Abstract
Objective
Euthymic bipolar disorder (BD) youths have a deficit in face emotion labeling that is present across multiple emotions. Recent research indicates that youths at familial risk for BD, but without a history of mood disorder, also have a deficit in face emotion labeling, suggesting that such impairments may be an endophenotype for BD. It is unclear if this deficit in at-risk youths is present across all emotions or if the impairment presents initially as an emotion-specific dysfunction that then generalizes to other emotions as the symptoms of BD become manifest.
Method
37 patients with pediatric BD, 25 unaffected children with a first-degree relative with BD, and 36 typically developing youths were administered the Emotional Expression Multimorph Task, a computerized behavioral task which presents gradations of facial emotions from 100% neutrality to 100% emotional expression (happiness, surprise, fear, sadness, anger, disgust).
Results
Repeated measures analysis of covariance revealed that, compared to healthy youths, patients and at-risk youths required significantly more intense emotional information to identify and correctly label face emotions. Patients with BD and at-risk youths did not differ from each other. Group-by-emotion interactions were not significant, indicating that the group effects did not differ based on the facial emotion.
Conclusions
Youths at risk for BD demonstrate non-specific deficits in face emotion recognition, similar to patients with the illness. Further research is needed to determine if such deficits meet all criteria for an endophenotype.
Introduction
Despite evidence that bipolar disorder (BD) is highly heritable, 1–5 the exact genetic profile remains unknown. Endophenotypes are biological or neuropsychological markers intermediate between clinical phenotype and genotype. 6, 7 The identification of risk-related genes for complex illnesses such as BD, which involve intricate modes of transmission, 8–10 could be aided by the identification of endophenotypes.
Youths with BD have deficits in face emotion labeling, 11–14 which might serve as an endophenotype for BD. This possibility is suggested by data finding face emotion labeling deficits to be 1) heritable in at least some populations, 15, 16 2) state-independent in BD, 13, 14 and 3) relatively unique to BD compared to childhood depression, anxiety, and behavioral disorders. 17 Most significantly, non-affected youths at risk for BD by virtue of having a first degree relative with the illness, but who themselves have no personal history of mood disorder, appear to have deficits in face emotion labeling similar to those seen in bipolar probands. 18
An outstanding question is whether face emotion processing deficits in at-risk youths, like those in pediatric BD patients, are present across all emotions. 14 Alternatively, face emotion labeling abnormalities may present initially as a specific deficit in labeling a narrow set of emotions, which then generalizes to others over time. The purpose of this study is to examine whether face emotion labeling deficits in youths with a first-degree relative with BD are specific to certain emotions, or whether they are present across all emotions. To that end, we used a task designed to detect deficits in labeling happiness, sadness, anger, fear, disgust, and surprise. Prior work in BD 13, 14 demonstrates a generalized impairment in face emotion labeling across these emotions. Thus, we a priori hypothesized that youths at risk for BD would similarly demonstrate deficits in face emotion processing across all emotions presented.
Method
Subjects included pediatric patients with BD (N=37), at-risk youths (N=25), and typically developing children (N=36). All participants, ages 7–18 years, were enrolled in an Institutional Review Board-approved study at the National Institute of Mental Health (NIMH). Parents and youths gave written informed consent/assent. None of the participants were biologically related. Pediatric bipolar patients were recruited through advertisements to support groups and psychiatrists.
At-risk youths were included if they had a parent and/or sibling in an NIMH IRB-approved study, in which a semi-structured interview confirmed a diagnosis of DSM-IV-TR bipolar disorder (BDI or BDII). Parental BD diagnosis was determined using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P) 19 or the Diagnostic Interview for Genetic Studies (DIGS). 20 Pediatric BD probands, at-risk youths, and controls were clinically assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL). 21 Interviewers were masters’ or doctoral level clinicians with excellent interrater reliability (kappa>0.9).
BD patients met criteria for “narrow phenotype” BD, with at least one full duration hypomanic or manic episode characterized by abnormally elevated mood and at least three “B” mania symptoms. 22 At-risk subjects with anxiety disorders or attention deficit hyperactivity disorder (ADHD) were included in order to avoid studying an unusually psychopathologically resilient group. At-risk youths with current or past mood disorders were excluded since BD can manifest first as depression. Healthy children were drawn from the community, had no lifetime psychiatric diagnoses, as determined by a K-SADS-PL interview with parent and child, and no first-degree relatives with a mood disorder. Psychopathology in first-degree relatives of controls was assessed via a telephone screening interview with a masters’ or doctoral level clinician.
Exclusion criteria for all subjects were: IQ<70, history of head trauma, neurological disorder, pervasive developmental disorder, unstable medical illness, or substance abuse/dependence. At-risk and healthy control youths were medication-free. Medicated patients with BD were included.
The Wechsler Abbreviated Scale of Intelligence (WASI) 23 was administered to determine IQ. To evaluate mood state in patients and at-risk youths, clinicians with inter-rater reliability (kappa>0.9) administered the Children’s Depression Rating Scale (CDRS), 24 and the Young Mania Rating Scale (YMRS). 25
Subjects performed the computerized Emotional Expression Multimorph Task. 14, 26 The face stimuli were taken from the empirically valid and reliable Pictures of Facial Affect Series. 27 During this task, subjects viewed a virtual series of neutral faces, each of which morphed 39 times until it reached 100% intensity (Figure 1). Subjects were told that the emotional expression would begin as neutral, but would slowly change to reveal one of the six emotions: happiness, surprise, fear, sadness, anger, or disgust. Subjects were asked to press the “stop” button on the computer as soon as they were able to identify the facial expression. This stopped the morphing image and the subjects were asked to identify one of the six emotional expressions listed on the screen. Upon selecting the emotion, the face would continue to morph through the remaining iterations. Subjects were told that they could change their emotional identification response at any time. When the face reached the final morph iteration (i.e., iteration #39, or “Morph #1”, the full emotional expression), subjects were asked to provide a final emotional identification response.
Figure 1.
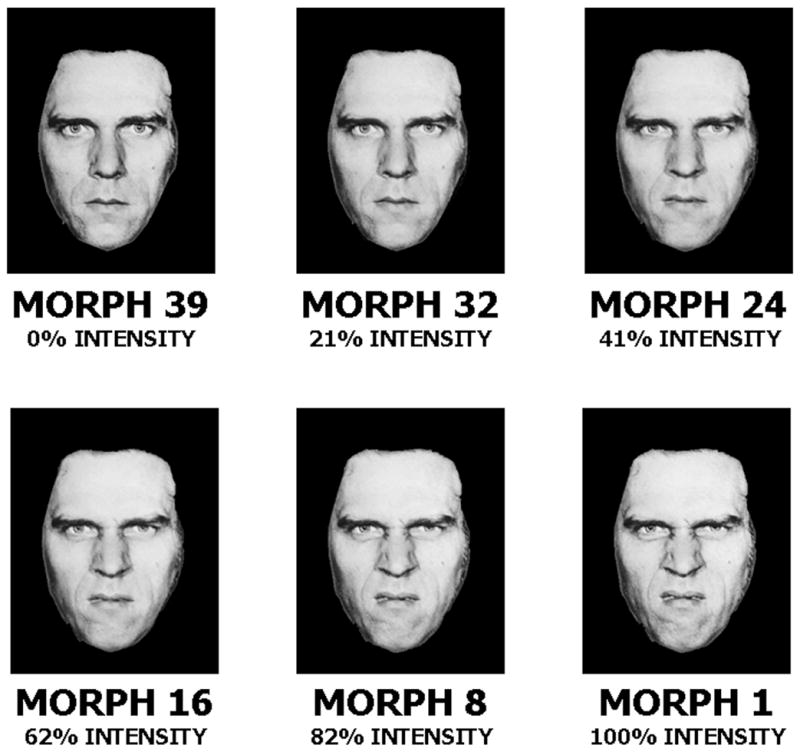
Gradations of Multimorph Emotional
Expression Examples of disgusted facial expressions across the 39 increment stages from 0% intensity (i.e. neutral) to 100% intensity (i.e. prototypical emotional expression). Reprinted from Rich et al. 14 with permission from Development and Psychopathology.
The response point along the 1–39 continua at which the subject stops the morphing process indicates the degree of facial intensity before the subject attempted to identify the emotion, with higher response points indicating better performance. There were two main dependent variables: (1) number of morphs before the subject’s first response (regardless of accuracy), and (2) number of morphs before the subject’s first correct response.
Data Analysis
Analyses of variance (ANOVA) assessed group differences in age and IQ and a chi-square determined sex differences. Age differed significantly (p<.01) and IQ differed at a trend level (p=.09) among the groups (Table 1). Therefore, for primary analyses, repeated measures analyses of covariance (ANCOVA) were performed, with group as the between-group factor, and age and IQ as covariates.
Table 1.
Demographic Characteristics and Performance in Bipolar Disorder (BD), At-risk, and Typically Developing Youths
| BD (N=37) | At-risk (N=25) | Typically Developing (N=36) | Analysis | P-value | |
|---|---|---|---|---|---|
| Age, years: Mean ± SD | 14.16 ± 2.92 | 12.15 ± 3.05 | 14.34 ± 2.28 | F=5.47 | <.0.1 |
| Range | 8.84–18.77 | 7.01–17.56 | 9.47–18.72 | ||
| Sex (male): N (%) | 17/37 (45.9) | 18/25 (72.0) | 20/36 (55.6) | X2=4.12 | .13 |
| IQ: mean ± standard deviation | 108.41 ± 14.05 | 113.83 ± 12.15 | 115.61 ± 14.21 | F=2.51 | .09 |
| Mood State: N (%) | |||||
| Euthymic | 17/37 (45.9) | 25/25 (100) | -- | -- | -- |
| Depressed | 5/37 (13.5) | 0 | -- | -- | -- |
| Hypomanic/manic/mixed | 15/37 (40.5) | 0 | -- | -- | -- |
| Any Axis I Diagnosis1: N (%) | 37/37 (100) | 7/25 (28.0) | 0 | -- | -- |
| Bipolar Disorder I | 33/37 (89.2) | 0 | -- | -- | -- |
| Any Anxiety Disorder | 15/37 (40.5) | 6/25 (24.0) | -- | -- | -- |
| Generalized Anxiety Disorder | 10/37 (27.0) | 2/25 (8.0) | -- | -- | -- |
| Separation Anxiety Disorder | 6/37 (16.2) | 4/25 (16.0) | -- | -- | -- |
| Social Phobia | 4/37 (10.8) | 2/25 (8.0) | -- | -- | -- |
| Attention Deficit Hyperactivity Disorder | 15/37 (40.5) | 3/25 (12.0) | -- | -- | -- |
| Oppositional Defiant Disorder | 9/37 (24.3) | 0 | -- | -- | -- |
| Conduct Disorder | 0 | 1/25 (4.0) | -- | -- | -- |
| Medicated: N (%) | 28/37 (75.7) | 0 | 0 | ||
| Anticonvulsants: N (%) | 21/28 (75.0) | -- | -- | -- | -- |
| Atypical Antipsychotics: N (%) | 20/28 (71.4) | -- | -- | -- | -- |
| Lithium: N (%) | 13/28 (46.4) | -- | -- | -- | -- |
| Antidepressants: N (%) | 11/28 (39.3) | -- | -- | -- | -- |
| Stimulants: N (%) | 10/28 (35.7) | -- | -- | -- | -- |
| Anxiolytics: N (%) | 3/28 (10.7) | -- | -- | -- | -- |
| Performance on Emotional Expression Multimorph Task: mean ± standard error2 | |||||
| Number of morphs before first response: | 12.65 ± .73 | 13.38 ± .92 | 16.00 ± .74 | F=5.53 | .005 |
| Number of morphs before first correct response | 10.97 ± .64 | 12.12 ± .84 | 14.22 ± .64 | F=6.44 | .002 |
Axis I diagnoses not mutually exclusive.
Analysis covaried for age and IQ. Higher number of morphs indicates less intensity of facial expression needed before identification and therefore better performance.
Post-hoc ANCOVAs, with age included as a covariate, compared at-risk children without an Axis I diagnosis and controls on the number of morphs before first response and number of morphs before first correct response. IQ was not included as a covariate in these analyses because it did not differ between these two groups (t=.40, p=.69). Cohen’s d effect sizes were calculated for task performance differences between controls and the entire at-risk sample (N=25), and between controls and the subset of at-risk youths without a diagnosis (N=18). Additional analyses in BD patients employed Pearson’s correlations and t-tests to examine relationships between performance and mood ratings, and between medication status and performance.
Results
ANOVA revealed significant group differences for age (p<.01); at-risk youths were significantly younger than both patients (p<.01) and typically developing youths (p<.01), who did not differ from each other (p=.78). IQ differed among the groups at a trend level (p=.09). Patients had a significantly lower IQ than typically developing youths (p=.03), but did not differ significantly from at-risk youths (p=.15). Typically developing and at-risk children’s IQ scores did not differ from each other (p=.64). Sex did not significantly differ across groups.
Table 1 presents demographic and clinical data. Among patients with BD, 45.9% were euthymic (i.e., CDRS<40 and YMRS≤12). Most (75.7%, N=28) BD patients were medicated; the mean number of medications was 3.0±1.2. All at-risk youths were euthymic and medication free.
Of the 25 at-risk youths, 11 had a parent and 14 had a sibling with BD. 72.7% (N=8) of the BD parents met criteria for BDI. Comorbidities in the adult probands included: anxiety disorders (27.3%), substance abuse/dependence (18.1%), and ADHD (9.1%). The remaining children had a sibling proband, all whom met criteria for BDI. Comorbidities in the pediatric BD youths were high and included: an anxiety disorder (78.6%), ADHD (42.9%), and ODD (28.6%).
Multimorph Results
Pearson correlations revealed that IQ was related to task performance across the entire group for first response (r=.19, p=.05) and at a trend level for first correct response (r=.18, p=.09). When these correlations were examined within each group, BD and control children did not show a significant relationship between number of morphs required and IQ (all p’s>.44). However, at-risk youths demonstrated a significant correlation between first response and IQ (r=.52, p=.01) and between first correct response and IQ (r=.56, p<.01).
Repeated measures ANCOVA, with age and IQ included as covariates, revealed a significant main effect of group for the first response point [F(2, 92)=5.53, p≤.01], with both patients (p≤.01) and at-risk youths (p≤.03) requiring higher emotional intensity before responding than healthy controls. Similarly, repeated measures ANCOVA for first correct response point revealed a significant main effect of group [F(2,88)=6.44, p≤.01). Compared to healthy youths, both patients (p≤.01) and at-risk (p≤.05) groups, required higher emotional intensity before correctly identifying the emotion being displayed. The performance of at-risk youths and patients did not differ (p’s>.28). For both analyses, the group-by-emotion interaction was not significant (for number of morphs, p=.56; for number of morphs until correct, p=.16), indicating that face emotion type did not moderate group differences (Table 1).
We used an ANCOVA, with age as a covariate, to compare the subset of at-risk youths without an Axis I diagnosis (N=18) to controls. At-risk children without diagnoses required significantly higher emotional intensity before first responding [F(1, 51)=5.65, p=.02] and before first correct response [F(1, 49)=5.86, p=.02], suggesting that Axis I pathology in the at-risk group does not account for the deficits observed.
For controls versus the entire group of at-risk youths (N=25), Cohen’s d=.76 and d=.75, respectively, for overall number of morphs and number of morphs until first correct. When the subset of at-risk youths (N=18) without an Axis I diagnosis was compared to controls, the effect size decreased to d=.54 and d=.53, respectively.
In BD patients, there was no relationship between scores on the YMRS or CDRS and performance on the morph task (all p’s>.33). When the analyses were repeated including only euthymic patients, patients required significantly higher emotional intensity than healthy controls on both the number of morphs before first response (t=−2.18, p=.03) and the number of morphs until correct (t=−2.13, p=.04), suggesting that poorer performance was not due to mood state. There was also no correlation between the number of medications and performance on the task (all p’s>.63). Among medicated patients, separate t-tests for each medication (e.g., anticonvulsant vs. no anticonvulsant) demonstrated no relationship between any medication and task performance (all p’s>.11).
Discussion
Similar to patients with BD, 11–13, 17, 28 youths at risk for the illness by virtue of having a parent and/or sibling with the diagnosis display a generalized deficit in facial emotion recognition. Specifically, relative to typically developing youths, both bipolar patients and youths at risk for BD required significantly greater intensity of emotional expression before first responding, and before correctly identifying the facial expression. In both bipolar and at-risk youths, these deficits were present across all emotions. The data presented here extend previous research in at-risk youths using a different face emotion identification paradigm, 18 and demonstrate that the face emotion identification deficit in at-risk youths is present across all emotions.
Are deficits in face emotion processing an endophenotype of BD? Evidence suggests that this deficit meets at least 3 of the 5 criteria, 6 including: association with illness in the population, 12, 17 state-independence, 13, 28 and presence in nonaffected family members. 18 Studies are necessary to determine whether impairments in facial emotion processing satisfy the remaining two criteria for an endophenotype of BD. 6 The heritability of face emotion processing needs further investigation, particularly in families with BD, and longitudinal studies are needed to assess if emotion identification deficits are more common in at-risk youths who ultimately develop BD, compared to those who do not. Finally, the neural correlates of these deficits should be explored, with a particular emphasis on examining amygdala hyperactivity. 29 If deficits in face emotion identification prove to be an endophenotype for BD, this knowledge could ultimately aid in efforts aimed at identifying risk-related genes for the illness, as well as in prevention and early intervention.
There are clinical implications for this work as well. BD youths are socially impaired. 30, 31 Building on this work, Rich et al. 28 found that impairment on the face emotion task used in this study is associated with psychosocial impairments in BD patients. Complementing studies which examine social functioning in at-risk youths 32 should investigate the role of face emotion labeling impairments as a potential mediator of the functional deficits observed in youths at risk for BD.
There are limitations to this work. First, the samples were relatively small. Second, the criteria for narrow phenotype BD is more stringent than DSM-IV-TR BD, making our findings less generalizable. However, it is important to note that in the Course and Outcome of Bipolar Youth (COBY) study, 86.4% of BDI youths demonstrated elated or expansive mood. 33 This suggests that our narrow phenotype criteria exclude less than 15% of the BDI population.
Third, the Ekman faces used in this paradigm were not developed for use in pediatric population. Fourth, it is possible that the increased number of morphs needed for BD and at-risk youths is due an overall slower performance or conservative response bias, as opposed to an emotion identification dysfunction. Prior work using this same task has shown deficits in euthymic BD youths. 28 Moreover, using a different task not designed to detect more subtle emotion specific impairments, Brotman et al. 18 demonstrated deficits in these groups. Nonetheless, additional studies are needed that use alternative designs, such as signal detection tasks.
Fifth, most BD patients were medicated. However, using two face emotion identification paradigms, Schenkel et al. 13 found that medication tended to diminish behavioral differences between BD youths and controls. In fact, for some emotions medicated youths did not differ from controls, whereas unmedicated youths did. Moreover, in this study, BD patients were in various mood states. It is important to note, however, that the findings remained when euthymic BD patients were compared to controls, consistent with prior work showing face emotion labeling deficits in euthymic BD youths. 12, 13, 18
Sixth, we excluded at-risk youths with a history of a mood disorder because a depressive episode can be the first presentation of BD. Prior work 34–37 indicates that mood disorders are among the most common diagnoses in offspring of BD parents. Therefore, our findings may not be generalizable to all at-risk youths. However, our approach is arguably conservative, in that it would decrease the likelihood of finding a difference between at-risk youths and controls on this face emotion processing task.
Finally, some at-risk youths had an anxiety disorder and/or ADHD. However, the majority (72%, N=18) had no diagnosis, and post-hoc analyses excluding children with Axis I diagnoses revealed the same pattern of deficits. Moreover, prior work 17 indicates that anxiety disorders and ADHD are not associated with emotion identification deficits. This suggests that these diagnoses in at-risk youths are unlikely to account for the impairments observed. In sum, the current study extends prior work demonstrating a non-specific face emotion processing deficit in pediatric BD patients and youths at risk for the illness, suggesting a potential endophenotypic marker.
Acknowledgments
Financial Support: This study was supported by the Intramural Research Program of the National Institute of Mental Health. The authors report no conflicts of interest.
References
- 1.Dick DM, Foroud T, Flury L, et al. Genomewide linkage analyses of bipolar disorder: A new sample of 250 pedigrees from the National Institute of Mental Health Genetics Initiative. Am J Hum Genet. 2003;73:107–114. doi: 10.1086/376562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faraone SV, Su J, Tsuang MT. A genome-wide scan of symptom dimensions in bipolar disorder pedigrees of adult probands. J Affect Disord. 2004;82S:S71–S78. doi: 10.1016/j.jad.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- 4.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 5.Smoller JW, Finn CT. Family, Twin, and Adoption Studies of Bipolar Disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 7.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 10.Lenox RH, Gould TD, Manji HK. Endophenotypes in bipolar disorder. Am J Med Genet. 2002;114:391–406. doi: 10.1002/ajmg.10360. [DOI] [PubMed] [Google Scholar]
- 11.McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003;160:1–3. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- 12.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 13.Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- 14.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJR, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pine DS, Klein RG, Mannuzza S, et al. Face-emotion processing in offspring at risk for panic disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- 16.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigeneralional family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 17.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 18.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2002. [Google Scholar]
- 20.Nurnberger JI, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 24.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- 27.Ekman P, Friesen WV. Pictures of facial affect. Palo Alto: Consulting Psychologists Press; 1976. [Google Scholar]
- 28.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJR, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein TR, Miklowitz DJ, Mullen KL. Social skills knowledge and performance among adolescents with bipolar disorder. Bipolar Disord. 2006;8:350361. doi: 10.1111/j.1399-5618.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 32.Hecht H, Genzworker S, Helle M, van Calker D. Social functioning and personality of subjects at familial risk for affective disorder. J Affect Disord. 2005;84:33–42. doi: 10.1016/j.jad.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 34.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 35.Chang KD, Steiner H, Ketter T. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39:453–460. doi: 10.1097/00004583-200004000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Reichart CG, Wals M, Hillegers MHJ, Ormel J, Nolen WA, Verhulst FC. Psychopathology in the adolescent offspring of bipolar parents. J Affect Disord. 2004;78:67–71. doi: 10.1016/s0165-0327(02)00178-7. [DOI] [PubMed] [Google Scholar]
- 37.Henin A, Biederman J, Mick E, et al. Psychopathology in the offspring in parents with bipolar disorder: A controlled study. Biol Psychiatry. 2005;58:554–561. doi: 10.1016/j.biopsych.2005.06.010. [DOI] [PubMed] [Google Scholar]


