Abstract
The anterior and intermediate lobes of the pituitary gland are formed from Rathke’s pouch. FGF, BMP and WNT signals emanating from the ventral diencephalon influence pouch growth and development. In order to examine the role of canonical WNT signaling during pituitary development we examined the pituitary expression of the TCF/LEF family of transcription factors, which mediate WNT signaling through the binding of β-catenin. We report here the expression of several members of this family during pituitary development and the functional role of one member, TCF4 (TCF7L2), in the induction of the pituitary primordium. TCF4 is expressed in the ventral diencephalon early in pituitary development, rostral to a domain of BMP and FGF expression. Tcf4 deficient mice express Fgf10 and Bmp4; however, the Bmp and Fgf expression domains are expanded rostrally. As a result, additional pituitary progenitor cells are recruited into Rathke’s pouch in Tcf4 mutants. Mutants also exhibit an expansion of the Six6 expression domain within Rathke’s pouch, which may increase the number of proliferating pouch cells, resulting in a greatly enlarged anterior pituitary gland. This suggests that TCF4 negatively regulates pituitary growth through two mechanisms. The first mechanism is to restrict the domains of BMP and FGF signaling in the ventral diencephalon, and the second mechanism is the restriction of Six6 within Rathke’s pouch. Thus, TCF4 is necessary both intrinsically and extrinsically to Rathke’s pouch to ensure the proper growth of the pituitary gland.
Keywords: pituitary, patterning, Wnt signaling, organogenesis, TCF, BMP, FGF
Introduction
Cell signaling through Wnt ligand activated pathways can regulate developmental processes such as cell proliferation, differentiation, and migration. Targeted disruption of Wnt genes leads to a variety of phenotypes including defects in brain, lung, limb, and kidney development (Bennett et al., 2005; Carroll et al., 2005; Kispert et al., 1996; Kubo et al., 2003; Liu et al., 1999; McMahon and Bradley, 1990; McMahon et al., 1992; McWhirter et al., 1999; Millar et al., 1999; Monkley et al., 1996; Parr et al., 2001; Parr and McMahon, 1995; Spater et al., 2006; Stark et al., 1994; Takada et al., 1994; Thomas and Capecchi, 1990). Pituitary gland development is also dependent on the proper expression of Wnt ligands. Wnt5a regulates the shape of the pituitary gland (Cha et al., 2004), and Wnt4 enhances pituitary growth (Treier et al., 1998), unpublished data). In addition, cell culture and pituitary explant studies have implicated β-catenin in the regulation of PITX2, NR5A1, and PROP1, transcription factors critical for pituitary development (Baek et al., 2003; Briata et al., 2003; Gummow et al., 2003; Hossain and Saunders, 2003; Kennell et al., 2003; Kioussi et al., 2002; Olson et al., 2006; Salisbury et al., 2007). These observations suggest that Wnt ligands signaling through the β-catenin dependent canonical pathway could be important for pituitary development; however, no specific Wnt ligand has been implicated in the regulation of these transcription factors.
The canonical Wnt pathway converts members of the TCF/LEF family of transcription factors from repressors to activators of downstream target genes. A wide range of single and double knockout models of the TCF/LEF family members have been used to decipher the important roles they play in mouse development (Galceran et al., 1999; Gregorieff et al., 2004; Korinek et al., 1998; Merrill et al., 2004; van Genderen et al., 1994; Verbeek et al., 1995). Several TCF/LEF family members are expressed during pituitary gland development (Olson et al., 2006), and the absence of Tcf4 (officially known as Tcf7l2) leads to pituitary gland hyperplasia, suggesting that canonical Wnt signaling could regulate pituitary gland growth through TCF4 (Brinkmeier et al., 2003).
The ventral diencephalon and Rathke’s Pouch develop from neighboring areas of the embryo that remains in intimate contact (Couly and Douarin, 1985). It is not known whether this is an active or passive process, but signaling between these structures is required for proper pituitary gland development (Gleiberman et al., 1999; Treier et al., 1998). The ventral diencephalon provides the inductive factors necessary for the invagination of the oral ectoderm, and the subsequent proliferation of the early progenitor cells to form Rathke’s pouch. BMP4 is a key signaling factor in the induction of the oral ectoderm. The invagination of Rathke’s pouch is absent in Bmp4−/− mice (Takuma et al., 1998), and excess BMP signaling in Noggin (BMP inhibitor) deficient mice, results in multiple invaginations of Rathke’s pouch (Davis and Camper, 2007). In addition, FGF signaling from the ventral diencephalon is necessary for both expression of Lhx3 and cell survival in Rathke’s pouch (De Moerlooze et al., 2000; Kimura et al., 1996; Norlin et al., 2000; Ohuchi et al., 2000; Takuma et al., 1998). Rathke’s pouch invaginates properly in the absence of Fgf8, Fgf10, or Fgfr2(IIIB), but Lhx3 is not activated, and cells in Rathke’s pouch undergo excessive apoptosis (De Moerlooze et al., 2000; Ohuchi et al., 2000; Takuma et al., 1998). Lhx3 and Lhx4 deficiencies cause extensive cell death within the pouch (Raetzman et al., 2002; unpublished observations). Thus, FGF and BMP signaling from the ventral diencephalon, as well as Lhx transcriptional regulation, are required for normal pituitary gland development (Treier et al., 1998).
Wnt signaling negatively regulates growth by antagonizing FGF and BMP signaling in multiple organs including the lung, teeth, and brain (Bellusci et al., 1997; Bellusci et al., 1996; Clark et al., 2001; Dean et al., 2005; Kratochwil et al., 2002; Kuschel et al., 2003; Sasaki et al., 2005; Shimogori et al., 2004; Weaver et al., 1999). For example, disruption of canonical Wnt signaling in the lung reduces BMP4 and FGF signaling, resulting in repression of distal airway epithelial differentiation and expansion of proximal airway development (Shu et al., 2005). In addition, overexpression of Wnt5a in the lung results in an increase in Fgf10 and Bmp4 expression and reduced epithelial branching (Li et al., 2005).
Despite the strong evidence of interactions between these pathways in multiple organs, the impact of Wnt signaling on BMP and FGF pathways has not been assessed in pituitary organogenesis. We report the temporal and spatial expression patterns of Tcf4, Tcf3, and Lef1 in the developing pituitary gland and ventral diencephalon. The results support the possibility of an interaction between TCF-mediated regulation of gene expression and BMP and FGF signaling. Consistent with this idea, we show that loss of Tcf4 permits expansion of FGF and BMP expression in the ventral diencephalon, with a concomitant increase in the number of progenitor cells that form Rathke’s pouch. Six6 transcripts and an extended region of proliferation can be detected throughout the expanded population of progenitor cells, suggesting Six6 may contribute to pituitary hyperplasia in Tcf4 mutant mice. The increased pouch size contributes to a 2 to 3 fold increase in the anterior pituitary volume of TCF4 deficient mice at birth (Brinkmeier et al., 2003). Thus, TCF4 may play a role as a repressor to regulate growth of Rathke’s pouch by influencing BMP and FGF signaling.
Materials and Methods
Generation of Mice
Dr. Hans Clevers, University Medical Center Utrecht, Heidelberglaan provided mice heterozygous for targeted disruption of the Tcf4 locus. They have been maintained at the University of Michigan in compliance with the guidelines set by the Unit of Laboratory Animal Medicine. The postnatal lethality of Tcf4−/− mice necessitates the maintenance of the strain through heterozygous matings. Genomic DNA, extracted from tail biopsies, was used to genotype mice at 2 weeks of age. PCR amplification of genomic DNA with primers specific for Tcf4 identified the wild type allele and primers recognizing hygromycin identified the knockout allele (Brinkmeier et al., 2003).
Preparation of Tissues
Timed pregnancies were set up with Tcf4+/− matings. The morning the copulation plug was observed is noted as embryonic day 0.5 (e0.5). For experiments analyzing the incorporation of 5-Bromo-2′ deoxyuridine (BrdU), pregnant females were injected intraperitoneally with 100mg/g body weight of BrdU two hours before embryo retrieval. Embryos were dissected at various time points during development and tail biopsies were used for genotyping as described above. Embryos were fixed in buffered 4% paraformaldehyde for 30 minutes to overnight. After fixation, the embryos were washed in phosphate buffered saline (PBS) and dehydrated. The embryos were embedded in paraffin, sectioned to 6μm thickness and then either stained with hematoxylin & eosin or processed as described below.
Immunohistochemistry
Sagittal sections of paraffin embedded embryos were used in all of the immunohistochemistry experiments. Prior to antibody application, slides were boiled for ten minutes in 10 mM citric acid and treated with a 1:1 mixture of methanol: 3% hydrogen peroxide. Mouse anti-TCF4 (1:50; Upstate Cell Signaling, Temecula, CA), rabbit anti-phospho-SMAD1 (1:200; Cell Signaling Technologies, Inc., Danvers, MA), mouse anti-p27 (1:150; Oncogene Research Products, Boston, MA), rabbit anti-phospho ERK (1:100; Cell Signaling Technologies, Inc. Danvers, MA), rabbit anti-human C FOXL2 (1:200, Dr. Reiner A. Veitia, University of Paris (Cocquet et al., 2002)), rabbit anti-PITX1 (1:200; Jacques Drouin, Laboratory of Molecular Genetics, University of Montreal), rabbit anti-PITX2 (1:200; Tord Hjalt, Department for Cell and Molecular Biology, Lund University, Sweden), mouse anti-NOTCH2 (1:150; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), and mouse anti-activated β-catenin (1:100; Upstate Cell Signaling, Temecula, CA) were incubated overnight at 4°C. The following day the sections were washed and then incubated with either a biotinylated anti-mouse IgG (1:200; Vectastain Mouse on Mouse Kit, Vector Laboratories, Burlingame, CA) or a biotinylated anti-rabbit IgG (1:200, Jackson ImmunoResearch Laboratories, Inc. West Grove, PA). The antibodies were detected using the reagents and protocols provided in the tyramide signal amplification (TSA) fluorescein kit (Perkin Elmer, Boston, MA). Rabbit anti-CyclinD2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-LHX3 (1:1000; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) were incubated overnight at 4°C. The following day, the sections were washed and incubated with the species appropriate biotin conjugated IgG as described above. This was followed by incubation with a streptavidin-conjugated Cy2 fluorophore (1:200; Jackson ImmunoResearch Laboratories). Mouse anti-ISL1 (1:600; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) was also incubated overnight at 4°C. Biotinylated secondary antibodies were used in conjunction with the TSA Fluorescein kit as described above or with avidin and biotinylated peroxidase (Vectastain Mouse On Mouse kit, Vector Laboratories). With this experiment, either diaminobenzidine (DAB) was used as the chromogen (Sigma, St. Louis, MO) or the tissues were processes with the TSA-fluorescein kit (Perkin Elmer, Boston, MA). For the cell proliferation studies, a rat anti-BrdU (1:100; Harlan Sera, Belton, UK) was incubated overnight at 4°C. After a series of washes, the sections were then incubated with a biotin-conjugated rat IgG (1:200; Jackson ImmunoResearch) followed by the streptavidin-conjugated Cy2 fluorophore as described above. Programmed cell death was detected by the terminal deoxyuridine triphosphate nick end labeling method (TUNEL) as previously described (Ward et al., 2006) using the In situ cell death detection kit (Roche, Indianapolis, IN).
In situ hybridization
In situ hybridization was performed on 6μm sections using riboprobes labeled with digoxigenin (Roche, Indianapolis, IN) (Douglas et al., 2001). A Lef1 cDNA clone containing the 3′ end of the coding sequence and the 3′UTR (1797–2455 bp) was a gift from Dr. Hans Clevers. Tcf4E cDNA was excised with XbaI from an expression plasmid received from Dr. Greg Dressler, (University of Michigan) and was subcloned into pBluescript KS+. It was linearized with BglI at 1738bp in order to specifically label the 3′UTR. A 1.4kb HindIII (1840 bp to 3040 bp) fragment from the 3′UTR of Tcf4B (a gift from Dr. Greg Dressler) was subcloned into pBluescript KS+. A Tcf4ΔDBD 900 bp cDNA clone, described in Douglas et al., 2001 was subcloned into the BamHI and XbaI sites of pBluescript KS+. A 1600 bp Six6 clone was obtained from an embryonic pituitary cDNA library RIKEN K720034H14 (Carninci et al., 2003). A 584 bp Fgf10 cDNA and a 1550 bp Bmp4 cDNA clone were gifts from Dr. Brigid Hogan, Duke University Medical Center. An 846 bp Prop1 cDNA clone was used for in situ hybridization as previously described (Cha et al., 2004).
Results
Tcf4 is expressed in the ventral diencephalon and Rathke’s pouch
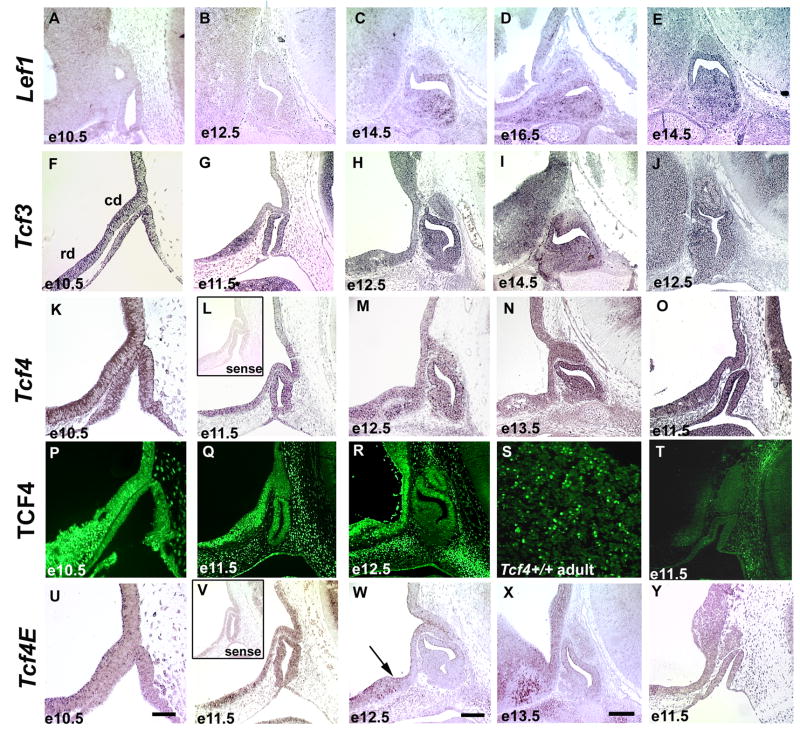
There are four members of the TCF/LEF family of transcription factors that share a highly conserved β-catenin binding domain and an HMG box type DNA binding domain (Figure 1A–F). The expression patterns of TCF/LEF family members were analyzed, expanding upon previously published data by examining the early stages of pituitary organogenesis (Olson et al., 2006). Lef1 transcripts are not detected in the ventral diencephalon, but are clearly present within the developing anterior pituitary gland at late stages, initiating at e14.5 and still detectable at e16.5 (Figure 2A–D). Tcf3 transcripts are present throughout the ventral diencephalon and Rathke’s pouch at e10.5 (Figure 2F). Tcf3 transcripts are more prevalent in the rostral domain of the ventral diencephalon than in the caudal domain from e11.5 through e14.5, and are also detectable in the dorsal aspect of Rathke’s pouch at during this period (Figure 2G–I). Tcf1 transcripts are not detectable in the ventral diencephalon or Rathke’s pouch from e10.5 through e16.5 (data not shown).
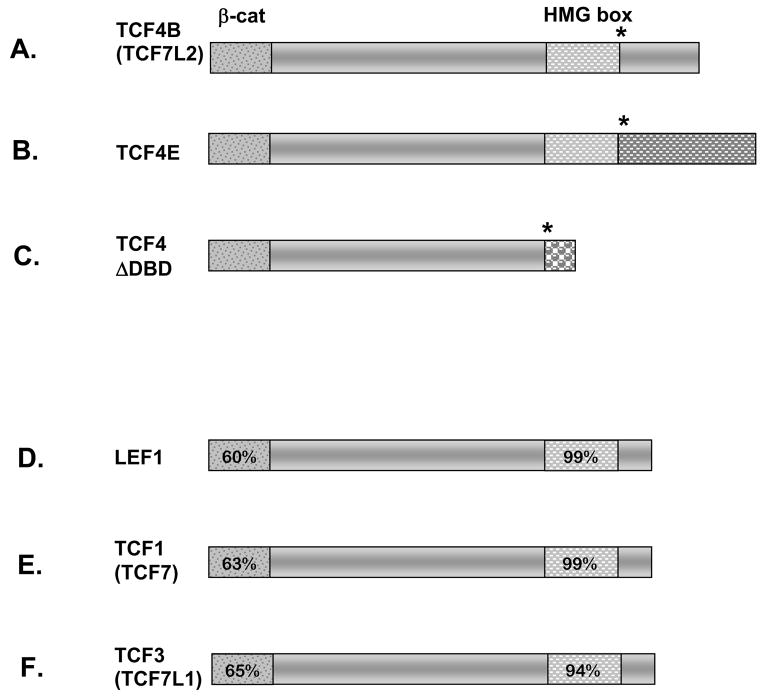
Figure 1.
TCF4 isoforms and conservation within the TCF/LEF family. Amino acid comparison of TCF/LEF family members in the mouse reveals a high degree of homology within the β-catenin binding domain and HMG box (patterned boxes in panels A–F). Percentages depicted in the conserved domains were determined by comparing the coding sequence to TCF4E (A–C). Alternative splicing results in three Tcf4 mRNAs and protein isoforms. TCF4, officially named TCF7L2, can be spliced (*) downstream of the HMG box to generate the TCF4B and TCF4E proteins, panels A and B. Panel C shows an alternative splice site (*) before the HMG box generates a protein that is missing the DNA binding domain, TCF4ΔDBD. The unique amino acid domains in the carboxy termini of TCF4B, TCF4E, and TCF4ΔDBD isoforms are depicted with different patterns.
Figure 2.
The temporal and spatial expression pattern of Lef1, Tcf3, and Tcf4 in the developing pituitary gland. In situ hybridization was used to analyze Lef1, Tcf3, Tcf4, and Tcf4E expression in the developing anterior pituitary gland and the adjacent dorsal, caudal domain (cd) and ventral, rostral domain (rd) of the ventral diencephalon. Immunohistochemistry utilizing an antibody that recognizes all TCF4 isoforms was used to confirm developmental expression. The scale bar in panel U represents 50μm and should be applied to e10.5 (A, F, K, P, and, U), and e11.5 (B, G, L, O, Q, T, V, and Y) pituitary sections. The scale bar for the e12.5 (B, H, J, M, R, and V), e13.5 (N and X) and e14.5 (C and I) pituitary sections represents 100μm and is shown in panel W. The arrow in e12.5 sections labeled with Tcf4E marks the boundary between the caudal and rostral domains of the ventral diencephalon. The insets in panels L and V represent sense probes from Tcf4 and Tcf4E, respectively. Panel S is a high magnification (400X) image of an adult pituitary gland.
Alternate splicing generates three protein isoforms of TCF4. TCF4B and TCF4E contain both conserved domains but differ in their carboxy termini (Figure 1A, B). TCF4ΔDBD lacks the DNA binding domain but retains the β-catenin binding domain, suggesting it could act as an inhibitor of the other isoforms (Figure 1C) (Douglas et al., 2001; Kennell et al., 2003). We report the temporal and spatial expression patterns of TCF4 using immunohistochemistry with an antibody reactive to all TCF4 isoforms and in situ hybridization with probes designed for the either the TCF4E isoform specifically or all isoforms combined. In situ hybridization using a probe that detects all Tcf4 isoforms reveals expression throughout the caudal and rostral domains of the ventral diencephalon at e10.5, through e12.5, in addition to high levels within Rathke’s pouch (Figure 2K–M). By e13.5 expression becomes localized to the rostral domain of the ventral diencephalon and the dorsal aspect of Rathke’s pouch (Figure 2N). At e14.5 and e16.5 expression is not detected in the developing pituitary gland, and it is diminished in the rostral domain of the ventral diencephalon (data not shown). These in situ results conflict with the previously described protein expression of TCF4 in the ventral aspect of Rathke’s pouch at e14.5 (Cha et al., 2004). We analyzed protein expression with an alternate antibody and found an overlapping expression pattern with Tcf4 mRNA in the pituitary and in other areas of the embryo, suggesting that the previous results were not specific. Using the new antibody, TCF4 immunoreactivity is present within the ventral diencephalon, Rathke’s Pouch, and the adult pituitary gland (Figure 2P–S), which corresponds to the location of the multi-isoform Tcf4 transcripts. Tcf4E specific transcripts are detectable throughout the caudal and rostral domains of the ventral diencephalon at e10.5 (Figure 2U). The caudal (and more dorsal) domain of the ventral diencephalon is defined by a region expressing BMP4 and FGF10 (Supplemental Figure 1), while a region of sonic hedgehog expression defines the rostral domain (Ericson et al., 1998; Treier et al., 1998)). Tcf4E expression becomes localized to the lower domain of the ventral diencephalon beginning at e11.5 and continuing through e12.5 (Figure 2V, W). The expression of Tcf4E remains high in the rostral domain of the ventral diencephalon at e13.5 (Figure 2X) but begins to diminish by e16.5 (data not shown). In situ hybridization using a probe that detects both Tcf4B and Tcf4E isoforms mirrors the expression pattern of the specific Tcf4E isoform probe (data not shown). The expression domain detected with the multi-isoform Tcf4 probe, but not the Tcf4E and Tcf4B probes, suggests that the Tcf4ΔDBD isoform is expressed in Rathke’s pouch in addition to the ventral diencephalon.
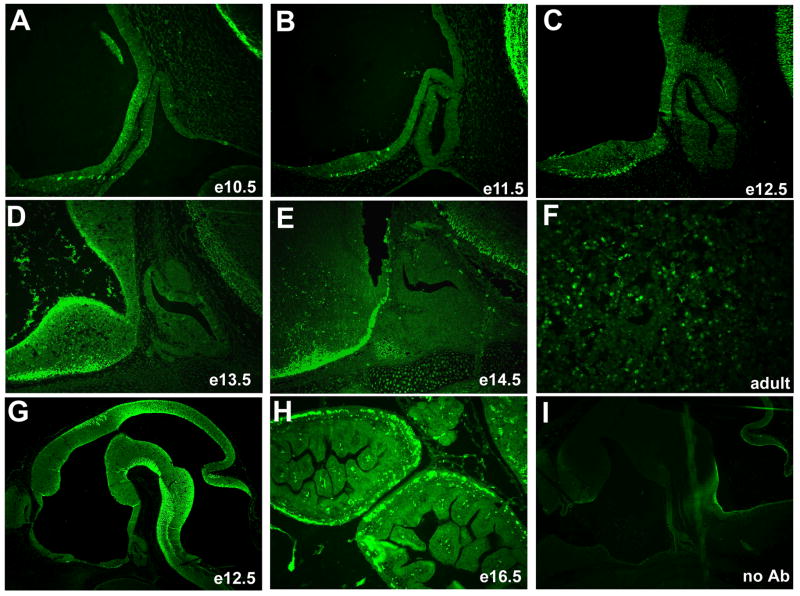
We determined the spatial and temporal pattern of activated β-catenin using immunohistochemistry (Figure 3). The strongest immunoreactivity is notable in the ventral diencephalon from e10.5 through e14.5 (Figure 3A–E). The level of staining is lower in the caudal domain of the ventral diencephalon and the infundibulum than in the more ventral and rostral area. The most prominent expression in Rathke’s pouch and its derivatives is in the rostral tip at e14.5 (Figure 3E). Selected cells in the adult anterior lobe contain strongly immunoreactive β-catenin (Figure 3F), which contrasts with embryonic pituitary stages.
Figure 3.
Activated, nuclear localized β-catenin predominates in the ventral diencephalon of developing mice. Immunohistochemistry reveals little or no staining in the developing pituitary gland from e10.5 through e13.5, with some staining of the rostral tip area at e14.5 (A–E, 200X). Activated β-catenin can be easily detected in the adult pituitary gland (F, 400X). Activated β-catenin can also be deteced in other known regions of WNT signaling such as e12.5 brain (G, 50X) and e16.5 intestine (H, 100X). Sections incubated without primary antibody do not show any nuclear staining (I, e13.5 brain, 50X).
The expression pattern of activated β-catenin, Tcf4 and Tcf3 isoforms suggests they could influence the development of Rathke’s pouch indirectly by regulating signals emanating from the ventral diencephalon, while the expression of Lef1 within Rathke’s pouch suggests it could have an intrinsic effect on pituitary cell maintenance or differentiation. Overexpression of Tcf4ΔDBD and an engineered dominant negative Tcf4E isoform missing the β-catenin binding domain, Tcf4Δβ-cat, in Rathke’s pouch of transgenic mice has no obvious effect on pituitary development (Supplementary Figure 2 and 3). The transient transgenic mouse analysis supports the hypothesis that the expression TCF4ΔDBD within Rathke’s pouch has little effect on pituitary development.
Mice with a disruption of Tcf4 that interrupts the HMG box DNA binding domain were previously described, but the expression of TCF4 isoforms in these mice has not been addressed (Brinkmeier et al., 2003; Korinek et al., 1998). In situ hybridization reveals that despite low levels of Tcf4 mRNA expression in the brain (data not shown), Tcf4E transcripts are not detectable in the ventral diencephalon at e11.5 in Tcf4−/− mice (Figure 2Y). mRNA was detected with the multi-isoform Tcf4 mRNA probe in the ventral diencephalon and Rathke’s pouch of Tcf4−/− mice at e11.5 (Figure 2O); however, no TCF4 protein was detected by immunohistochemistry, impling the targeted disruption affects all TCF4 isoforms and is a null allele (Figure 2T). Other members of the TCF/LEF family do not appear to compensate for the loss of TCF4, as the expression of Tcf3 and Lef1 is unchanged in Tcf4−/− mice (Figure 2E, J).
Dysmorphology of Rathke’s pouch is evident in Tcf4−/− mice by 10.5
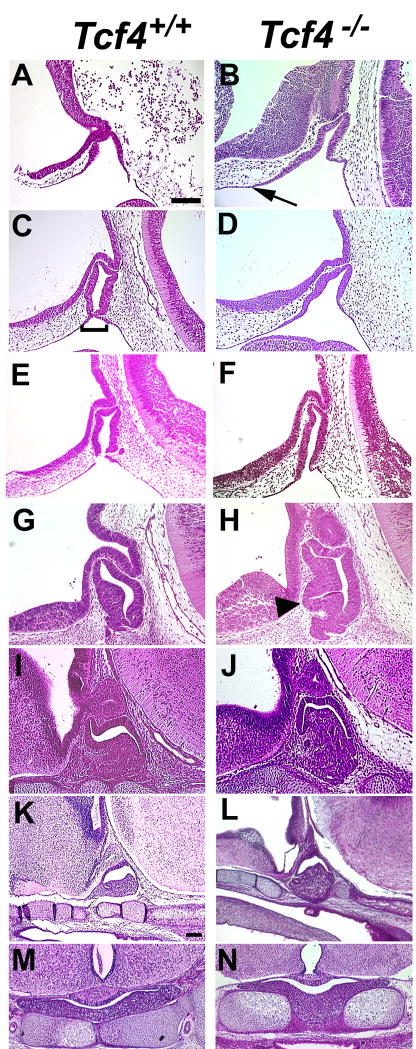
The absence of TCF4 results in an increase in size of the developing anterior pituitary gland at e14.5, leading to a 2–3 fold larger volume by e18.5 (Brinkmeier et al., 2003). This suggests that TCF4 is necessary for regulation of pituitary growth. In order to discover the onset of the pituitary growth abnormality, hematoxylin and eosin stained pituitary sections from Tcf4−/− mice were compared to similar sections from Tcf4+/+ littermates at e9.5 through e18.5 (Figure 4). There are no obvious morphological changes in Tcf4−/− pituitary glands at e9.5 relative to wild type (data not shown). By e10.5, the invaginating Rathke’s pouch in mutants appears to extend farther rostrally on the side adjacent to the ventral diencephalon (Figure 4A, B). This expanded region is more evident at e11.5, as wild type cells that are separating from the oral ectoderm thin out and pinch together (Figure 4C, D). This characteristic thinning of cells occurs in Tcf4−/− mice at the appropriate location on the caudal side of the pouch but the thinning on the rostral side adjacent to the ventral diencephalon is displaced farther rostrally in the mutants. The characteristic pinching off of Rathke’s pouch is usually delayed in Tcf4 mutants, but in rare cases it occurs at e11.5 (n=1 out of 6) (Figure 4E, F). The size of the mutant pouch appears larger suggesting that excess oral ectoderm is incorporated into the developing pituitary gland. Excess cells in the mutant are even more obvious at e12.5 and e14.5 (Figure 4G–J). The size of the mutant anterior pituitary gland is consistently larger than wild type, and it protrudes through the cartilage plate at e16.5 and e18.5 (Figure 4K–N). This suggests that TCF4 normally influences the location at which Rathke’s pouch separates from the oral ectoderm, which affects the overall size of the pituitary gland. In the absence of TCF4, additional oral ectoderm appears to be recruited to form Rathke’s pouch.
Figure 4.
Pituitary dysmorphology is evident by e10.5 in Tcf4−/− mice. Hematoxylin and eosin staining were used to determine the onset of pituitary dysmorphology in Tcf4−/− mice compared to Tcf4+/+ controls. Rathke’s pouch is thickened and expanded rostrally beginning at e10.5 (panel A, B, arrow). Thinning of the oral ectoderm and the separation of Rathke’s pouch (panel C, bracket) is evident in Tcf4+/+ mice at e11.5 (panel C, E) but is typically delayed in Tcf4−/− mice (panel D). One example of the separation of the pouch from the oral ectoderm has been identified in a Tcf4−/− mouse at e11.5 (panel F). The thickened and expanded Rathke’s pouch is separated from the oral ectoderm by e12.5 in Tcf4−/− mice and the additional cells are incorporated into the anterior pituitary (panel H, arrowhead). Sagittal sections from fetuses at e10.5 through e16.5 (panels A–L) are oriented with rostral on the left and e18.5 (panels M, N) are coronal sections. Panels from e10.5 through e14.5 are the same magnification; e16.5 and e18.5 are at a lower magnification. The scale bars in panels A and K represent 100μm.
A reduction in cell death and an extended region of proliferation in Tcf4−/− mice contributes to an increase in the anterior pituitary gland size
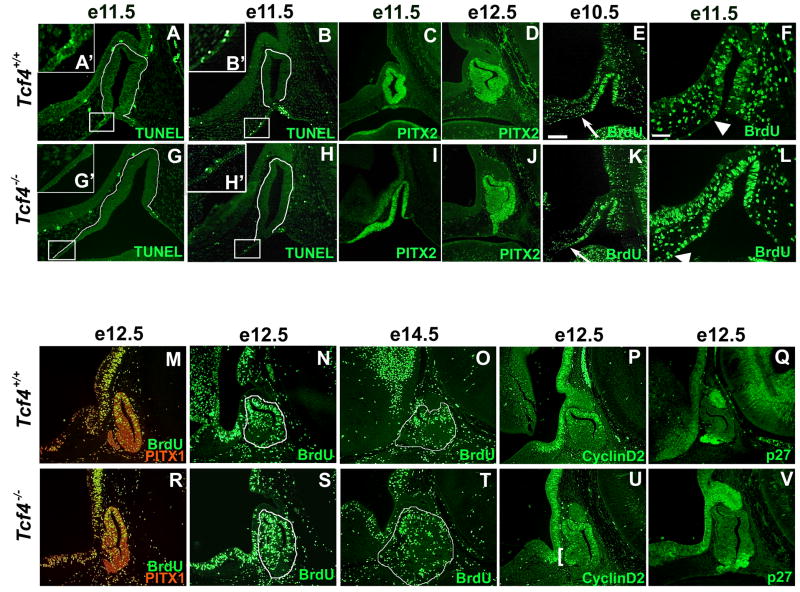
Apoptosis normally occurs at the sites where Rathke’s pouch separates from the oral ectoderm (Charles et al., 2005). TUNEL staining of Tcf4+/+ mice at e11.5 confirmed the presence of apoptotic cells in normal mice at the rostral and caudal aspects of Rathke’s pouch where separation from the oral ectoderm occurs (Figure 5A, B). In Tcf4−/− mice the apoptotic cells on the caudal side of Rathke’s pouch are present at the same time as in wild type mice; however, apoptotic cells on the rostral side are rarely detected (Figure 5G). This rostral region is thicker than normal and evidence of apoptosis cannot always be detected in Tcf4−/− mice (none, n=3; some, n=3, Figure 5G, H). Apoptotic cells, when detectable, are localized more rostrally in mutants than in normal littermates. The homeobox transcription factor PITX2 is expressed in a rostrally expanded manner in Tcf4−/− mice (Figure 5C, I). The separation of Rathke’s pouch from the oral ectoderm is consistently complete by e12.5 in wildtype and mutant mice (Figure 5D, J). No PITX2 staining is detected in the oral ectoderm at e12.5, indicating that the enlarged area of PITX2 staining at e11.5 is incorporated into the pouch by e12.5.
Figure 5.
Rathke’s pouch dysmorphology in Tcf4−/− mice is caused by an inappropriate separation from the oral ectoderm and an increased zone of proliferating cells. TUNEL staining showed apoptotic cells at the rostral and caudal edges where Rathke’s pouch separates from the oral ectoderm in e11.5 Tcf4+/+ mice (A, B). TUNEL staining is absent on the rostral side of Rathke’s pouch in the majority of Tcf4−/− mice (panel G and inset G′). Separation of Rathke’s pouch from the oral ectoderm and TUNEL positive cells can be identified on rare occasions Tcf4−/− mouse (panel H, inset H′). The rostral edge of apoptosis is boxed in panel A, B, G, H and shown in the higher magnification insets A′, B′, G′, H′. The cells forming Rathke’s pouch are outlined in white in the TUNEL panels (A, B, G, H). The expression of PITX2 at e11.5 and e12.5 was used to mark Rathke’s pouch cells (C, D, I, J). Proliferation of cells in Rathke’s pouch between e10.5 and e14.5 was analyzed in Tcf4+/+ and Tcf4−/− mice (E, F, K, L–O. R–T). BrdU immunohistochemistry reveals a zone of increased proliferation on the rostral side of Rathke’s pouch in Tcf4−/− mice (K, L, R. S). The arrows in e10.5 denote the rostral ventral boundary of BrdU detection (E, K), which is expanded in Tcf4−/− mice. The arrowhead in e11.5 panels F and L denote the expanded boundary between proliferating and nonproliferating cells in Rathke’s pouch of Tcf4−/− mice compared to Tcf4+/+ mice. The additional proliferating cells in Tcf4−/− mice are incorporated into the rostral ventral side of Rathke’s pouch by e12.5 in both parasagittal (R) and midsagittal (S) sections. Proliferating cells are absent in comparable regions of Rathke’s pouch in Tcf4+/+ mice (M, N). Parasagittal sections were co-immunostained with PITX1 in red to clearly identify Rathke’s pouch cells (M, R). There were no differences in proliferation at e14.5 between Tcf4+/+ and Tcf4−/− mice (O, T). Rathke’s pouch is outlined in e12.5 and e14.5 midsagittal sections (N, O, S, T). CyclinD2 immunoreactivity, marking the G1 phase of the cell cycle, was used to confirm the additional region of proliferation at e12.5 (P vs. U, bracket). p27 immunoreactivity, marking post mitotic cells, is present in the portion of Rathke’s pouch that ceases proliferation in both Tcf4+/+ and Tcf4−/− mice at 12.5 (Q, V). Panels C, D, E, I, J, K, and M–V were taken at the same magnification, and the scale bar in panel E represent 100μm. Panels A, B, F, G, H, and L were taken at the same magnification, and the scale bar in panel F represents 50μm.
Cell proliferation was analyzed by the incorporation of BrdU in normal and Tcf4−/− pituitary glands. The most striking proliferation difference is evident between e10.5 and e11.5 (Figure 5E, F, K, L). In Tcf4+/+ mice, BrdU staining is more prominent in Rathke’s pouch than in the oral ectoderm rostral and caudal to it. Tcf4−/− mice exhibit a larger region of prominent BrdU staining that extends much farther rostrally at e10.5 (n=2) and at e11.5 (n=4). The extended area of cell proliferation is evident within Rathke’s pouch even after it completely separates from the oral ectoderm in Tcf4−/− embryos at e12.5 (n=3) (Figure 5N vs. S). Parasagittal sections from the same embryos were co-immunostained with BrdU and another homeobox transcription factor PITX1 to clearly identify Rathke’s pouch material (Suh et al., 2002). Additional pouch tissue is evident at a midsagittal level and more parasagittally on both sides of the midline, suggesting that extra proliferating cells are present throughout Rathke’s pouch at e12.5 (Figure 5M, R). The increase in proliferation is not evident in, Tcf4−/− pituitary glands, at e14.5 (Figure 5O, T). Immunohistochemistry using antibodies against CyclinD2, a marker of the G1 phase of the cell cycle, confirms an additional domain of proliferating cells at e12.5 in Tcf4−/− pituitary glands that is not present in normal mice (n=3) (Figure 5P, U bracket). Cessation of proliferation in the anterior pituitary gland appears normal at e12.5, as assessed by expression of the post-mitotic cell marker p27 in the ventral aspect of the gland (n=3) (Figure 5Q, V) (Nakayama et al., 1996).
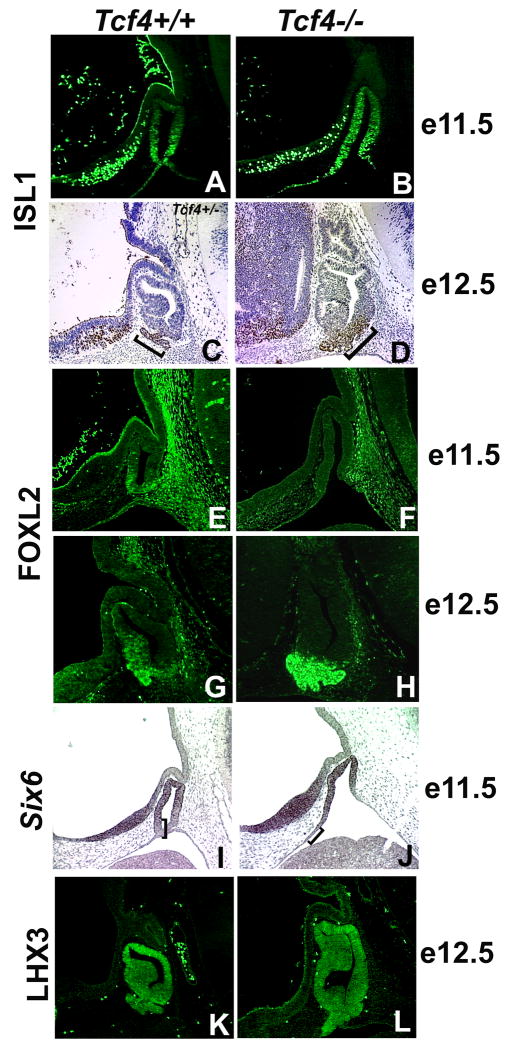
Differentiation begins in the ventral region of Rathke’s pouch after the separation from the oral ectoderm around e11.5 in wild type mice (Mullis, 2001). ISL1, which is initially expressed throughout Rathke’s pouch, becomes concentrated in the ventral cells at e11.5 (Figure 6A) (Treier et al., 1998). In Tcf4−/− mice, ISL1 is detectable throughout Rathke’s pouch at e11.5 and does not become localized to the ventral cells until e12.5 (Figure 6B–D). Another marker of differentiation, FOXL2, is readily detected in the ventral aspect of Rathke’s pouch cells of normal mice by e11.5 (Figure 6E) (Ellsworth et al., 2006). FOXL2 is not detectable in the pouch cells of Tcf4−/− mice at e11.5, but its expression recovers completely by e12.5 (Figure 6F–H). Both ISL1 and FOXL2 expression suggest a delay in the ventral cell differentiation program. Six6 is expressed in a reciprocal pattern to that of ISL1 and FOXL2. Six6 is a transcription factor that stimulates progenitor cell proliferation in the pituitary (Li et al., 2002). Six6 transcripts are detected in the rostral domain of the ventral diencephalon and the dorsal and middle regions of Rathke’s pouch in normal mice at e11.5 (Figure 6I, bracket). In Tcf4−/− mice the expression of Six6 is appropriately concentrated in these regions, but expression on the side adjacent to the ventral diencephalon is expanded rostrally and ventrally (n=3) (Figure 5J, bracket). The extended area of Six6 expression in Tcf4 mutants corresponds to the extended area of proliferating cells (Figure 5L, Figure 6J). The expression of the early markers LHX3, NOTCH2, and Prop1, are not obviously changed in Tcf4−/− mice compared to Tcf4+/+ mice (Figure 6K, L and data not shown).
Figure 6.
Differentiation of ventral Rathke’s pouch cells is delayed in Tcf4−/− mice. Immunohistochemistry for ISL1 at e11.5 (A, B) and e12.5 (C, D) as well as FOXL2 at e11.5 (E, F) and e12.5 (G, H) were used to determine the onset of cell differentiation in the ventral aspect of Rathke’s pouch. In situ hybridization for Six6 at e11.5 (I, J) and immunohistochemistry for LHX3 at e12.5 (K, L) were used to analyze the progress of cell differentiation in the dorsal aspect of Rathke’s pouch. The brackets in panels I and J mark the region void of Six6 expression.
TCF4 mutants exhibit increased FGF and BMP signaling
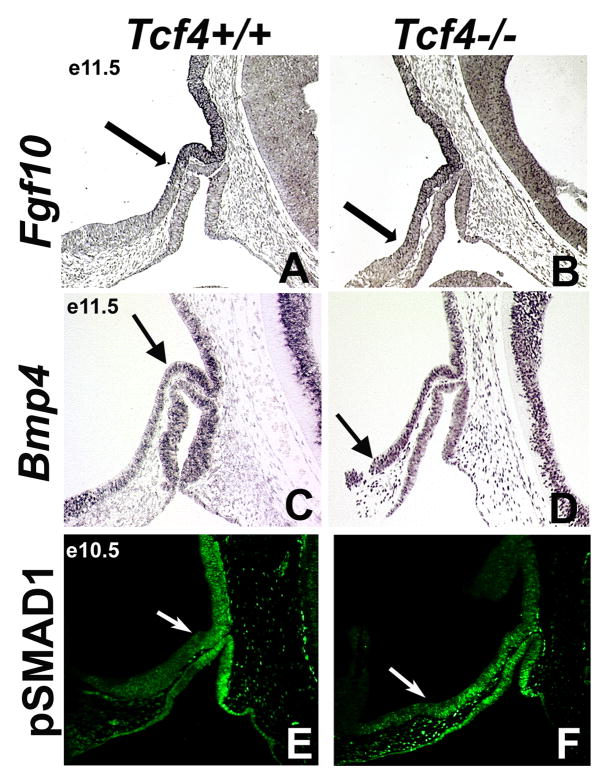
Wnt signaling regulates FGF and BMP signaling in the organogenesis of many tissues (Jiang and Harrison, 2005; Litsiou et al., 2005; Shu et al., 2005). FGF10 and BMP positively regulate pituitary gland growth and cell survival (Ericson et al., 1998; Norlin et al., 2000; Ohuchi et al., 2000). This contrasts with TCF4, which negatively regulates pituitary growth (Brinkmeier et al., 2003). Based on these previous studies and on the expression patterns of FGF, BMP, and TCF4 in the ventral diencephalon and Rathke’s pouch (Supplementary Figure 1), we hypothesize that WNT signaling negatively regulates FGF and BMP mediated stimulation of pituitary gland growth and cell survival. To test this idea, we compared expression patterns of Fgf10, Bmp4, and pSMAD1 in Tcf4+/+ and Tcf4−/− embryos. Fgf10 transcripts are most prominent in the dorsal and caudal domain of the ventral diencephalon at e11.5 in Tcf4+/+ mice (Figure 7A, arrow). The expression domain of Fgf10 is expanded rostrally in the ventral diencephalon in Tcf4−/− mice at e11.5 (n=3) (Figure 7B, arrow). In some tissues, FGF signaling causes the phosphorylation of ERK. However, pERK does not appear to mark FGF signaling in the pituitary gland (Supplemental Figure 4). Bmp4 mRNA is detected in a region of the ventral diencephalon that is expanded rostrally and ventrally in Tcf4−/−mice at e11.5 relative to wild type (n=3) (Figure 7C, D, arrows). Consistent with this observation, immunohistochemistry with antibodies against phospho-SMAD1, a protein activated by BMP signaling, reveals a rostral and ventral expansion of the BMP signaling domain in TCF4 mutants relative to wild type at e10.5 (n=3) (Figure 7E, F, arrows). The expansion of FGF and BMP signaling in the ventral diencephalon is implicated as a mechanism for recruiting additional oral ectoderm tissue into Rathke’s pouch and enhancing pituitary growth and size.
Figure 7.
The boundaries of BMP and FGF signaling are expanded rostrally in Tcf4−/− mice. In situ hybridization for Fgf10 at e11.5 (A, B), Bmp4 at e11.5 (C, D), and immunohistochemistry for the phosophorylated form of SMAD1, pSMAD1, a protein activated by BMP, at e10.5 (E, F) were examined in Tcf4+/+ and Tcf4−/− mice. The boundaries Fgf10, Bmp4, and pSMAD1 expression in the ventral diencephalon are expanded rostrally in Tcf4−/− mice (A–F, arrows).
Discussion
Several lines of investigation have implicated canonical Wnt signaling as an important process in pituitary development (Ai et al., 2007; Brinkmeier et al., 2003; Cha et al., 2004; Douglas et al., 2001; Kioussi et al., 2002; Olson et al., 2006; Salisbury et al., 2007; Treier et al., 1998). The foundation for understanding this process requires an in-depth knowledge of the temporal and spatial expression patterns of the genes regulated by canonical WNT signaling during pituitary development, such as the TCF/LEF family. From our expression analysis we predict that TCF3 and TCF4 may have overlapping roles and act both extrinsically and intrinsically on pituitary development. Extrinsic effects of TCF3 and TCF4 may involve regulation of genes expressed in the ventral diencephalon that induce development of the pituitary primordium, as we illustrate here for TCF4 and the BMP and FGF signaling pathways. TCF3 and TCF4 may also act intrinsically during pituitary development to regulate pituitary precursor cells exiting the cell cycle to begin differentiation. This idea is supported by our demonstration that nuclear localized β-catenin predominates in the ventral diencephalon during the time that Rathke’s pouch formation is being induced. LEF1 likely has a later, intrinsic role, as the individualized hormone-producing cells within the anterior lobe complete their differentiation process (Olson et al., 2006).
These ideas for the roles of TCF/LEF family members in pituitary development are supported by their known roles in other organs where TCF4 and TCF3 are important in the advancement of early precursor cell populations (Korinek et al., 1998; Merrill et al., 2004). TCF4 is crucial in maintaining the proliferative compartment of the crypts in between the villi of the small intestine (Korinek et al., 1998), and TCF3 is required to restrict the induction of the anterior-posterior axis during early gastrulation (Merrill et al., 2004). In contrast, LEF1 is not required for early induction or proliferation in the tooth bud, but it is required for cell survival in the early tooth bud and advancement from the late bud stage (Sasaki et al., 2005).
TCF4 mutants have normal proportions of differentiated pituitary cell types at birth, but the anterior lobe of the organ is dramatically enlarged (Brinkmeier et al., 2003). The effects of the targeted disruption of Tcf4 on expression of TCF4 isoforms and the mechanism whereby TCF4 regulates pituitary growth had not been investigated previously. We report that two of the TCF4 isoforms (Tcf4B and E) are expressed primarily in the rostral domain of the ventral diencephalon, while Tcf4ΔDBD, an isoform that lacks the HMG box DNA binding domain, is expressed in the dorsal cells of Rathke’s pouch. We show that the targeted disruption of Tcf4 is essentially a null allele, with no detectable protein isoforms. Using these mice, we demonstrate that TCF4 is necessary for normal expression of other critical pituitary factors in both the ventral diencephalon and in the dorsal aspect of Rathke’s pouch.
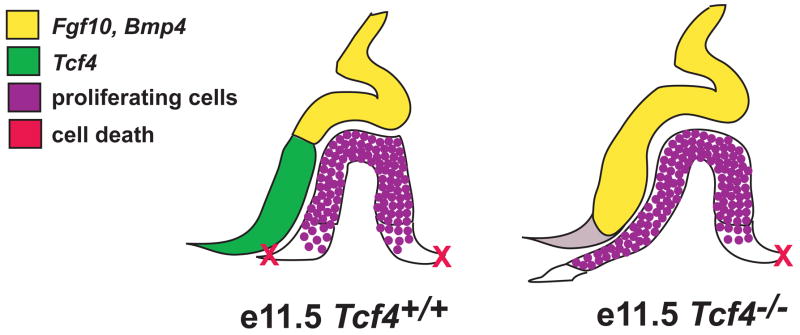
The overgrowth of the anterior lobe of the pituitary gland in TCF4 deficient mice is associated with an expansion of the area of oral ectoderm that is recruited to form pituitary precursors. Previous studies have shown that BMP and FGF signals emanating from the ventral diencephalon are critical for induction of Rathke’s pouch (Davis and Camper, 2007; Takuma et al., 1998). We show that both of these expression domains are expanded in TCF4 mutants. It is plausible that excessive signaling causes the recruitment of excess oral ectoderm tissue to form the pouch and that canonical WNT signaling, via regulation of TCF4 activity, influences the output of the BMP and FGF signaling pathways (Figure 8). This idea is consistent with studies that reveal antagonism between canonical WNT signaling and the BMP and FGF pathways in other organs. For example, the balance of WNT, BMP, and FGF signaling pathways regulates growth and development of the cerebral cortex and neural crest induction (Shimogori et al., 2004) reviewed in (Basch and Bronner-Fraser, 2006). WNT and BMP signals emanating from the cerebral cortex antagonize FGF signals from the anterior telencephalon. The balance within these signaling centers establishes the appropriate expression of transcription factors that initiate differentiation and organ development. The interaction of the WNT, FGF, and BMP signaling pathways influences neural crest induction, possibly by a gradient of BMP signaling that establishes a zone of ectoderm competent to respond to WNT and FGF signals, or by modulating effects of WNT and FGF signaling on BMP signaling activity (reviewed in (Litsiou et al., 2005; Raible and Ragland, 2005). We have shown that TCF4, a key element in the WNT signaling pathway, restricts the domain of FGF and BMP expression in the ventral diencephalon, reducing the induction of pituitary progenitor cells.
Figure 8.
The rostral expansion of the FGF and BMP boundary in Tcf4−/− mice increases the amount of oral ectoderm specified to invaginate and become Rathke’s pouch. As Rathke’s pouch invaginates at e11.5, opposing boundaries of FGF/BMP and TCF4 expression are established within the ventral diencephalon. This pattern of expression in the ventral diencephalon likely influences the adjacent cells within Rathke’s pouch to proliferate. The ventral boundary on each side of Rathke’s pouch establishes the pinch off point from the oral ectoderm and determines the subsequent size of the pituitary gland. In Tcf4−/− mice, the absence of TCF4 signal in the ventral diencephalon results in ventral expansion of the FGF/BMP expression boundary. This establishes a larger region of proliferating cells in Rathke’s pouch, potentially leading to recruitment of additional oral ectoderm, and ultimately a larger pituitary gland.
Negative regulation of organ size through Wnt antagonism of FGF and BMP action has also been seen in the lung (Dean et al., 2005). In this study, canonical Wnt signaling negatively regulates branching of the lung bud, and is important for controlling FGF and BMP signals, which positively regulate this process. We hypothesize that upon activation of canonical Wnt signaling in the ventral diencephalon during early development, TCF4, either directly or indirectly, represses Bmp4 and Fgf10 within the rostral domain of the ventral diencephalon to promote the proper growth and shape of Rathke’s pouch.
The expanded FGF and BMP signaling domain in TCF4 mutants is associated with an extended region of proliferating cells that compose Rathke’s pouch and that express markers of pituitary cell commitment such as PITX genes and Six6 (Figure 6). Six6 enhances proliferation of pituitary cells by inhibiting cyclin-dependent kinase inhibitors (Li et al., 2002), and the expansion of Six6 expression in TCF4 mutants coincides with the area of additional proliferating cells in Tcf4−/− mice. It is intriguing that Six6 is expressed normally within the ventral diencephalon of Tcf4 mutant mice, despite the expanded expression of Six6 in Rathke’s pouch. This means that Six6 expression is regulated differently in neural and oral ectoderm. The expression of Six6 in the ventral and rostral aspect of Rathke’s pouch may be established by WNT signaling emanating from the neural ectoderm. These results imply that the role of Tcf4 in pituitary development is to down regulate pituitary gland size by restricting or antagonizing FGF and BMP signaling.
Several other genes are involved in limiting the rostral expansion of Rathke’s pouch. Rx, a homeobox gene required for development of the brain and eye, is expressed throughout the ventral diencephalon early in mouse development (Voronina et al., 2005); Mathers, personal communication). In Rx mutant mice, Rathke’s pouch is expanded and exhibits a similar extension of Six6 expression that coincides with increased cell proliferation. In addition, Rx−/− and Tcf4−/− mice both have a rostral extension of the FGF and BMP boundary in the ventral diencephalon (Mathers, personal communication; Figure 7A–D). The maintenance of this boundary is important for inducing an appropriately shaped and sized Rathke’s pouch. For example Sox3+/− mice exhibit rostral extension of the FGF and BMP expression region and altered pituitary gland morphology (Rizzoti et al., 2004). The pituitaries of Wnt5a−/− mice exhibit dysmorphology similar to that of Sox3+/− mice (Camper, 2004; Cha et al., 2004), and the disruption of the BMP and FGF boundary is also evident in these mutants (unpublished observations). Finally, lack of the BMP inhibitor, noggin, results in altered FGF signaling and abnormal pituitary shape (Davis and Camper 2007). Taken together, these results support the idea that balancing the BMP, FGF, and WNT signaling pathways in the ventral diencephalon is important for normal induction of Rathke’s pouch, and pituitary growth.
The relevance of integrating WNT, BMP and FGF signaling for maintenance of normal pituitary gland for normal pituitary gland growth may extend to adulthood. TCF4, noggin, and activated β-catenin are active in a subset of cells in the adult pituitary gland (Figure 2, 3; Davis et al., 2007). The role of TCF4 or noggin at this stage cannot be addressed with the current knockout mouse models because of neonatal lethality. However, floxed alleles would be useful for determining whether WNT signaling regulates the expansion of specialized pituitary cell populations in response to hormonal feedback from target organs (Pakarinen et al., 1994; Torres et al., 1995) and to test whether dysregulation of TCF/LEF and/or WNT signaling could contribute to abnormalities in adult pituitary gland function such as adenoma formation. Indeed, there is a high incidence of accumulation of nuclear β-catenin in pituitary adenomas (Semba et al., 2001). Consistent with this, TCF4 expression is reduced in a subset of breast cancers, and it is hypothesized to repress proliferation of normal breast cells by suppressing expression of the cell cycle gene CD24 (Shulewitz et al., 2006). The loss of TCF4 may relieve the suppression of the cell cycle gene CD24, thus driving proliferation. This supports the idea that excess canonical Wnt signaling, or inappropriate TCF function could contribute to pituitary hyperplasia.
Supplementary Material
Acknowledgments
This work is supported by NIH grants R37-HD30428-10 and R01-HD34283-09. We would like to thank Drs. Hans Clevers, Alex Gregorieff, Greg Dressler, Brigid Hogan, the Developmental Studies Hybridoma Banks, and the National Hormone Pituitary Program for providing reagents and resources, Julie Minns for technical help, and Donna Martin for comments on the manuscript. We acknowledge Galina Gavrilina and Maggie Van Keuren for preparation of transgenic mice and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities. Core support was provided by The University of Michigan Cancer Center, NIH grant number, CA465952, The University of Michigan Multipurpose Arthritis Center, NIH grant number, AR20557, and The University of Michigan Gut Peptide Research Center, NIH grant number, DK34933. The authors gratefully acknowledge the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program (Grant 085P100815).
References
- Ai D, Wang J, Amen M, Lu M, Amendt B, Martin J. Nuclear Factor-1 and TCF/LEF recognition elements regulate Pitx2 transcription in pituitary development. Mol Cell Biol. 2007 doi: 10.1128/MCB.01848-06. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SH, Kioussi C, Briata P, Wang D, Nguyen HD, Ohgi KA, Glass CK, Wynshaw-Boris A, Rose DW, Rosenfeld MG. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci U S A. 2003;100:3245–50. doi: 10.1073/pnas.0330217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch M, Bronner-Fraser M. Neural crest inducing signals. Adv Exp Med Biol. 2006;589:34–31. doi: 10.1007/978-0-387-46954-6_2. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–78. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen CY, Gherzi R. The Wnt/beta-catenin-->Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol Cell. 2003;12:1201–11. doi: 10.1016/s1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–61. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Camper SA. Sox3 and sexual dysfunction: it’s in the head. Nat Genet. 2004;36:217–9. doi: 10.1038/ng0304-217. [DOI] [PubMed] [Google Scholar]
- Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a complex transcriptome: the construction of the mouse full-length cDNA encyclopedia. Genome Res. 2003;13:1273–89. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–94. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19:1893–903. doi: 10.1210/me.2005-0052. [DOI] [PubMed] [Google Scholar]
- Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, Whitsett JA. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol. 2001;280:L705–15. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–922. doi: 10.1136/jmg.39.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G, Douarin NL. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Developmental Biology. 1985;110:422–39. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Developmental Biology. 2007 doi: 10.1016/j.ydbio.2007.02.001. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–86. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr, MacDougald OA, Camper SA. Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome. 2001;12:843–51. doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the Pituitary: Molecular, Genetic, and Developmental Analysis. Mol Endocrinol. 2006;20:2796–2805. doi: 10.1210/me.2005-0303. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–15. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–17. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1999;213:340–53. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. Embo J. 2004;23:1825–33. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummow BM, Winnay JN, Hammer GD. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin alpha gene. J Biol Chem. 2003;278:26572–9. doi: 10.1074/jbc.M212677200. [DOI] [PubMed] [Google Scholar]
- Hossain A, Saunders GF. Synergistic cooperation between the beta-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J Biol Chem. 2003;278:26511–6. doi: 10.1074/jbc.M300804200. [DOI] [PubMed] [Google Scholar]
- Jiang FX, Harrison LC. Convergence of bone morphogenetic protein and laminin-1 signaling pathways promotes proliferation and colony formation by fetal mouse pancreatic cells. Exp Cell Res. 2005;308:114–22. doi: 10.1016/j.yexcr.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Kennell JA, O’Leary EE, Gummow BM, Hammer GD, MacDougald OA. T-cell factor 4N (TCF-4N), a novel isoform of mouse TCF-4, synergizes with beta-catenin to coactivate C/EBPalpha and steroidogenic factor 1 transcription factors. Mol Cell Biol. 2003;23:5366–75. doi: 10.1128/MCB.23.15.5366-5375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–9. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–85. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–37. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev. 2002;16:3173–85. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–98. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kuschel S, Ruther U, Theil T. A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol. 2003;260:484–95. doi: 10.1016/s0012-1606(03)00252-5. [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–3. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–62. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–5. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–85. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–95. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C. Oncogenic homeodomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc Natl Acad Sci U S A. 1999;96:11464–9. doi: 10.1073/pnas.96.20.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–74. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–49. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–53. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Mullis PE. Transcription factors in pituitary development. Molecular and Cellular Endocrinology. 2001;185:1–16. doi: 10.1016/s0303-7207(01)00617-7. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–20. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Norlin S, Nordstrom U, Edlund T. Fibroblast growth factor signaling is required for the proliferation and patterning of progenitor cells in the developing anterior pituitary. Mech Dev. 2000;96:175–82. doi: 10.1016/s0925-4773(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Pakarinen P, Niemimaa T, Huhtaniemi IT, Warren DW. Transcriptional and translational regulation of LH, prolactin and their testicular receptors by hCG and bromocriptine treatments in adult and neonatal rats. Mol Cell Endocrinol. 1994;101:37–47. doi: 10.1016/0303-7207(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev Biol. 2001;237:324–32. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D–V and A–P axes of mouse limb. Nature. 1995;374:350–3. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Raible DW, Ragland JW. Reiterated Wnt and BMP signals in neural crest development. Semin Cell Dev Biol. 2005;16:673–82. doi: 10.1016/j.semcdb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–55. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- Salisbury T, Binder A, Grammer J, Nilson J. Maximal activity of the luteinizing hormone beta-subunit gene requires beta-catenin. Mol Endocrinol. 2007;21:963–71. doi: 10.1210/me.2006-0383. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Xu X, Han J, Bringas P, Jr, Maeda T, Slavkin HC, Grosschedl R, Chai Y. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278:130–43. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Semba S, Han SY, Ikeda H, Horii A. Frequent nuclear accumulation of beta-catenin in pituitary adenoma. Cancer. 2001;91:42–8. doi: 10.1002/1097-0142(20010101)91:1<42::aid-cncr6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–47. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–39. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shulewitz M, Soloviev I, Wu T, Koeppen H, Polakis P, Sakanaka C. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene. 2006;25:4361–9. doi: 10.1038/sj.onc.1209470. [DOI] [PubMed] [Google Scholar]
- Spater D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133:3039–49. doi: 10.1242/dev.02471. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–83. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–37. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–89. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–40. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–50. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Torres AI, Pasolli HA, Maldonado CA, Aoki A. Changes in thyrotroph and somatotroph cell populations induced by stimulation and inhibition of their secretory activity. Histochem J. 1995;27:370–9. [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–4. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Voronina VA, Kozlov S, Mathers PH, Lewandoski M. Conditional alleles for activation and inactivation of the mouse Rx homeobox gene. Genesis. 2005;41:160–4. doi: 10.1002/gene.20109. [DOI] [PubMed] [Google Scholar]
- Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–90. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–15. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.