Abstract
This fMRI study investigates neural activity associated with the interfering effects of emotional distracters. While in the scanner, participants made simple motor responses to target stimuli that were preceded and followed by positive, negative, or neutral images. Despite instructions to disregard the pictorial images, participants were slower to respond in the presence of positive or negative relative to neutral distracters, and significantly slower for negative relative to positive distracters. Enhanced activity in the amygdala and visual cortex was evident during trials that included positive and negative distracters. In contrast, increased activity in inferior frontal gyrus (BA 47) was only observed during trials that involved negative distracters. Connectivity analysis showed that activity in right amygdala correlated with activity in cingulate gyrus, posterior cingulate, middle temporal cortex, and was negatively correlated with activity in lateral superior frontal gyrus, middle frontal/orbital gyrus, and parietal cortex. The pattern of neural activity observed was interpreted within the framework of current cognitive models of attention. During a task demonstrating behavioural interference in the context of emotional distracters, increased activity in neural regions implicated in emotional processing (the amygdala) was associated with reduced activity in regions thought to be involved in exerting attentional control over task-relevant sensory representations (a frontoparietal network).
Keywords: Amygdala, emotional attention, emotional bias, conditioned suppression, biased competition model of attention
Introduction
The ability to quickly orient attention to positive or negative emotional cues can enhance a range of complex behaviours. However, an unhealthy preoccupation with emotional cues can hinder functioning. For example, a cognitive bias for negative material is thought to contribute to mood and anxiety disorders (Beck et al., 1979; Mathews & Macleod, 1994; Bradley & Mogg, 1994). At the other end of the spectrum, impoverished processing of emotional material is seen as a key feature of psychopathic disorder (Blair, 2004; Hare, 1991; Mitchell et al., 2006). Adaptive responding therefore requires striking a balance between attending to emotional representations when they are relevant, and minimizing their influence when they are not. However, we know relatively little about the neural regions involved when goal-directed behaviours are influenced by negative distracters. Even less is known about the neurocognitive systems important for regulating the impact of positive distracters. One method of indexing the level of interference generated by emotional distracters on goal-directed behaviour is through the use of the “Emotional Interrupt Task,” which measures the impact of irrelevant emotional stimuli on the speed of operant responding. The purpose of the present study was to pair this task with fMRI to delineate the neural activity associated with operant responding influenced by positive and negative distracters. Specifically, we examined whether there would be an antagonistic relationship between neural activity associated with emotional versus executive processes.
The biased competition model of attention predicts a common neurocognitive pathway for the emotional interference of simple operant behaviour, and that of more complex forms of executive function (e.g., memory or cognitive control). This model suggests that attending to one stimulus or class of stimuli decreases the availability of cognitive resources available for others (Desimone & Duncan, 1995). In this way, stimuli compete for neural representation and control over behaviour. Stimuli that are relevant to ongoing behaviour can be “selected” for processing through the influence of executive attention mechanisms, thereby reducing the impact of distracters. However, other factors such as stimulus salience or emotion also play a role in selection. The competitive advantage that emotional stimuli have for attention is thought to result from reciprocal functional connections between the amygdala, occipital, and temporal cortices (Morris et al., 1998; Pessoa et al., 2002; Vuilleumier et al., 2004). Specifically, the amygdala is thought to be activated by valenced stimuli, and subsequently to strengthen the representation of emotional stimuli in sensory representation areas of temporal and occipital cortex (Morris et al., 1998; Pessoa et al., 2002; Vuilleumier, 2005).
The Emotional Interrupt task provides a test of the biased competition model in that it involves operant responding that can be influenced by both emotional processes, and top-down executive attention processes. The data concerning the impact of emotional distracters on behaviour has largely been derived from studies involving two behavioural paradigms: 1) tasks involving the emotional interference of executive function (i.e., higher order cognitive function such as working memory or Stroop task performance); and 2) “conditioned suppression” paradigms. With regard to the impact of emotion on executive function, it has been shown that the presentation of a CS+ that predicts an aversive auditory stimulus interferes with executive task performance (Salgado et al., 2000). At the neural level, increased amygdala activity and decreased lateral prefrontal and parietal cortex activity is associated with the emotional interference of working memory (Dolcos & McCarthy, 2006). Interference of Stroop task performance by positive and negative distracters is associated with increased amygdala activity and compensatory recruitment of a frontopolar region of middle frontal cortex (Blair et al., 2007). Studies have also shown that increased processing load reduces emotional distracter-related activity in the amygdala, superior temporal gyrus, and ventromedial prefrontal cortex (Mitchell et al., 2007; Pessoa et al., 2005). Taken together, these studies suggest that an antagonistic relationship exists between regions of prefrontal cortex and the amygdala. However, relatively little work has specifically tested this. Furthermore, whereas these studies explore the impact of emotion on more complex executive operations, less is known at the neural level about how emotion interferes with more basic forms of operant behaviour.
Conditioned suppression paradigms model the interference of basic operant behaviour by emotional stimuli. Early studies demonstrating conditioned suppression revealed that presenting a conditioned stimulus (CS+) predicting shock interferes with operant responding in rats (Bouton & Bolles, 1980; Estes & Skinner, 1941). Conditioned suppression is neurally dissociable from freezing. Lesions of the periaqueductal gray area impair freezing, but not conditioned suppression (Amorapanth, Nader, & Ledoux, 1999). However, lesions of the amygdala do interfere with conditioned suppression (Killcross et al., 1997; Lee et al., 2005). Furthermore, whereas freezing occurs in response to threatening or aversive stimuli (Blanchard et al., 1977), conditioned suppression also occurs to a CS+ that predicts a rewarding outcome (Schindler et al., 1999). Although the interference of operant behaviour by complex emotional stimuli has been demonstrated behaviourally in humans (Hartikainen, Ogawa, & Knight, 2000; Mitchell et al., 2006; Tipples & Sharma, 2000), its neural correlates remain unclear.
The current fMRI study involves a laboratory task that, like measures of conditioned suppression used in animal studies, uses emotional distracters to interfere with basic goal-directed behaviour. In line with current models of attentional competition (Desimone & Duncan, 1995), and cognitive control (Botvinick et al., 2004), we predicted that positive and negative distracters would activate the amygdala, be more strongly represented in sensory representation areas, and interfere with operant behaviour. We also predicted that a mutually antagonistic relationship (negative connectivity) would exist between activity in regions implicated in attributing emotional salience to stimuli (i.e., the amygdala), and those thought to be involved in attentional selection (i.e., lateral prefrontal cortex and parietal cortex).
Methods
Subjects
Twenty-one subjects took part in the study. Data from two subjects were discarded due to head movement greater than 4mm; consequently, data were analyzed from nineteen healthy adults (9 women) with a mean age of 26.37 (aged 21 to 44; standard deviation 5.96). All subjects granted informed consent, were in good health, and had no past history of psychiatric or neurological disease as determined by a medical exam performed by a licensed physician. The study was approved by the National Institute of Mental Health Institutional Review Board.
MRI data acquisition
Subjects were scanned during task performance using a 1.5 Tesla GE Signa scanner. Functional images were taken with a gradient echo-planar imaging (EPI) sequence (repetition time = 2500 ms, echo time = 40 ms, 64 × 64 matrix, flip angle 90°, FOV 24 cm). Whole brain coverage was obtained with 29 axial slices (thickness, 4-mm; in-plane resolution, 3.75 × 3.75 mm). A high-resolution anatomical scan (three-dimensional Spoiled GRASS; repetition time = 8.1 ms, echo time = 3.2 ms; field of view = 24 cm; flip angle = 20°; 124 axial slices; thickness = 1.0 mm; 256 × 256 matrix) in register with the EPI dataset was obtained covering the whole brain.
Emotional Interrupt task and experimental procedure
Figure 1 depicts the trial structure and conditions of the Emotional Interrupt Task. In this event-related study, subjects completed a total of three 5 minute runs presented in counterbalanced order. The task was programmed using E-Prime software (Schneider et al., 2002). Within each run, subjects were presented with a separate version of the Emotional Interrupt task (which differed only in the visual stimuli used to minimize habituation). The subjects were placed in a light head restraint within the scanner to limit head movement. Before entering the scanner, subjects performed a 40-trial training run consisting of four neutral IAPS stimuli that were not included in the experimental runs. A total of 36 positive, 36 negative and 36 neutral images were used taken from the International Affective Picture System (IAPS; Lang et al., 2005). The IAPS is a large set of normative emotional pictorial stimuli for experimental investigations of emotion and attention. The IAPS provides normative 9-point ratings from a large sample of healthy adults on the dimensions of pleasantness (higher scores corresponding to greater pleasantness) and arousal (higher scores corresponding to greater arousal; Lang et al., 2005). The images were selected on the basis of these normative ratings. The mean pleasantness ratings for negative, neutral, and positive images were 2.80, 4.87, and 7.19 respectively. This difference was statistically significant (F(2,105) = 417, p < 0.001); negative < neutral < positive, p < 0.001). The mean arousal ratings for negative, neutral, and positive images were 5.98, 2.81, and 5.40 respectively. This difference was also statistically significant (F(2,105) = 154; p < 0.001; negative > positive > neutral, p < 0.005).
Figure 1. Components of the Emotional Interrupt Task.
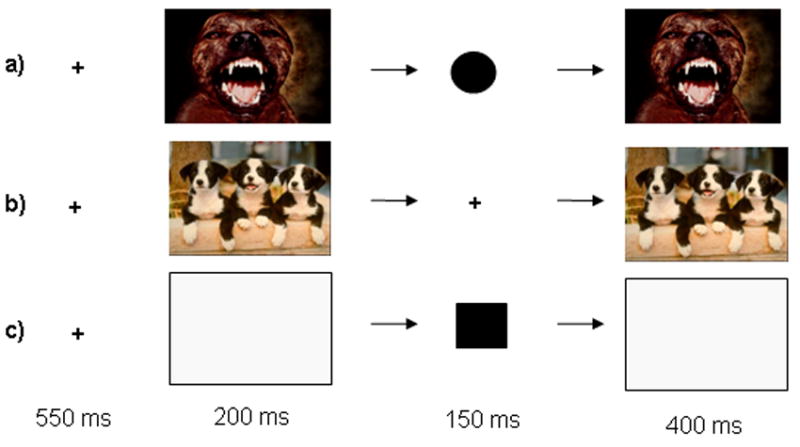
Emotional Interrupt Task procedures. Each row represents a trial type. Each trial consisted of 4 serially presented events: i) a fixation cross (550ms); ii) a negative, neutral, positive or blank image (200ms); iii) a circle, square, or fixation cross (150ms); iv) the same image that preceded the shape or cross (400ms); v) a blank screen for (1200ms). Participants responded during either the second picture or blank and reaction times were calculated from the onset of the shape stimulus. The hemodynamic response was modeled and averaged for the duration of each trial. The trials displayed correspond to: a) a negative response-with-distracter trial; b) a positive image-only trial; c) a response-only trial.
The task consisted of 7 total conditions: three response-with-distracter conditions (positive, negative and neutral images), 3 image-only conditions (positive, negative, and neutral images), and one response-only condition. Response-with-distracter trials consisted of sequential presentations of a fixation cross for 550 ms, a positive, negative or neutral IAPS image for 200 ms, a small circle or square printed on a grey background for 150 ms, the same IAPS image that preceded the shape for 400 ms, and a blank screen for 1200 ms. Image-only trials consisted of a fixation point (550ms), a positive or negative IAPS image (200ms), another fixation point (150ms), the same IAPS image that preceded it, and a blank screen (1200ms). These stimulus timings were selected in the course of pilot testing. Each condition consisted of 36 trials lasting 2500ms for a total of 252 trials presented in random order. The task was broken up into three runs of equal length. Participants were instructed to respond as quickly as possible with the left button to the small circles, and the right button to the squares. Speeded responding was emphasized, and participants were instructed not to respond to the images in any way. The stimuli were presented on a Dell Inspiron laptop computer display projected onto a mirror through a data projector onto a screen that could be seen by the subject through mirrors positioned above the coil of the MRI scanner. Subjects were able to respond with right and left button presses during either the second image or the blank period. All other responses were not recorded.
Regressors were time-locked to the course of the entire trial (2500 ms). In addition to the test stimuli, 63 fixation trials were presented per block to serve as a baseline. Each IAPS stimulus was repeated twice (once in the experimental condition, and once in the control condition); the order of the test stimuli was randomized across blocks. At the beginning and end of each run a fixation cross was displayed for 15 s.
FMRI analysis
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI) (Cox, 1996). Both individual and group-level analyses were conducted. The first six volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected shortly before acquisition of the high-resolution anatomical dataset. The EPI datasets for each subject were spatially smoothed (using an isotropic 6mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps in generating group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean. Following this, regressors depicting each of the seven trial types were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response (Cohen, 1997). Linear regression modeling was performed fitting the BOLD response to the seven regressors of interest described above. To control for voxel-wise correlated drifting, a baseline plus linear drift and quadratic trend were modeled in each voxel’s time series. This produced for each voxel and each regressor, a beta coefficient and its associated t-statistic. Voxel-wise group analyses involved transforming single subject beta coefficients into the standard coordinate space of Talairach and Tournoux (Talairach & Tournoux, 1988) followed by a 2(Operant response: response-with-distracter versus image-only conditions) X 3(Emotion: negative, neutral and positive) ANOVA on the imaging data. For the main effects, regions significantly active at a threshold of p < 0.001 were examined as were regions showing an interaction at a level of p < 0.005. To correct for multiple comparisons we performed a spatial clustering operation using AlphaSim (Ward, 2000) with 1,000 Monte Carlo simulations taking into account the entire EPI matrix (p <0.05). A subset of clusters showing significant differential activation for each main effect (p < 0.001, for operant response and emotion) and the interaction (p < 0.005) were selected according to a priori predictions about the regions involved in the representation or manipulation of operant responding and emotion. These clusters were used to define functional regions of interest (ROIs). In addition, a priori predictions about the involvement of the amygdala in the modulation of attention by emotion justified the use of an ROI approach to investigate activation within this region. Thus, standardized bilateral ROIs of the amygdala (identified using a predefined AFNI template) were applied to the data. Following this, areas of significant differential activation within the template were sampled, resulting in a ROI encompassing only significant activation within the right amygdala (p < 0.005).
Connectivity Analysis
We measured functional connectivity by examining covariation across the whole brain with the activation within a functionally-defined ROI. This analysis examines how the BOLD within a specified seed region correlates with activity in the rest of the brain, across all conditions. Each individual subject’s time series was converted to common Talairach space according to their structural data set. Within our primary ROI within the amygdala, the voxel with the peak signal change for the main effect of emotion was identified across subjects. This voxel with peak signal change became our “seed” voxel, and the time series within it was extracted. Baseline plus linear and quadratic trend were modeled in each voxel’s time series to control for voxel-wise correlated drifting. The global signal (average signal across the whole brain) was used as a covariate in the correlation analysis to control for global drifting. A voxel-wise correlation analysis was conducted between each individual voxel’s time series and that of the identified seed. The proportion of the variation in the signal that could be explained by the correlation with the seed was determined by squaring the resulting correlation coefficient. Correlation coefficients were converted to a Gaussian variable using a Fisher transformation formula in order to reduce the skew and normalize the sampling distribution. To identify regions significantly positively or negatively correlated with the target voxel at group level, a one-sample t-test was performed on the transformed correlation coefficients (p < 0.005).
Results
Behavioural Results
Response Latency and Error Data
Repeated-measures ANOVAs were conducted on the reaction time and error data. The reaction time analysis revealed a significant main effect of condition (F(3,54) = 11.02; p < 0.001). Subsequent planned pair-wise comparisons revealed that participants responded significantly more slowly for positive (p = 0.05) and negative (p < 0.001) stimuli than neutral stimuli. Further, mean reaction time to target stimuli temporally-flanked by negative stimuli was significantly slower than those presented with positive stimuli (p < 0.05). In contrast, the mean reaction time for neutral versus response-only conditions was not significant (p = 0.23). A repeated-measures ANOVA conducted on the error data revealed no significant main effects or interactions.
fMRI Results
Main Effect of Operant Response
A significant main effect of operant response was observed in medial frontal/cingulate gyrus, motor cortex, parietal cortices, caudate, and cerebellum (p < 0.001; p < 0.05 corrected for multiple comparisons). In each case, activity was greater during the response versus view-only conditions. Table 1 provides a summary of all regions showing significant main effects and interaction of the operant response and emotion conditions. Figure 2 shows activity in regions significantly modulated by operant response.
Table 1.
Areas showing main effects and interaction for task and emotion
| Anatomical Location | L/R | BA | x | y | z | F-value |
|---|---|---|---|---|---|---|
| Main Effect of Task* | ||||||
|
| ||||||
| Medial frontal/cingulate gyrus | R | 24 | 9 | −10 | 52 | 29.62 |
| Precentral gyrus | R | 6 | 33 | −17 | 55 | 28.84 |
| Precentral gyrus | L | 6 | −63 | 3 | 9 | 31.19 |
| Postcentral gyrus | R | 5 | 44 | −45 | 67 | 29.67 |
| Superior parietal lobule | R | 7 | 25 | −58 | 69 | 31.71 |
| Inferior parietal lobule | R | 40 | 66 | −41 | 32 | 39.02 |
| Inferior parietal lobule | L | 40 | −40 | −39 | 53 | 31.79 |
| Caudate body | R | - | 15 | −9 | 23 | 32.5 |
| Culmen (cerebellum) to caudate | L | - | −11 | −51 | −22 | 87.84 |
|
| ||||||
| Main Effect of Emotion | ||||||
|
| ||||||
| Inferior frontal gyrus2 | R | 47 | 32 | 29 | −1 | 11.73 |
| Inferior frontal gyrus2 | L | 13 | −37 | 29 | 12 | 11.32 |
| Lentiform/putamen/amygdala1 | R | - | 26 | −9 | −8 | 18.28 |
| Lentiform nucleus/globus pallidus2† | L | - | −16 | −6 | −6 | 11.00 |
| Parahippocampal gyrus1 | L | - | −37 | −20 | −18 | 12.83 |
| Middle occipital gyrus1+ | R | 19 | 51 | −75 | 2 | 65.71 |
| Middle occipital gyrus1 | L | 37 | −53 | −71 | 2 | 20.99 |
|
| ||||||
| Task by Emotion Interaction | ||||||
|
| ||||||
| Inferior parietal lobule | R | 7/39 | 35 | −62 | 40 | 8.69 |
Activity is significant at p < 0.001; corrected for multiple comparison at p < 0.05.
Did not survive correction
All Task > Images
Negative > Positive > Neutral, p < 0.05;
p = 0.05
Negative > (Positive = Neutral), p < 0.05
Figure 2. Main Effect of Task.
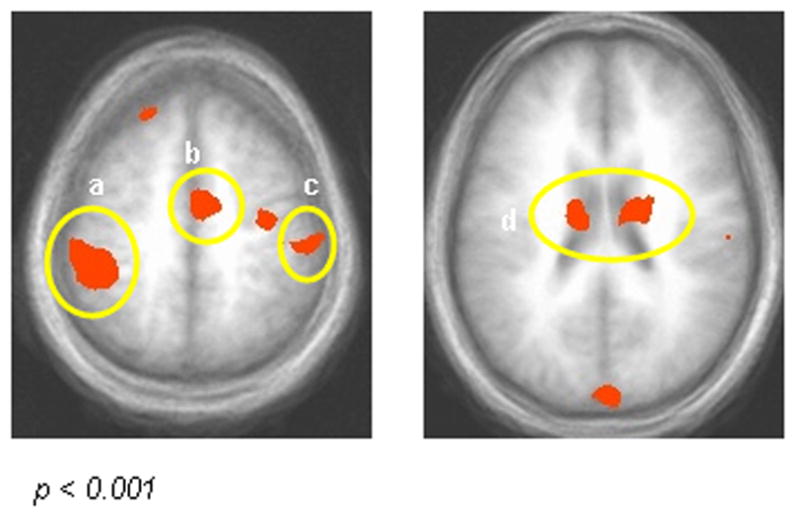
Areas showing a significant main effect of operant responding (p < 0.001; p < 0.05 corrected): a) Left inferior parietal lobule (BA 40); b) Medial frontal/cingulate gyrus (BA 24); c) Right postcentral gyrus (BA 5); d) Bilateral caudate. Activity was significantly greater across response-with-distracter conditions than image-only conditions.
Main Effect of Emotion
A significant effect of emotion (p < 0.001; p < 0.05 corrected) was revealed bilaterally in inferior frontal gyrus (ventrolateral prefrontal cortex, BA 47), lentiform nucleus extending to the amygdala (lentiform nucleus/amygdala), and middle occipital gyrus. Inferior frontal gyrus showed significantly greater activity bilaterally to negative relative to positive or neutral stimuli (p < 0.05). Pair-wise comparisons of the percent signal change data reveal that the main effect of emotion in the occipital gyri and right lentiform nucleus/amygdala was characterized by greater activity in the presence of negative and positive relative to neutral stimuli (p < 0.05). Figure 3 shows bilateral activity and percent signal change across conditions in inferior frontal gyrus and middle occipital gyrus.
Figure 3. Main Effect of Emotion.
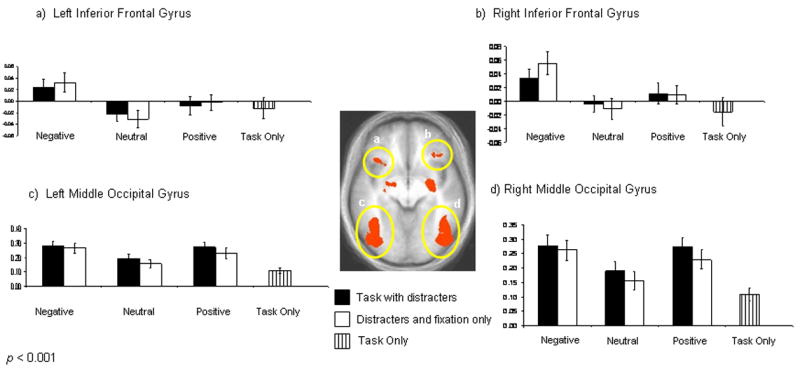
Areas showing significant activity to emotion (p < 0.001; p < 0.05 corrected). Graphs depict the percent signal change relative to fixation across conditions. Graphics depicted by a and b show significantly enhanced bilateral activity in left and right inferior frontal gyrus to negative stimuli relative to neutral or positive stimuli (p < 0.05). Images c and d show significantly enhanced bilateral activity in middle occipital gyrus to positive and negative stimuli relative to neutral stimuli (p < 0.05).
An ROI approach was used to investigate percent signal change restricted to an anatomically-defined area of right amygdala across conditions. The ROI consisted of all voxels within the right amygdala that showed a main effect of emotion (p < 0.005). Significantly active voxels outside of right amygdala were excluded. Figure 4 shows an axial slice of this ROI and its percent signal change across conditions. Pair-wise comparisons of the percent signal change revealed significantly greater activity within right amygdala to both positive and negative stimuli relative to neutral stimuli (p < 0.01). Furthermore, this activity was significantly greater for negative than for positive distracters (p < 0.05).
Figure 4. Functional ROI: Main Effect of Emotion within Right Amygdala.
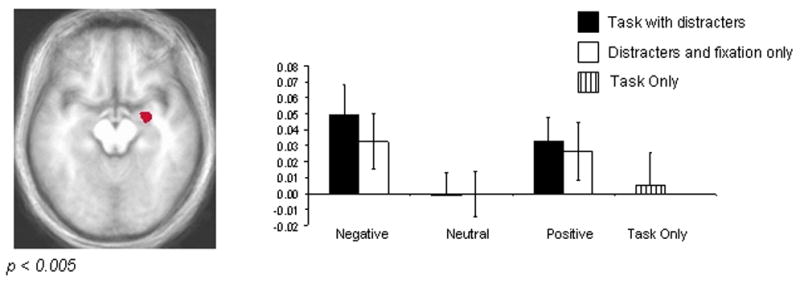
Significantly active voxels (p < 0.005) within an anatomically defined area of the right amygdala are displayed. The graph depicts the percent signal change relative to the mean across conditions. The right amygdala shows significantly enhanced activity to both negative and positive stimulus conditions relative to neutral conditions (p < 0.01). Activity in this region in the context of negative distracters was significantly greater than that of positive distracters (p < 0.05).
Response by Emotion Interaction
A significant interaction was observed in right inferior parietal lobule (p < 0.005). When participants engaged in the response-with-distracters conditions, activity was greatest for positive stimuli and least for negative stimuli. However, during image-only trials, activity was greatest to negative stimuli, and least to positive stimuli. Thus, the BOLD response in right inferior parietal cortex was higher to positive than negative stimuli only in the context of performing an operant response. In contrast, activity in this region was highest to negative stimuli only in the absence of an operant stimulus. Activity in this region to neutral distracters remained the same regardless of whether operant stimuli were present. The response by emotion interaction is depicted in Figure 5.
Figure 5. Task by Emotion Interaction in Right Inferior Parietal Lobule.

A response by emotion condition interaction in right inferior parietal lobule (p < 0.005). The graph shows that percent signal change was greater for image-only trials including negative distracters versus positive distracters (p < 0.005). During the response-with-distracter conditions, the opposite was true (positive > negative; p < 0.05).
Connectivity Analysis
Functional connectivity is a measure of significant correlated activity between a target neural region or voxel and the whole brain. The analysis examines correlated activity across the entire task irrespective of condition, and therefore provides an additional test of hypotheses concerning functional connectivity. Based on our interest in the amygdala as a critical source for augmenting the representation and effects of positive and negative distracters, we chose the voxel of peak intensity within the amygdala region as our “seed.” The voxel with peak intensity was located in the right amygdala. Activity in right amygdala was positively correlated with activity in middle temporal cortex, cingulate gyrus, extended amygdala, cuneus, and posterior cingulate (p < 0.005). Activity in right amygdala was negatively correlated with activity within areas of lateral superior frontal gyrus, middle frontal/orbital gyrus, parietal cortex, occipital gyrus, angular gyrus, and culmen (p < 0.005). Table 2 and Figure 6 provide a summary of the regions showing significant positive and negative connectivity with the amygdala.
Table 2.
Areas significantly correlated with the voxel of peak intensity within right amygdala
| Anatomical Location | L/R | BA | x | y | z | t-value* |
|---|---|---|---|---|---|---|
|
Regions Showing Positive Functional Connectivity with Right Amygdala
| ||||||
| Cingulate gyrus | R | 24 | 3 | 2 | 31 | 4.18 |
| Posterior cingulated | R | 30 | 13 | −65 | 7 | 3.91 |
| Cuneus | R | 18 | 11 | −72 | 16 | 4.39 |
| Middle temporal gyrus | L | 21 | −39 | −1 | −41 | 3.84 |
| Extended Amygdala | R/L | - | 23 | −1 | −12 | 27.53 |
|
| ||||||
|
Regions Showing Negative Functional Connectivity with Right Amygdala
| ||||||
| Middle frontal/orbital gyrus | R | 10 | 19 | 62 | 12 | −5.91 |
| Superior frontal gyrus | R | 8 | 23 | 46 | 48 | −3.88 |
| Superior frontal gyrus | L | 9 | −29 | 49 | 37 | −3.67 |
| Inferior parietal lobule | R | 40 | 56 | −38 | 48 | −3.86 |
| Superior parietal lobule | L | 7 | −3 | −74 | 57 | −5.05 |
| Middle occipital gyrus | L | 19 | −29 | −78 | −17 | −5.61 |
| Angular gyrus | R | 39 | 43 | −77 | 31 | −4.49 |
| Culmen (cerebellum) | R | - | 31 | −51 | −32 | −3.69 |
All regions significant at p < 0.005, uncorrected
Figure 6. Connectivity analysis.
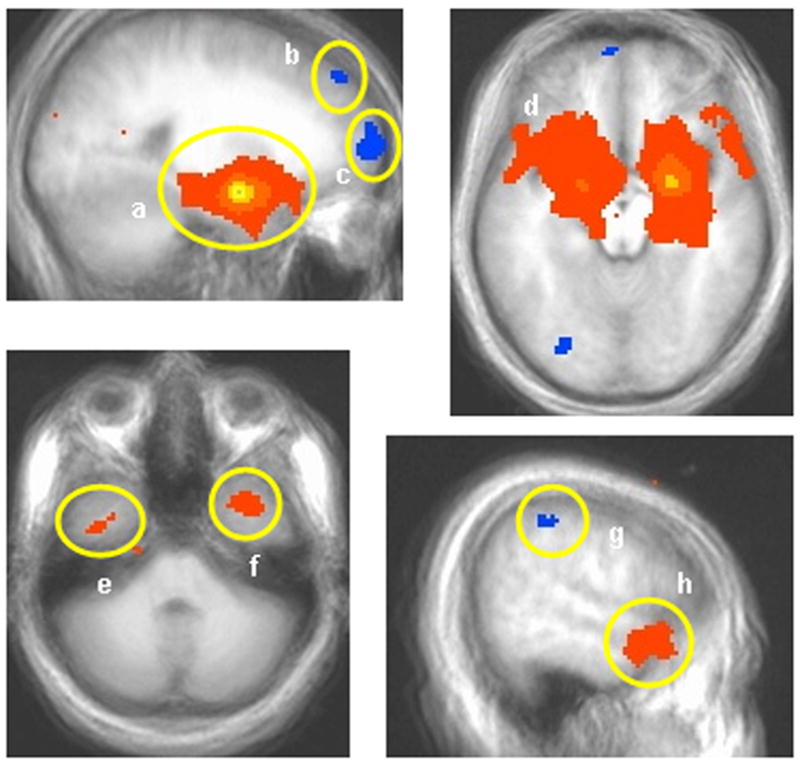
Results of the connectivity analysis with the voxel of peak intensity in right amygdala (p < 0.005) as the seed (a). Regions showing positive connectivity with the seed voxel include the extended amygdala (a and d), and anterior temporal cortex (e, f, and h). Regions showing negative correlation with right amygdala include the lateral superior frontal gyrus (b), middle frontal/orbital gyrus (c), and parietal cortex (g).
Discussion
The present study examined neural activation associated with the emotional interference of goal-directed behaviour. This task has been shown to differentiate behaviourally between groups of patients with psychopathic disorder (i.e., a disorder involving reduced empathy) and incarcerated controls (Mitchell et al., 2006). Operant responding was associated with increased BOLD signal change in motor cortex, caudate, and areas implicated in attention including the medial frontal/cingulate gyrus and parietal cortex. A main effect of emotion was observed in the inferior frontal gyrus (BA 47), right amygdala, and occipital gyri. This effect was characterized by enhanced activity in middle occipital gyrus, and the right amygdala in the presence of positive and negative relative to neutral distracters. Inferior frontal gyrus showed increased activity only to negative images. An area of inferior parietal cortex showed a response by emotion condition interaction characterized by greater activity in the presence of negative relative to neutral or positive images during distracter-only conditions, and the opposite pattern of activity whilst engaging in response-with- distracter conditions. The functional connectivity analysis showed that activity in the right amygdala correlated positively with activity in middle temporal cortex, cingulate gyrus, cuneus, and posterior cingulate gyrus. Negative correlations were observed between right amygdala and lateral superior frontal gyrus, parietal cortex, occipital cortex, angular gyrus and cerebellum.
Neurocognitive models of emotional interference
Emotional stimuli may have an impact on behaviour through at least two dissociable neurocognitive pathways. Emotional material may interfere with operant behaviour by eliciting an incompatible innate fear response such as freezing. At low-levels of threat in the environment, freezing occurs as a natural defensive reaction (Blanchard et al., 1977). However, like previous studies of emotional interference (Hartikainen et al., 2000; Tipples & Sharma, 2000), we found that both negative and positive stimuli interfered with ongoing behaviour. This suggests that a mechanism other than freezing was involved. Furthermore, animal studies implicate the periaqueductal grey area as the neural substrate critical for freezing behaviour (Amorapanth et al., 1999), but in the present study, significant activity in this region was not observed. In contrast, the amygdala is critical for conditioned suppression (Killcross et al., 1997; Lee et al., 2005), and significant activity in this region was observed in the context of the present task. How might affect-related activity in the amygdala interact with other cortical structures to give rise to the kind of behavioural interference by positive and negative distracters observed here and in other studies of conditioned suppression?
The biased competition model of attention would suggest that distracters interfere with operant behaviour by preferentially drawing upon (or “capturing”) attentional resources that might otherwise be devoted to the primary task (Desimone & Duncan, 1995). We suggest that the results of the current and similar studies are best understood within this framework. Emotional material is thought to capture attention through interactions between the amygdala and visual processing areas (Pessoa et al., 2002; Vuilleumier & Schwartz, 2001). Data suggest that the amygdala is particularly responsive to negative stimuli (e.g., Whalen et al., 1998; Dannlowski et al., 2007). However, both neuroimaging (e.g., Garavan et al., 2001; Liberzon et al., 2003; Siebert et al., 2003; Kensinger & Schacter, 2006) and animal (e.g., Paton et al., 2006; for a review, Baxter & Murray, 2000) studies also implicate the amygdala in processing positive stimuli or reward. In the present study, we observed increased reaction times to trials that contained positive or negative distracters relative to neutral distracters, and on trials containing negative relative to positive distracters. Enhanced activity to both positive and negative stimuli was found in occipital cortex and the right amygdala. We also report increased functional connectivity between the right amygdala and regions implicated in representing object attributes and concepts in temporal cortex (e.g., Martin et al., 1996). Finally, significant functional connectivity was seen between right amygdala and posterior cingulate. The posterior cingulate is thought to be involved in both visuospatial orientation (Vogt, 2005) and processing emotionally salient stimuli (Maddock, 1999; Nakic et al., 2006). Although speculative, we suggest that the posterior cingulate may interact with the amygdala and parietal cortices to facilitate orientation to the spatial location of emotional stimuli. Indeed, it has also been suggested that an adjacent region of intraparietal cortex plays a role in directing and maintaining attention to the spatial location of threatening stimuli (Pourtois et al., 2006). Further studies will help delineate more precisely how these posterior cortical areas may interact with subcortical and prefrontal cortical areas during emotional interference.
An important caveat in the current study should be noted. One limitation of the picture set used here is that affective valence may be confounded with visual complexity (neutral pictures in the set may be less complex and colourful). This would be problematic for contrasts between emotional and neutral pictures, particularly with respect to activity in visual processing areas. It is important to note that participants were not significantly slower to respond to response-only versus response-with-neutral-distracter trials. If the behavioural effect were driven by visual complexity rather than emotion, one would predict that the difference in visual complexity between no image and a neutral image would be greatest, and therefore should create the largest effect size. This was not observed. At the neural level, the enhanced activity to emotional stimuli observed in visual cortex may have been driven by visual complexity rather than emotion. However, other studies using stimuli that differ minimally in visual complexity (e.g., fearful versus neutral faces) also report increased activity in visual processing to emotion (e.g., Morris et al., 1998). For these reasons, it is unlikely that the pattern of results can be accounted for entirely by differences in visual complexity.
Implications for emotional regulation
In addition to an adaptive system that increases the salience of emotional material (LeDoux, 1995), a counteractive system likely regulates the impact of emotional stimuli when they interfere with task-demands (Pessoa et al., 2005; Vuilleumier, 2005). A key component of this system is thought to be the prefrontal cortex, particularly middle frontal gyrus, which has modulatory connections with the amygdala (Ongur & Price, 2000). The regulatory role of areas in middle frontal and orbital gyrus over amygdala responding has been supported by data from non-human (Quirk & Gehlert, 2003; Rosenkranz, Moor, & Grace, 2003) and human subjects (Drevets, 2000; Pezawas et al., 2005). Neuroimaging data implicate anterior cingulate cortex (Bishop et al., 2004; Johnson et al., 2005) and orbitofrontal cortex (Pourtois et al., 2006; Blair et al., 2007) in regulating the impact of emotional information under high conflict conditions. Consistent with this suggestion, functional connectivity analysis in the present study revealed that activity in right amygdala was negatively correlated with a region extending from middle frontal gyrus to orbital gyrus. This area corresponds closely with regions of middle frontal or orbital gyrus implicated in voluntary emotional regulation studies (e.g., Beauregard et al., 2001; Levesque et al., 2003).
A second possible mechanism of emotional regulation involves regions of prefrontal cortex, particularly lateral prefrontal cortex, which are thought to manipulate representations of task relevant stimuli in temporal and occipital regions at the expense of distracters (Botvinick et al., 2004; Desimone & Duncan, 1995). Direct functional connections between lateral prefrontal gyrus and temporal cortex, or indirect connections via parietal cortex (see Petrides, 2005), may be involved in increasing the representational strength of task-relevant stimuli (Botvinick et al., 2004; Desimone & Duncan, 1995). A recent study suggests that increasing attentional load in the context of emotional distracters results in increased activity in a frontoparietal network, and reduced activity in areas associated with emotion or face processing including the amygdala and areas of occipital temporal cortex (Mitchell et al., 2007). In the current study, the response by emotion interaction observed in parietal cortex may indicate a role for this region in adaptive attentional selection. Furthermore, a functional connectivity analysis showed that activity in parietal and lateral superior frontal gyrus was negatively correlated with activity in right amygdala. This pattern of activation is consistent with the idea that cognitive control over distracting emotional material results from activity in a frontoparietal network that manipulates the strength of object representation in visual processing areas (Mitchell et al., 2006; Mitchell et al., 2007; Vuilleumier, 2005). These newly emerging findings raise the possibility that clinically relevant emotional regulation may also be facilitated by augmenting the strength of alternative (neutral or positive rather than negative) sensory representations. This system may operate either in parallel to, or in place of (depending on situational or individual factors), a partially dissociable regulatory system involving regions of medial prefrontal cortex and frontal pole that facilitate extinction or emotional suppression. We suggest that this alternative “attentional” pathway may be particularly relevant for cognitive therapy interventions, which emphasize replacing maladaptive biases towards symptom-relevant emotions with healthier representations (Beck, 1979).
Another candidate structure for emotional regulation is the inferior frontal gyrus (BA 47), which has been implicated in emotional and behavioural modification. For example, inferior frontal gyrus is active during cognitive reappraisal tasks (Ochsner, 2005; Phan et al., 2005), during unwanted implicit associations (Cunningham et al., 2004; Luo et al., 2006), during placebo-induced analgesia (Petrovic et al., 2002), and during response reversal decision-making tasks (Cools et al., 2002; O’Doherty et al., 2003). In the present study, significant left and right inferior frontal gyrus activity was observed in the presence of negative, but not positive or neutral images. Participants were also significantly slower to respond in the presence of negative images relative to positive or neutral images. One possibility is that inferior frontal gyrus activity (or activity in the adjacent insular cortex) may be involved in enhancing the representation of negative emotional stimuli. This would explain the increased interference of negative relative to positive images. A second possibility is that inferior frontal gyrus is involved in resolving response conflict generated by negative stimuli (Blair, 2004). Although speculative, this account is compatible with the collection of studies that implicate inferior frontal gyrus in emotional regulation (e.g., Ochsner, 2005; Cunningham et al., 2004; Luo et al., 2006; Monk et al., 2006) response change (e.g., Budhani et al., 2007; Cools et al., 2002; O’Doherty et al., 2003), response inhibition (Casey et al., 2001), and viewing negative images irrespective of whether an operant response is made, as was observed in this study. These findings are consistent with the idea that the inferior frontal gyrus is involved in the regulation of both maladaptive motor and emotional responses. However, further work is required to better delineate the functional contribution of this region of prefrontal cortex to decision-making and attention to emotional representations.
Conclusion
The current study examined the neural correlates of operant behaviour in the context of emotional distracters in healthy individuals. A previous study had showed that patients with psychopathy, who may exhibit a pathological form of emotional resilience, demonstrated reduced emotional interference relative to controls on the same behavioural measure (Mitchell et al., 2006). Given the importance of attention in emotional biases (Mogg, Bradley, Williams, 1995; Monk et al., 2004), determining the neural systems involved in resolving attentional competition may prove relevant to a spectrum of affective disorders. We are currently conducting further studies that examine emotional interference and regulation across a range of paediatric and adult affective disorders. We predict that relative to their control groups, patients with psychiatric disorders involving anxiety will be associated with increased behavioural interference by negative distracters coupled with reduced activity in frontoparietal cortex. Here we found significant activity within motor cortex, caudate, cingulate, and parietal cortex during trials involving operant responding. Occipital gyrus and amygdala showed significantly enhanced activity to positive and negative stimuli. Enhanced inferior frontal gyrus activity that occurred only in the context of negative distracters was consistent with suggestions that this area either reflects or resolves competition generated by aversive material. Connectivity analysis showed a positive correlation between activity in right amygdala and areas within posterior cingulate and middle temporal gyrus. In contrast, negative functional connectivity was observed between right amygdala and regions of lateral superior frontal gyrus and parietal cortices. Together, these results are consistent with suggestions that the amygdala increases the salience of emotional distracters through interactions with posterior cingulate and visual processing areas. Furthermore, they suggest the potential involvement of an attentional frontoparietal cortical system (in addition to a potentially suppressive influence of middle frontal/orbital gyrus) for regulating the impact of emotion on behaviour. Further studies that delineate the neurocognitive factors that give rise to and resolve biased attention will likely provide important clues to the neurobiology of a range of affective disturbances from psychopathy to mood and anxiety disorders.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH: NIMH. The authors would like to extend special thanks to Gang Chen and Ziad Saad for assistance with the fMRI connectivity analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport DA. Attention and control: Have we been asking the wrong questions? A critical review of twenty-five years. In: Meyer DE, Kornblum S, editors. Attention and Performance XIV. Cambridge, MA: MIT Press; 1993. pp. 183–218. [Google Scholar]
- Amorapanth P, Nader K, LeDoux JE. Lesions of periaqueductal gray dissociate-conditioned freezing from conditioned suppression behaviour in rats. Learn Mem. 1999;6(5):491–499. doi: 10.1101/lm.6.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: The Guilford Press; 1979. [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24(46):10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbitofrontal cortex in the modulation of antisocial behaviour. Brain and Cognition. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DGV, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJR. Modulation of emotion by cognition and cognition by emotion. NeuroImage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Takahashi LK. Attack and defensive behaviour in the albino rat. Animal Behaviour. 1977;25:197–224. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Conditioned fear assessed by freezing and by the suppression of three different baselines. Animal Learning and Behaviour. 1980;8(3):429–434. [Google Scholar]
- Bradley BP, Mogg K. Mood and personality in recall of positive and negative information. Behav Res Ther. 1994;32(1):137–141. doi: 10.1016/0005-7967(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Budhani S, Marsh AA, Pine DS, Blair RJR. Neural correlates of response reversal: Considering acquisition. NeuroImage. 2007;34:1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, et al. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins T. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15(12):806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Arolt V, Heindel W, Suslow T. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. 2007;154(1):13–20. doi: 10.1016/j.pscychresns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Estes WK, Skinner BF. Some quantitative properties of anxiety. Journal of Experimental Psychology. 1941;29:390–400. [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. NeuroReport. 2001;12(12):2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Transient interference of right hemispheric function due to automatic emotional processing. Neuropsychologia. 2000;38(12):1576–1580. doi: 10.1016/s0028-3932(00)00072-5. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci. 2005;5(3):339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. The Journal of Neuroscience. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lee JL, Dickinson A, Everitt BJ. Conditioned suppression and freezing as measures of aversive Pavlovian conditioning: effects of discrete amygdala lesions and overtraining. Behav Brain Res. 2005;159(2):221–233. doi: 10.1016/j.bbr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53(6):502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Luo Q, Nakic M, Wheatley T, Richell R, Martin A, Blair RJ. The neural basis of implicit moral attitude--an IAT study using event-related fMRI. NeuroImage. 2006;30(4):1449–1457. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379(6566):649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Richell RA, Leonard A, Blair RJR. Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115:559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Nakic M, Pine DS, Blair RJR. The impact of processing load on emotion. NeuroImage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, Woldehawariat G, Montgomery LA, Zarahn E, McClure EB, et al. Experience-dependent plasticity for attention to threat: Behavioural and neurophysiological evidence in humans. Biological Psychiatry. 2004;56:607–610. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nakic M, Smith BW, Busis S, Vythilingam M, Blair RJ. The impact of affect and frequency on lexical decision: The role of the amygdala and inferior frontal cortex. NeuroImage. 2006 doi: 10.1016/j.neuroimage.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioural control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol. 2005;15(2):188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. NeuroImage. 2006;31(2):920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23(35):11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado JV, Vidal M, Oberling P, Graeff FG, Danion JM, Sandner G. Associative learning and latent inhibition in a conditioned suppression paradigm in humans. Behavioural Brain Research. 2000;117:53–60. doi: 10.1016/s0166-4328(00)00280-1. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Ma JD, Goldberg SR. Conditioned suppression with cocaine as the unconditioned stimulus. Pharmacology Biochemistry and Behaviour. 1999;65(1):83–89. doi: 10.1016/s0091-3057(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime User’s Guide (Version 1) Pittsburgh: Psychology Software Tools Inc; 2002. [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tipples J, Sharma D. Orienting to exogenous cues and attentional bias to affective pictures reflect separate processes. Br J Psychol. 2000;91(Pt 1):87–97. doi: 10.1348/000712600161691. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6(7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56(2):153–8. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manuals/AlphaSim.pdf.
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


