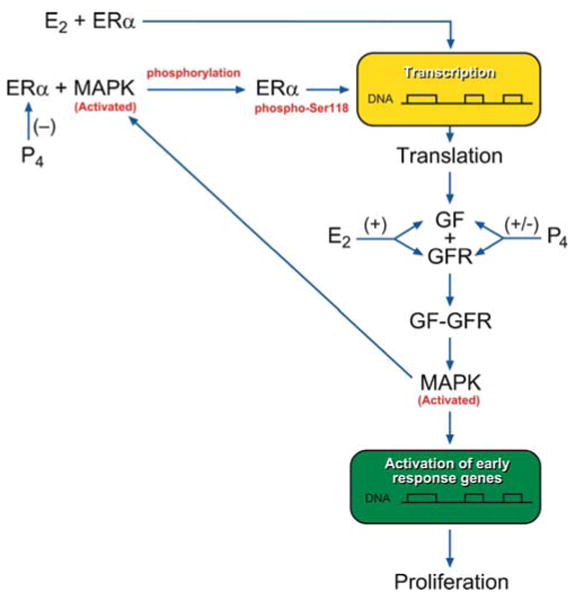
Proposed pathway for the interaction between ERα and growth factor (GF) signaling pathways and regulation by estradiol (E2) and progesterone (P4). The ERα is activated by classical E2 binding and/or by phosphorylation at serine 118 by activated MAPK. Activated ERα then activates gene transcription and translation of a GF. The GF will bind to its receptor (GFR), and the activated GFR will in turn activate the MAPK pathway, which can result in proliferation and also possible phosphorylation of ERα at serine 118. E2 can up-regulate GFs and GFRs with an increase in downstream MAPK activation and possible ERα-phospho-Ser118 phosphorylation, whereas P4 can decrease or increase ERα, GF, and GFR production, which in turn results in up- or down-regulation of activated MAPK and ERα-phospho-Ser118 expression
Acknowledgments
The authors would like to thank Norris Flagler, Elizabeth Ney, Paul Cacioppo, and C. Jeffrey Tucker for their technical assistance with imaging. This research was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Contributor Information
Tonia L. Hermon, Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences (NIEHS), National Toxicology Program (NTP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), P.O. Box 12233, MD C2-09, Research Triangle Park, NC 27709, USA Department of Physiological Sciences, Division of Biochemistry, Eastern Virginia Medical School, Norfolk, VA 23507, USA.
Alicia B. Moore, Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences (NIEHS), National Toxicology Program (NTP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), P.O. Box 12233, MD C2-09, Research Triangle Park, NC 27709, USA
Linda Yu, Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences (NIEHS), National Toxicology Program (NTP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), P.O. Box 12233, MD C2-09, Research Triangle Park, NC 27709, USA.
Grace E. Kissling, Biostatistics Branch, National Institute of Environmental Health Sciences (NIEHS), National Toxicology Program (NTP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), Research Triangle Park, NC 27709, USA
Frank J. Castora, Department of Physiological Sciences, Division of Biochemistry, Eastern Virginia Medical School, Norfolk, VA 23507, USA
Darlene Dixon, Cellular and Molecular Pathology Branch, National Institute of Environmental Health Sciences (NIEHS), National Toxicology Program (NTP), National Institutes of Health (NIH), Department of Health and Human Services (DHHS), P.O. Box 12233, MD C2-09, Research Triangle Park, NC 27709, USA, e-mail: dixon@niehs.nih.gov.
References
- 1.Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186(3):414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Beinfeld MT, Bosch JL, Isaacson KB, Gazelle GS. Cost-effectiveness of uterine artery embolization and hysterectomy for uterine fibroids. Radiology. 2004;230(1):207–213. doi: 10.1148/radiol.2301021482. [DOI] [PubMed] [Google Scholar]
- 3.Bourlev V, Pavlovitch S, Stygar D, Volkov N, Lindblom B, Olovsson M. Different proliferative and apoptotic activity in peripheral versus central parts of human uterine leiomyomas. Gynecol Obstet Invest. 2003;55(4):199–204. doi: 10.1159/000072074. [DOI] [PubMed] [Google Scholar]
- 4.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. Embo J. 1996;15(9):2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 5.Buttram VC., Jr Uterine leiomyomata—aetiology, symptomatology and management. Prog Clin Biol Res. 1986;225:275–296. [PubMed] [Google Scholar]
- 6.Chegini N, Kornberg L. Gonadotropin releasing hormone analogue therapy alters signal transduction pathways involving mitogen-activated protein and focal adhesion kinases in leiomyoma. J Soc Gynecol Investig. 2003;10(1):21–26. [PubMed] [Google Scholar]
- 7.Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly JM, Coombes RC, Ali S. Phosphorylation of human estrogen receptor alpha at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21(32):4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 8.Chwalisz K, DeManno D, Garg R, Larsen L, Mattia-Goldberg C, Stickler T. Therapeutic potential for the selective progesterone receptor modulator asoprisnil in the treatment of leiomyomata. Semin Reprod Med. 2004;22(2):113–119. doi: 10.1055/s-2004-828617. [DOI] [PubMed] [Google Scholar]
- 9.Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26(3):423–438. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]
- 10.Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med. 2004;22(2):105–111. doi: 10.1055/s-2004-828616. [DOI] [PubMed] [Google Scholar]
- 11.Denton RR, Koszewski NJ, Notides AC. Estrogen receptor phosphorylation. Hormonal dependence and consequence on specific DNA binding. J Biol Chem. 1992;267(11):7263–7268. [PubMed] [Google Scholar]
- 12.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon D, Flake GP, Moore AB, He H, Haseman JK, Risinger JI, Lancaster JM, Berchuck A, Barrett JC, Robboy SJ. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Arch. 2002;441(1):53–62. doi: 10.1007/s00428-001-0568-7. [DOI] [PubMed] [Google Scholar]
- 14.Emembolu JO. Uterine fibromyomata: presentation and management in northern Nigeria. Int J Gynaecol Obstet. 1987;25(5):413–416. doi: 10.1016/0020-7292(87)90349-3. [DOI] [PubMed] [Google Scholar]
- 15.Farquhar CM, Steiner CA, Sozen I, Arici A. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99(2):229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 16.Hodges LC, Houston KD, Hunter DS, Fuchs-Young R, Zhang Z, Wineker RC, Walker CL. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol. 2002;196(1–2):11–20. doi: 10.1016/s0303-7207(02)00230-7. [DOI] [PubMed] [Google Scholar]
- 17.Jasonni VM, La Marca A, Santini D. Progestin effects on epidermal growth factor receptor (EGFR) endometrial expression in normal and hyperplastic endometrium. Int J Gynaecol Obstet. 2005;89(3):297–298. doi: 10.1016/j.ijgo.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 19.Katzenellenbogen BS. Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig. 2000;7(1 Suppl):S33–S37. doi: 10.1016/s1071-5576(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 20.Kayisli UA, Berkkanoglu M, Kizilay G, Senturk L, Arici A. Expression of proliferative and preapoptotic molecules in human myometrium and leiomyoma throughout the menstrual cycle. Reprod Sci. 2007;14(7):678–686. doi: 10.1177/1933719107305866. [DOI] [PubMed] [Google Scholar]
- 21.Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, Ryu HS, Min CK. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Hum Reprod. 2005;11(11):801–808. doi: 10.1093/molehr/gah240. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs KA, Oszter A, Gocze PM, Kornyei JL, Szabo I. Comparative analysis of cyclin D1 and oestrogen receptor (alpha and beta) levels in human leiomyoma and adjacent myometrium. Mol Hum Reprod. 2001;7(11):1085–1091. doi: 10.1093/molehr/7.11.1085. [DOI] [PubMed] [Google Scholar]
- 23.Kraus WL, Katzenellenbogen BS. Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology. 1993;132(6):2371–2379. doi: 10.1210/endo.132.6.8504742. [DOI] [PubMed] [Google Scholar]
- 24.Kraus WL, Weis KE, Katzenellenbogen BS. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol Cell Biol. 1995;15(4):1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68(1):1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 26.Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33(1):59–67. doi: 10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Maruo T, Ohara N, Matsuo H, Xu Q, Chen W, Sitruk-Ware R, Johansson ED. Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception. 2007;75(6 Suppl):S99–103. doi: 10.1016/j.contraception.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10(3):207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Dechelotte PJ, Mage G. Both GnRH agonist and continuous oral progestin treatments reduce the expression of the tyrosine kinase receptor B and mu-opioid receptor in deep infiltrating endometriosis. Hum Reprod. 2007;22(1):124–128. doi: 10.1093/humrep/del368. [DOI] [PubMed] [Google Scholar]
- 30.Parazzini F. Risk factors for clinically diagnosed uterine fibroids in women around menopause. Maturitas. 2006;55(2):174–179. doi: 10.1016/j.maturitas.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Rein M. Biology of uterine myomas and myometrium in vitro. In: Barbieri RL, editor. Seminars in reproductive endocrinology. Thieme; New York: 1992. pp. 310–319. [Google Scholar]
- 32.Reis FM, Lhullier C, Edelweiss MI, Spritzer PM. In vivo assessment of the regulation of transforming growth factor alpha, epidermal growth factor (EGF), and EGF receptor in the human endometrium by medroxyprogesterone acetate. J Assist Reprod Genet. 2005;22(1):19–24. doi: 10.1007/s10815-005-0816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato F, Mori M, Nishi M, Kudo R, Miyake H. Familial aggregation of uterine myomas in Japanese women. J Epidemiol. 2002;12(3):249–253. doi: 10.2188/jea.12.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83(6):2192–2198. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- 35.Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78(1):1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 36.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79(3):900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 37.Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11(6):441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- 38.Vereide AB, Kaino T, Sager G, Arnes M, Orbo A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol. 2006;101(2):214–223. doi: 10.1016/j.ygyno.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Vollenhoven B. Introduction: the epidemiology of uterine leiomyomas. Baillieres Clin Obstet Gynaecol. 1998;12(2):169–176. doi: 10.1016/s0950-3552(98)80059-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, DeManno DA, Chwalisz K, Maruo T. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- 41.Washburn T, Hocutt A, Brautigan DL, Korach KS. Uterine estrogen receptor in vivo: phosphorylation of nuclear specific forms on serine residues. Mol Endocrinol. 1991;5(2):235–242. doi: 10.1210/mend-5-2-235. [DOI] [PubMed] [Google Scholar]
- 42.Yamada T, Nakago S, Kurachi O, Wang J, Takekida S, Matsuo H, Maruo T. Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod. 2004;19(4):815–821. doi: 10.1093/humrep/deh146. [DOI] [PubMed] [Google Scholar]
- 43.Zaslawski R, Surowiak P, Dziegiel P, Pretnik L, Zabel M. Analysis of the expression of estrogen and progesterone receptors, and of PCNA and Ki67 proliferation antigens, in uterine myomata cells in relation to the phase of the menstrual cycle. Med Sci Monit. 2001;7(5):908–913. [PubMed] [Google Scholar]


