Abstract
Pheochromocytoma is an endocrine tumor that can uniquely mimic numerous stress-associated disorders, with variations in clinical manifestations resulting from different patterns of catecholamine secretion and actions of released catecholamines on physiological systems.
Keywords: pheochromocytoma, stress, general adaptation syndrome, adrenoceptors, catecholamines, hypothalamic pituitary adrenocortical axis, glucocorticoids
Introduction
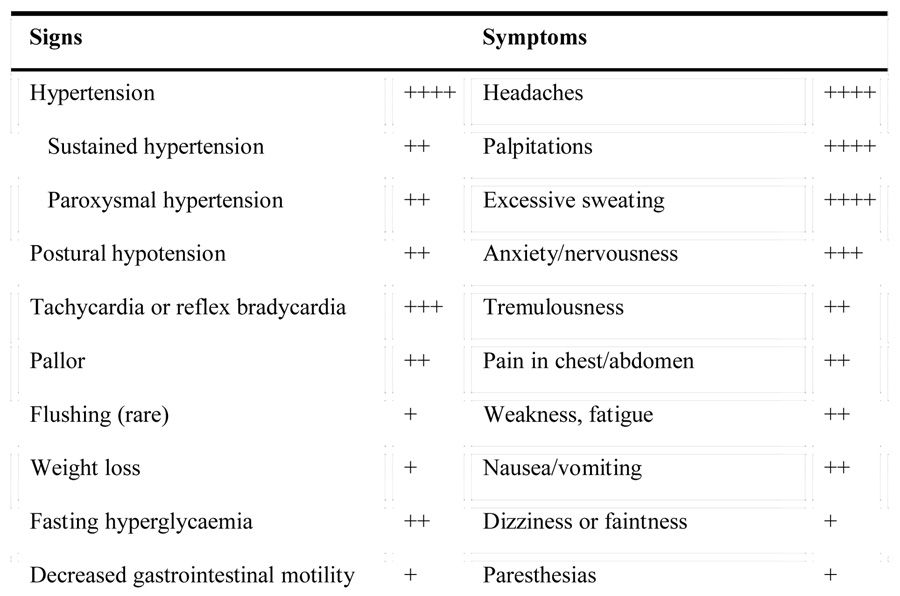
Pheochromocytomas are rare but treacherous catecholamine-producing tumors that, if not diagnosed or appropriately treated, will almost invariably prove fatal. The tumors may display numerous clinical manifestations similar to psychological or physiological stress. These clinical manifestations result from the hemodynamic and metabolic actions of high circulating catecholamine levels and include hypertension, headache, spontaneous sweating, palpitations, anxiety or panic attacks, as well as the presence of pallor.
The catecholamines, norepinephrine and epinephrine, act on α- and β-adrenoceptors at effector sites throughout the body with different organ-specific distributions. Both catecholamines have overlapping but also distinct actions on the different groups of adrenoceptors (1). In particular, epinephrine has more potent actions on β2-adrenoceptors than norepinephrine, while norepinephrine is a more potent β1-adrenoceptor agonist than epinephrine. However, the proximity of sites of norepinephrine and epinephrine release to adrenoceptors and the resulting concentrations at effector sites are also important determinants of adrenoceptor-mediated responses to these two catecholamines.
Stimulation of cardiac β-adrenoceptors by catecholamines released by a pheochromocytoma can elicit severe arrhythmias, aseptic myocarditis or cardiomyopathy. Catecholamine-induced dilating cardiomyopathy is most frequently reported when catecholamine excess is very high. Norepinephrine released locally from sympathetic nerve endings within the vasculature causes α1-adrenoceptor-mediated vasoconstriction resulting in hypertension. In contrast, epinephrine acts potently on β2-adrenergic receptors of the skeletal muscle vasculature causing vasodilation which can result in hypotension. Catecholamines, particularly epinephrine, stimulate lipolysis, ketogenesis, thermogenesis and glucose production (2).
Similar to stress-related situations, pheochromocytoma-induced metabolic or hemodynamic attacks or episodes may last from a few seconds, up to one or more hours or even extend to much longer intervals in patients with chronically elevated catecholamine levels. Rarely, severe acute release of catecholamines (e.g. during severe stressful situations such as surgery, acute severe psychological stress) may result in multisystem crisis — defined as hypertension and/or hypotension, high fever, encephalopathy and signs of multiple organ failure.
Background
a.Stress
Walter B. Cannon introduced concepts of “homeostasis” and “fight or flight” responses in early 1900’s, initiating development of our understanding of relationships between individuals and their surroundings; however, it was Hans Selye, who borrowed the word “stress” from mechanics to transform the term into a definition for the nonspecific adaptation to the environment (3). Despite a still relatively vague definition with a myriad of symptoms and signs, the concept of stress has steadily evolved over the last half century and is now recognized to play a significant role in human health and disease (Table 1 and Table 2). Responses to stress are mediated through 3 main effector systems: the hypothalamic-pituitary adrenocortical (HPA) axis and adrenomedullary and sympathoneuronal systems. While cortisol is the mediating hormone for the first system, the catecholamines are responsible for actions of two last systems. Although initially seen as a non-specific response, stress is now established to elicit highly specific effector system responses depending on the nature of stressor (4–5).
Table 1.
Stress Warning Signs and Symptoms*
| Cognitive Symptoms | Emotional Symptoms |
| • Memory problems | • Moodiness |
| • Indecisiveness | • Anxiey/Agitation |
| • Inability to concentrate | • Restlessness |
| • Trouble thinking clearly | • Short temper |
| • Poor judgment | • Irritability, impatience |
| • Seeing only the negative | • Inability to relax |
| • Anxious or racing thoughts | • Feeling tense and “on edge” |
| • Constant worrying | • Feeling overwhelmed |
| • Loss of objectivity | • Sense of loneliness and isolation |
| • Fearful anticipation | • Depression or general unhappiness |
| Physical Symptoms | Behavioral Symptoms |
| • Headaches or backaches | • Eating more or less |
| • Muscle tension and stiffness | • Sleeping too much or too little |
| • Diarrhea or constipation | • Isolating yourself from others |
| • Nausea | • Procrastination, neglecting responsibilities |
| • Dizziness, lightheadedness | • Using alcohol, cigarettes, or drugs to relax |
| • Insomnia | • Nervous habits (e.g. nail biting, pacing) |
| • Chest pain, rapid heartbeat | • Teeth grinding or jaw clenching |
| • Weight gain or loss | • Overdoing activities (e.g. exercising, shopping) |
| • Skin breakouts (hives, eczema) | • Overreacting to unexpected problems |
| • Loss of sex drive | • Picking fights with others |
| • Frequent colds | |
| • Pallor or blushing | |
| • Profuse sweating | |
| • Tachypnea | |
| • Hypertension or hypotension |
Table 2.
Medical conditions, caused or exacerbated by stress*
| • Chronic pain | • Heart disease | • Infertility |
| • Migraines | • Diabetes | • Autoimmune diseases |
| • Ulcers | • Asthma | • Irritable bowel syndrome |
| • Heartburn | • PMS | • Skin problems |
| • High blood pressure | • Obesity/weight loss |
Adapted with changes from www.helpguide.org and www.stress.org (The American Institute of Stress).
b. Pheochromocytoma
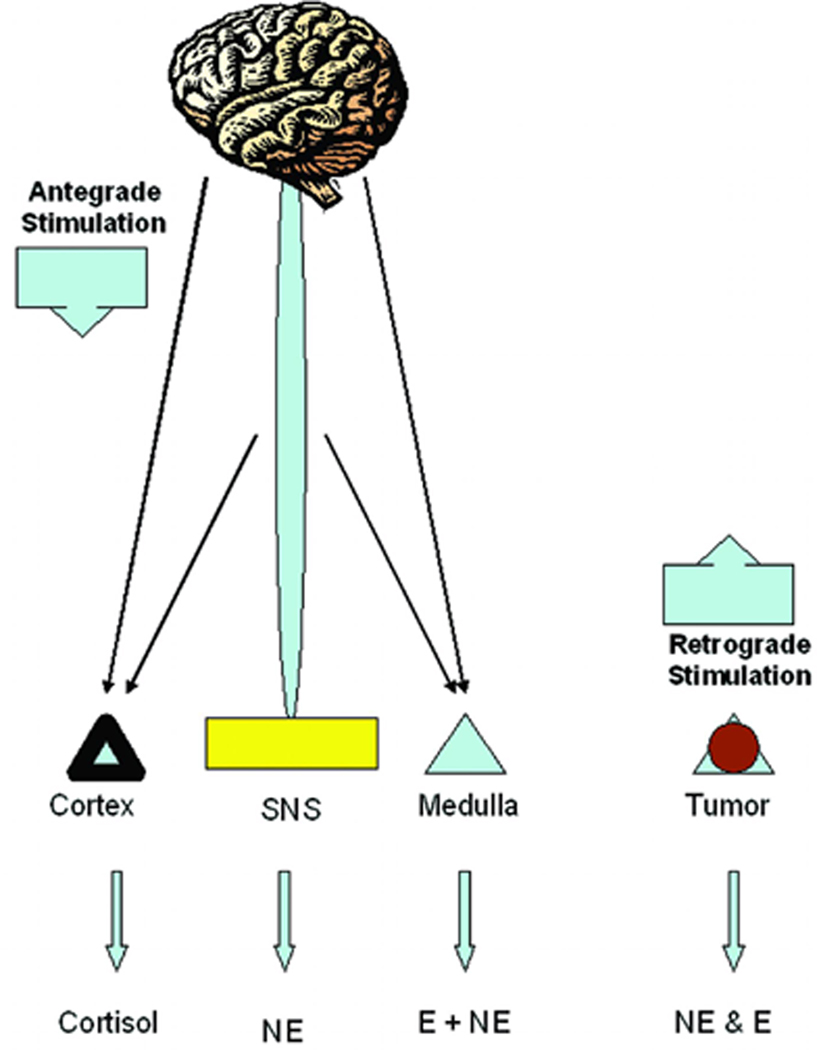
Pheochromocytomas are catecholamine-producing neuroendocrine tumors arising mainly from the adrenal gland. These chromaffin cell-derived tumors can result in significant morbidity and mortality related to catecholamine oversecretion as described above (Table 3) (6–7). The tumors can manifest with highly variable presentations, from rare and progressive hypercatecholaminergic episodes, through relatively sustained hypertension, to severe and clinically malignant disease, complicated by hypertensive crises, stroke, acute myocardial infarction and numerous other conditions (Figure 1). However, in contrast to stress where stressor-specific responses of the three main effector systems typically arise through CNS-mediated mechanisms, in pheochromocytoma the signs and symptoms occur secondary to release of catecholamines that is autonomous of any nervous system input
Table 3.
Signs and symptoms of pheochromocytoma
 |
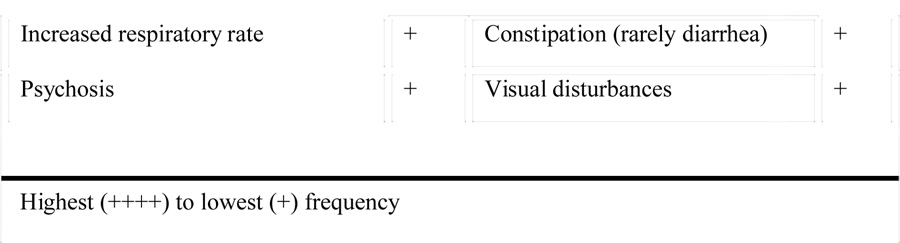 |
Figure 1.
Pathophysiology of stress and pheochromocytoma.
Overlap in clinical signs and symptoms
a. General
General signs and symptoms of both conditions are strikingly similar during acute episodes and can be difficult to differentiate based on severity. Anxiety, pallor, sweating, tremor, palpitations and fatigue can be severe in either case.
b. Pulmonary, GI, GU
Increased respiratory rate, diffuse abdominal pain and diarrhea are common in both situations, while more severe disturbances, such as acute mesenteric ischemia with bleeding and perforation, paralytic ileus, intestinal obstruction are mainly restricted to patients with highly functional pheochromocytoma and would perhaps be seen only in patients with very severe stress.
c. Cardiovascular
Hypertension, as well as hypotension can be seen in both conditions. Paroxysmal hypertension is more characteristic for both conditions, while sustained hypertension may be more typical for pheochromocytoma. Hypertensive crisis, shock, or stroke can occur in situations associated with severe stress and also in patients with a poorly controlled pheochromocytoma. Hypotension is vasovagal in stress and postural in pheochromocytoma.
Although rarely seen as a significant overall contributor to severe cardiovascular morbidity, stress is known to associate with arrhythmias, cardiomyopathy and sudden cardiac death. The existence of stress-related cardiac death was first described by Cannon in 1942 (8), who together with Richter (9) suggested its relation to acute overactivity of both sympatoadrenal and autonomic parasympathetic nervous systems. Hans Selye, on the other hand, suggested steroids to induce electrolyte-steroid-cardiomyopathy with necrosis (ESCN), a particular type on neurologically-driven cardiomyopathy (10). At least descriptively, these studies were supported by finding of focal cardiac damage in patients with acute neurologic disorders (11–12). The fact that epinephrine infusion causes cardiac hypertrophy was described in 1907 (13–14) and stress-induced myocardial damage can be prevented with adrenergic blockade (15). Finally, there is a recently described “broken heart syndrome”, which is identical to takotsubo syndrome and represents an acute LV ballooning and sudden death, associated with acute stress or grief (16–17).
Cardiovascular abnormalities can be acute and severe, and associate with structural myocardial changes that may or may not resolve after tumor resection (18–22). Cardiac manifestations of pheochromocytoma are thought to be frequent and potentially catastrophic in majority of patients. Structural cardiac manifestations are seen in up to 30% of patients with pheochromocytoma and include left ventricular hypertrophy, catecholamine myocarditis, dilated or obstructive cardiomyopathy, which can associate with myocardial necrosis and/or fibrosis and resultant heart failure (19, 21, 23–31).
Discussion
The present article describes some of the similarities between pheochromocytoma situations of stress. Therefore, pheochromocytoma can be considered as endocrine stress mimicking disorder. Both stress and pheochromocytoma can present with either acute or chronic symptoms and signs related to catecholamine excess and in stress additionally to elevated glucocorticoid levels. Hypertension, tachyarrhythmia, sweating, pallor and anxiety are common to both disorders, but particularly in acute emergency situations. Only careful and detailed history and physical examination will point correctly to either stress-related problems or pheochromocytoma.
As evident from numerous symptoms and signs, both disorders can present with elevated catecholamine levels. Interestingly, as stress-specific catecholamine responses exist, the same is valid for pheochromocytoma-specific catecholamine release. For example, physical and cold stress preferentially activates the sympathoneuronal system while hypoglycemia activates the adrenal medullary system. Multiple endocrine neoplasia type 2-related pheochromocytoma have an adrenergic phenotype while von Hippel-Lindau syndrome- and succinate dehydrogenase subunit B--related pheochromocytoma have noradrenergic phenotype (Table 5). However, stress-related disorders are characterized by the activation of the hypothalamic-pituitary-adrenocortical axis, which is not the case in pheochromocytoma patients.
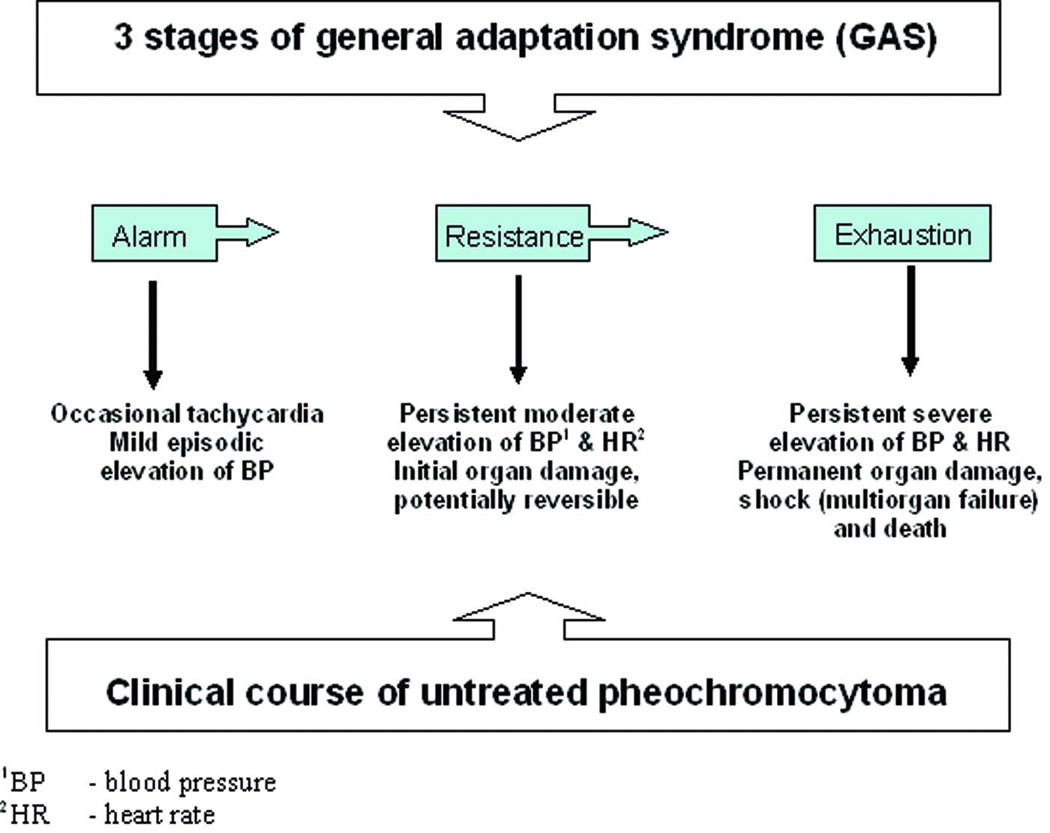
Some pheochromocytoma patients may experience a clinical course similar to Selye’s stress-induced General Adaptation Syndrome (GAS) (Figure 2). The initial stage includes sporadic surges of catecholamines that produce in both conditions episodic clinical symptomatology. The pathology of the second – resistance – stage is unique for both conditions. While clinical symptomatology is more persistent and severe, and associates with structural organ damage, some patients may show a paradoxical lack of damage and apparent adaptation to the condition. At least in pheochromocytoma, such adaptation has been shown to be related to desensitization of adrenergic receptors (32–35). Such adapation in pheochromocytoma appears to be associated mainly with chronically elevated catecholamine levels, rather than paroxysmal secretion (36–38). Although sometimes seen as advantageous, this stage inevitably progresses to exhaustion of compensatory mechanisms and results in irreversible organ damage and failure. This analogy with GAS further support similarities of both conditions and strengthens the importance of understanding of the phenotypic and biochemical mimicry.
Figure 2.
Similarities between stress and pheochromocytoma disease course
It is important to understand similarities and differences between both conditions, because of potential morbidity of untreated pheochromocytoma and the fact that when appropriately suspected and timely treated, this disease can be cured. Recognizing the differences between pheochromocytoma and stress-associated conditions, such as panic disorder, can be difficult. However, once a tumor is suspected diagnosis is made relatively straightforward by use of suitable biochemical tests (e.g., measurements of plasma free metanephrines). The cardinal rule is to first think of the tumor. While anxiolytics, antidepressants and beta-blockers can be effective in stress-associated conditions, these drugs can cause adverse reactions in pheochromocytoma.
Table 4.
Dominant biochemical phenotype
| • Sporadic pheochromocytoma – epinephrine or norepinephrine or both |
| • von Hippel-Lindau syndrome – norepinephrine |
| • Multiple endocrine neoplasia – epinephrine or epinephrine and norepinephrine |
| • Succinate dehydrogenase subunit B – norepinephrine |
Acknowledgements
This work was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development. The authors have no conflict of interest to disclose.
References
- 1.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 2.Cryer PE. Adrenaline: a physiological metabolic regulatory hormone in humans? Int J Obes Relat Metab Disord. 1993;17 Suppl 3:S43–S46. [PubMed] [Google Scholar]
- 3.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 4.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye's doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS. Catecholamines and stress. Endocrine Regulations. 2003;37:69–80. [PubMed] [Google Scholar]
- 6.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 7.Dluhy RG, Lawrence JE, Williams GH. Larsen: Williams Textbook of Endocrinology. 10th ed. Saunders; 2003. Pheochromocytoma. [Google Scholar]
- 8.Cannon WB. "Voodoo" death. Am Anthropologist. 1942;44(new series):169–118. doi: 10.2105/ajph.92.10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter CP. On the phenomenon of sudden death in animal and man. Psychosomatic Med. 1957;19:191–198. doi: 10.1097/00006842-195705000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Selye H. The Chemical Prevention of Cardiac Necrosis. New York, NY: Ronald Press; 1958. [Google Scholar]
- 11.Koskelo P, Punsar SO, Sipila W. Subendocardial haemorrhage and ECG changes in intracranial bleeding. BMJ. 1964;1:1479–1483. doi: 10.1136/bmj.1.5396.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor RCR. Myocardial damage secondary to brain lesions. Am Heart J. 1969;78:145–148. doi: 10.1016/0002-8703(69)90001-5. [DOI] [PubMed] [Google Scholar]
- 13.Josué O. Hypertrophie cardiaque cause par l’adrenaline and la toxine typhique. C R Soc Biol (Paris) 1907;63:285–287. [Google Scholar]
- 14.Samuels MA. The brain-heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- 15.Raab W, Stark E, MacMillan WH, Gigee WR. Sympathogenic origin and anti-adrenergic prevention of stress-induced myocardial lesions. Am J Cardiol. 1961;8:203–211. doi: 10.1016/0002-9149(61)90207-7. [DOI] [PubMed] [Google Scholar]
- 16.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–544. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 17.Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: a review of the current literature. Postgrad Med J. 2007;83:261–264. doi: 10.1136/pgmj.2006.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dabrowska B, Dabrowski A, Pruszczyk P, et al. Heart rate variability in pheochromocytoma. Am J Cardiol. 1995;76:1202–1204. doi: 10.1016/s0002-9149(99)80340-3. [DOI] [PubMed] [Google Scholar]
- 19.Gatzoulis KA, Tolis G, Theopistou, et al. Cardiomyopathy due to a pheochromocytoma: a reversible entity. Acta Cardiol. 1998;53:227–229. [PubMed] [Google Scholar]
- 20.Bravo EL. Pheochromocytoma: new concepts and future trends. Kidney Int. 1991;40:544–556. doi: 10.1038/ki.1991.244. [DOI] [PubMed] [Google Scholar]
- 21.Liao WB, Liu CF, Chiang CW, et al. Cardiovascular manifestations of pheochromocytoma. Am J Emerg Med. 2000;18:622–625. doi: 10.1053/ajem.2000.7341. [DOI] [PubMed] [Google Scholar]
- 22.McManus BM, Fleury TA, Roberts WC. Fatal catecholamine crisis in pheochromocytoma: curable cause of cardiac arrest. Am Heart J. 1981;102:930–932. doi: 10.1016/0002-8703(81)90045-4. [DOI] [PubMed] [Google Scholar]
- 23.Nanda AS, Feldman A, Liang CS. Acute reversal of pheochromocytoma-induced catecholamine cardiomyopathy. Clin Cardiol. 1995;18:421–423. doi: 10.1002/clc.4960180712. [DOI] [PubMed] [Google Scholar]
- 24.Huddle KR, Kalliatakis B, Skoularigis J. Pheochromocytoma associated with clinical and echocardioagraphic features simulating hypertrophic obstructive cardiomyopathy. Chest. 1996;109:1394–1397. doi: 10.1378/chest.109.5.1394. [DOI] [PubMed] [Google Scholar]
- 25.Scott IU, Gutterman DD. Pheochromocytoma with reversible focal cardiac dysfunction. Am Heart J. 1995;130:909–911. doi: 10.1016/0002-8703(95)90096-9. [DOI] [PubMed] [Google Scholar]
- 26.Kline IK. Myocardial alterations associated with pheochromo-cytoma. Am J Pathol. 1961;38:539–551. [PMC free article] [PubMed] [Google Scholar]
- 27.Simon M, Downin SE. Coronary vasoconstriction and catecholamine cardiomyopathy. Am Heart J. 1985;109:297–304. doi: 10.1016/0002-8703(85)90597-6. [DOI] [PubMed] [Google Scholar]
- 28.Sardesai SH, Mourant AJ, Sivathandon Y, Farrow R, Gibbons DO. Pheochromocytoma and catecholamine-induced cardiomyopathy presenting as heart failure. Br Heart J. 1990;63:234–237. doi: 10.1136/hrt.63.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuiki ER, Jenni R, Amann FW, et al. A reversible form of apical left ventricular hypertrophy associated with pheochromocytoma. J Am Soc Echocardiogr. 1993;6:327–331. doi: 10.1016/s0894-7317(14)80072-2. [DOI] [PubMed] [Google Scholar]
- 30.Hamada N, Akamatsu A, Joh T. A case of pheochromocytoma complicated with acute renal failure and cardiomyopathy. Jpn J Circulation. 1993;57:84–90. doi: 10.1253/jcj.57.84. [DOI] [PubMed] [Google Scholar]
- 31.Suga K, Tsukamoto K, Nishigauchi K, Kume N, Matsunaga N, Hayano T, Iwami T. Iodine-123-MIBG imaging in pheochromocytoma with cardiomyopathy and pulmonary edema. J Nucl Med. 1996;37:1361–1364. [PubMed] [Google Scholar]
- 32.Bravo E, Fouad-Tarazi F, Rossi G, et al. A reevaluation of the hemodynamics of pheochromocytoma. Hypertension. 1990;15:1128–1131. doi: 10.1161/01.hyp.15.2_suppl.i128. [DOI] [PubMed] [Google Scholar]
- 33.Snavely MD, Ziegler MG, Insel PA. Subtype-selective down-regulation of rat renal cortical alpha- and beta-adrenergic receptors by catecholamines. Endocrinology. 1985;117:2182–2189. doi: 10.1210/endo-117-5-2182. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimoto G, Manger WM, Hoffman BB. Desensitization of beta-adrenergic receptors by pheochromocytoma. Endocrinology. 1984;114:1272–1278. doi: 10.1210/endo-114-4-1272. [DOI] [PubMed] [Google Scholar]
- 35.Greenacre JK, Conolly ME. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and patients with phaeochromocytoma: studies in vivo. Br J Clin Pharmacol. 1978;5:191–197. doi: 10.1111/j.1365-2125.1978.tb01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause M, Reinhardt D, Kruse K. Phaeochromocytoma without symptoms: desensitization of the alpha- and beta-adrenoceptors. Eur J Pediatr. 1988;147:121–122. doi: 10.1007/BF00442207. [DOI] [PubMed] [Google Scholar]
- 37.Ratge D, Wisser H. Alpha- and beta-adrenergic receptor activity in circulating blood cells of patients with phaeochromocytoma: effects of adrenalectomy. Acta Endocrinol (Copenh) 1986;111:80–88. doi: 10.1530/acta.0.1110080. [DOI] [PubMed] [Google Scholar]
- 38.Cases A, Bono M, Gaya J, Jimenez W, Calls J, Esforzado N, Rivera F, Revert L. Reversible decrease of surface beta 2-adrenoceptor number and response in lymphocytes of patients with pheochromocytoma. Clin Exp Hypertens. 1995;17:537–549. doi: 10.3109/10641969509037423. [DOI] [PubMed] [Google Scholar]