Abstract
The α2-adrenoreceptor agonist, medetomidine, which exhibits dose-dependent sedative effects and is gaining acceptance in small-animal functional magnetic resonance imaging (fMRI), has been studied. Rats were examined on the bench using the classic tail-pinch method with three infusion sequences: 100 μg/kg/hr, 300 μg/kg/hr, or 100 μg/kg/hr followed by 300 μg/kg/hr. Stepping the infusion rate from 100 to 300 μg/kg/hr after 2.5 hours resulted in a prolonged period of approximately level sedation that cannot be achieved by a constant infusion of either 100 or 300 μg/kg/hr. By stepping the infusion dosage, experiments as long as six hours are possible. Functional MRI experiments were carried out on rats using a frequency dependent electrical stimulation protocol—namely, forepaw stimulation at 3, 5, 7, and 10 Hz. Each rat was studied for a four-hour period, divided into two equal portions. During the first portion, rats were started at a 100 μg/kg/hr constant infusion. During the second portion, four secondary levels of infusion were used: 100, 150, 200, and 300 μg/kg/hr. The fMRI response to stimulation frequency was used as an indirect measure of modulation of neuronal activity through pharmacological manipulation. The frequency response to stimulus was attenuated at the lower secondary infusion dosages 100 or 150 μg/kg/hr but not at the higher secondary infusion dosages 200 or 300 μg/kg/hr. Parallel experiments with the animal at rest were carried out using both electroencephalogram (EEG) and functional connectivity MRI (fcMRI) methods with consistent results. In the secondary infusion period using 300 μg/kg/hr, resting-state functional connectivity is enhanced.
Keywords: Medetomidine anesthesia, rat, blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI), physiological fluctuations, resting-state functional connectivity magnetic resonance imaging (fcMRI), forepaw stimulation
Introduction
Proper anesthetic dosage is crucial for the success of blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) small-animal experiments. Recently, medetomidine was introduced as an anesthetic for noninvasive longitudinal fMRI experiments (Sommers et al., 2002; Weber et al., 2006). A bench study in the present work showed that a constant infusion of medetomidine is not sufficient to maintain sedation for fMRI protocols longer than three hours. In several previous fMRI studies, the muscle relaxant pancuronium bromide was added to a medetomidine infusion mixture in order to limit animal motion over the course of a four-hour protocol (Cho et al., 2007; Pawela et al., 2008a; Pawela et al., 2008b). The use of a paralyzing agent complicates recovery following the conclusion of the experiment. It may also mask the need for additional anesthetic. The goal of this work was to develop a more robust medetomidine anesthesia protocol for longer fMRI experiments (> 3 hrs) that maintains a steady state of sedation throughout the experiment.
Medetomidine is an α2-agonist shown to have dose-dependent sedative effects. It is primarily used for short-term procedures in veterinary medicine. This anesthetic has a short in vivo half-life and can be easily reversed with an α2-antagonist. The active enantiomer, dexmedetomidine, is used in human medicine. Medetomidine and dexmedetomidine have many beneficial effects in anesthesia practice, including reliable sedation, analgesia, muscle relaxation, and anxiolysis (Sinclair, 2003). Side effects include initial hypertension, bradycardia, and diminished cardiac output followed by a reflex-mediated hypotension (Sinclair, 2003).
Medetomidine exhibits rapid distribution, on the order of a minute, that includes distribution to the brain (Salonen, 1989). Elimination occurs by biotransformation in the liver. After a single bolus, the half-life of the drug in plasma is approximately 60–100 min. The dose-dependent effect of medetomidine on functional BOLD response has not been systematically evaluated. Likewise, the dose-dependent effect of medetomidine on resting-state functional connectivity in the brain has not been studied.
Hayton et al. (Hayton et al., 1999) demonstrated that medetomidine anesthesia causes reduction in somatosensory evoked potentials (SEPs) in rat brain compared to other anesthetic regimes. However, Li et al. (Li et al., 2003) showed that increase of the infusion dosage produces no further reduction in evoked somatosensory potentials. A recent fMRI study comparing alpha-chloralose- and urethane-anesthetized rats suggests that each anesthetic influences SEPs in a unique manner, although the relationship between SEPs and the BOLD response remains the same (Huttunen et al., 2008). The limited suppressive effect of medetomidine on SEPs makes it an ideal anesthetic to study the coupling between neuronal activation and the hemodynamic properties of the BOLD signal. For this study, we chose forepaw stimulation because it has been studied with fMRI using different dosages across a variety of anesthetics (Goloshevsky et al., 2008; Masamoto et al., 2007; Sommers et al., 2008; Zhao et al., 2008). A previous medetomidine study demonstrated a stepwise increase in BOLD response to increasing forepaw stimulation frequencies from 1–9 Hz (Zhao et al., 2008). The results from our bench study show that the sedative effects of medetomidine infusion at a constant rate wear off over time but can be maintained by increasing the infusion dosage. In this paper, we demonstrate that a stepwise increase in BOLD response to increasing stimulation frequency can be used as a marker for the level of sedation and can be modulated by the medetomidine infusion dosage.
Functional connectivity detected by BOLD contrast MRI in the rodent brain has been recently demonstrated (Kannurpatti et al., 2008; Lu et al., 2007; Pawela et al., 2008a; Zhao et al., 2008). Spontaneous neuronal activity is implicated as the physiological basis for low-frequency fluctuations (LFFs) in the BOLD signal (Lu et al., 2007; Shmuel and Leopold, 2008). It has been shown that LFFs in rest are decreased under isoflurane anesthesia (Vincent et al., 2007). In rat electroencephalograms (EEG), dexmedetomidine induces intense recurrent low-frequency activity that becomes more pronounced at deeper levels of sedation (Bol et al., 1997). Because task-activated and resting-state BOLD signals are believed to have similar neurovascular coupling mechanisms, we hypothesize that the correlations in BOLD signals between regions will be increased or decreased based on the level of medetomidine sedation.
The purpose of this study was to determine the effect of the level of medetomidine anesthesia on the BOLD response to somatosensory stimulation, as well as on resting-state correlations between brain regions in BOLD-based physiological fluctuations.
Materials and methods
General protocols
All procedures and protocols were in compliance with the Medical College of Wisconsin Institutional Animal Care and Use Committee (IACUC). Twenty male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Massachusetts, USA) weighing 300–400 g were used in the fMRI portion of the study. Twelve male Sprague-Dawley rats (300–400 g) were used in the bench-top tail-pinch study, and eight male Sprague-Dawley rats (325–375 g) were used in the EEG study.
Surgical protocol
The rat was placed in a gas anesthesia box, and anesthesia was induced with 2.5% isoflurane (Halocarbon Laboratories, River Edge, New Jersey, USA). The rat was then placed on a surgical table equipped with a heated surface. Isoflurane was continued at 1.5% for maintenance during surgery. PE-50 tubing (Stoelting, Wood Dale, Illinois, USA) was used as catheters and inserted into both the right femoral artery and vein. A tracheostomy was performed, and the animal was mechanically ventilated for the duration of all experiments.
Anesthetic depth tail-pinch bench study
The rats underwent the protocol described in the Surgical protocol section. Using an infusion pump (Harvard Apparatus, Boston, Massachusetts, USA), medetomidine (Domitor, Pfizer Animal Health, New York, New York, USA) was infused continuously through the venous femoral catheter. The isoflurane gas anesthesia was discontinued once the medetomidine was started. Three different infusion protocols were used: 100 μg/kg/hr constant infusion (n=4), 300 μg/kg/hr constant infusion (n=4), or 75 min of 100 μg/kg/hr infusion followed by an increase in concentration to 300 μg/kg/hr (n=4). The level of anesthesia was recorded every 10 min by recording the heart rate and reaction to a tail pinch with a toothed surgical forceps. The tail was pinched 2 cm from the tip of the tail. The tail pinch measures spinal nociceptive reflex and is an accepted measure of anesthetic depth (Jugovac et al., 2006; Savola et al., 1991). The investigator attempted to apply the same consistent light pressure for all experiments. The endpoint of the study was determined by either a heart rate greater than 350 beats per min or spontaneous movement of the rat. At this point, the rat was euthanized with an overdose of pentobarbital.
EEG measurements
Rats underwent the procedures outlined in the Surgical protocol section. Afterward, heads were secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, California, USA). Burr holes were made above the frontal cortex (+3.5 ± 1.1 mm (SD) rostral and ± 2.4 ± 0.3 mm (SD) lateral with respect to bregma) with a manual drill. In order to record the EEG signal from the frontal cortex, two stainless-steel screw electrodes (3 mm long, 1.5 mm diameter) (Small Parts, Inc., Miami Lakes, Florida, USA) were placed through the burr holes until the screw tips rested on the dura. Following the placement of the electrodes, infusion of a mixture of 100 μg/kg/hr medetomidine and 2 mg/kg/hr pancuronium bromide was started (Syringe Pump Model 22, Harvard Apparatus, Holliston, Massachusetts, USA), and the isoflurane was gradually tapered to zero over several minutes. Thirty minutes after the start of the medetomidine/pancuronium infusion, resting-state data acquisition began.
Mean arterial blood pressure (MAP), stimulation timing signals, and EEG were digitally sampled at 1000 Hz (WinDaq DataQ Instruments, Akron, Ohio, USA) and recorded on a PC. Inspired and expired gases were continuously monitored with an online gas analyzer. The EEG signals were analyzed offline with a fast Fourier transform (Matlab, The Math Works, Boston, Massachusetts, USA) to determine the power in five frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–20 Hz), and gamma (20–40 Hz).
The ratio of the square root of the power of the beta and delta bands was chosen as the EEG gauge of wakeful versus sleeping rhythms. Several processed EEG indices have been used to measure anesthetic depth in the past (Rampil, 1998), but they generally reflect relative power in high and low frequencies (Miller et al., 2004). Transition to the waking state is accompanied by a reduction in slow waves (especially delta) and by an increase in high frequency (especially beta), reflecting cortical activation, and can be assessed by the beta/delta ratio. This ratio performs similarly to the beta ratio used in the Bispectral Index anesthesia depth monitor (Rampil, 1998). Statistical testing was performed using SigmaStat 3.0 (SPSS, Chicago, Illinois, USA). Statistical significance between the average beta/delta ratios was determined using a one-way RM-ANOVA.
MRI hardware and physiologic monitoring
A Bruker 9.4 Tesla (AVANCE, Billerica, Massachusetts, USA) small-animal scanner was used for the fMRI protocol. The procedures outlined in the Surgical protocol section were performed on the subject rat. The rat was then loaded onto a custom-built G-10 fiberglass rodent cradle equipped with a recirculating water pump (Meditherm-III, Gaymar Industries, Orchard Park, New York, USA). The temperature was maintained at 37°C throughout the course of the experiment. An MRI-compatible mechanical ventilator (MRI-1, CWE, Ardmore, Pennsylvania, USA) was set between 55 and 65 breaths per min depending on underlying physiology. Initially, a 70/30 N2/O2 gas mixture was used for respiration. Gases were adjusted by monitoring both inspired and end-tidal isoflurane, O2, and CO2 (POET-IQ2, Criticare Systems, Waukesha, Wisconsin, USA). Invasive blood pressure (Model 1025, SA-Instruments, Stony Brook, New York, USA) and pulse oximetry (Model 8600V, Nonin Medical, Plymouth, Minnesota, USA) were monitored during the entire experiment. A digital recording system was used to record all physiologic parameters (Windaq Pro, DataQ Instruments, Akron, Ohio, USA). Beryllium-copper electrodes were inserted into the left forepaw between the second and fourth digits of the rat and attached to a square-pulse generator (S88, Grass Technologies, West Warrick, Rhode Island, USA) equipped with a constant-current power supply (CCU, Grass Technologies, West Warrick, Rhode Island, USA). Images were acquired with a Bruker linear transmit coil (T10325) and a Bruker rodent surface receiving coil (T9208) placed on the head.
Anesthesia and fMRI protocol
Isoflurane was discontinued and an intravenous infusion of 100 μg/kg/hr medetomidine hydrochloride anesthesia with 2 mg/kg/hr pancuronium bromide (Hospira Inc., Lake Forest, Illinois, USA) was started using an MRI-compatible infusion pump. The rat was allowed to equilibrate for 30 min. Initially, a rapid acquisition with relaxation enhancement (RARE) anatomic image was acquired with 10 contiguous slices with a 40 mm FOV and 1 mm slice thickness. The third slice was located over the anterior commissure centered -0.36 mm from bregma. The RARE anatomy was obtained with a 256 × 256 matrix and a TE = 50.8 ms. After RARE acquisition, two “resting-state” echo planar imaging (EPI) scans were obtained prior to stimulation. EPI parameters were 96 × 96 (zero-filled to 128 × 128), TR = 2 s, TE = 18.76 ms, and 110 repetitions, total scan time of 3 min 40 s with the same geometry as the RARE images. Square stimulation pulses of 2.0 mA, 3 ms duration, and frequencies of 3, 5, 7, and 10 Hz were applied in random order. Two BOLD fMRI experiments for each stimulation frequency were conducted. A block-designed paradigm was used with an initial 40 s off period followed by three on/off cycles (20 s on/40 s off) for a total of 3 min 40 s. Ten EPI acquisitions were obtained with a 5 min rest period between scans for the 100 μg/kg/hr dosage. Total time for this experimental portion was around 1.5 hrs. Rats were then switched either to 150/200/300 μg/kg/hr medetomidine infusion or maintained at 100 μg/kg/hr medetomidine infusion at five rats per group for a total of 20 rats. A 30 min equilibration period was allowed between dosages. Two initial “resting-state” acquisitions were obtained followed by a repeat of the stimulation experiments in the same random order as before for an additional 1.5 hrs. Total experimental time including surgery was around 4.0–4.5 hrs. Figure 1 is a timing diagram for the fMRI protocol used in this work. The two gray shaded areas plot the fMRI/fcMRI acquisition periods.
Fig. 1.
fMRI protocol time courses. Data acquisition occurred during the shaded time periods. Resting-state scans were performed prior to each time course stimulation sequence.
fMRI analysis
Datasets were detrended to eliminate linear drifts, and the separate slices were aligned to the same temporal origin. “Ideal” RARE anatomic images showing the best gray/white matter contrast were selected, and EPI images were registered to the corresponding RARE images with the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain's (FMRIB) Linear Image Registration Tool (FLIRT) (Jenkinson and Smith, 2001). The EPI images were then transferred to Analysis of Functional NeuroImages (AFNI) (Cox and Hyde, 1997) format and further analysis was performed with this software suite. A total of ten runs (five subjects and two experiments) for each stimulation frequency and dosage were averaged with the AFNI tool 3dcalc. The averaged EPI acquisitions were analyzed with the 3dDeconvolve tool, with an F-test performed with the block design as the only regressor. Activation maps were generated and plotted for the averaged data, with a p-value of 0.005 as the threshold for display. The activation maps were overlaid on the “ideal” RARE anatomy.
fMRI comparisons
Each animal was analyzed on an individual basis for both the area (number of activated voxels) and intensity of activation (percent signal change) in the S1FL region. Voxels above a p-value threshold of 0.005 were used in further analysis. The frequency dependence for the number of activated voxels was tested with a one-way repeated measures analysis of variance (ANOVA) with a Tukey comparison (p < 0.05, 95% confidence intervals). The slope of the number of activated voxels as a function of frequency was determined by linear regression. The slopes for different dosages were compared by calculating a p-value (two-tailed) by testing the null hypothesis that the two slopes are identical.
The percent signal change was determined by standardizing the baseline to the mean signal of each animal. The three stimulation events were averaged for display. The intensity was then examined using area-under-the-curve analysis. However, this intensity metric failed to reveal significant differences between infusion dosages.
Resting-state connectivity analysis
Consultation with the Paxinos atlas (Paxinos G, 2005) helped define regions of interest (ROIs). ROIs included the corpus callosum (CC), primary/secondary motor cortex (M1/M2), primary somatosensory forepaw region (S1FL), primary somatosensory trunk region (S1Tr), secondary somatosensory region (S2), sensory motor thalamus (SMT), posterior thalamic nucleus (PO), posterior lateral thalamic nucleus (VP), caudate putamen (CP), and globus pallidus (GP). The superior colliculus (SC) was included as a control because it is part of the visual system and should demonstrate no connectivity to the sensorimotor regions.
Data sets were detrended to eliminate linear drifts. A low-pass filter with a cutoff at 0.1 Hz was applied to all regional time courses. Principal component analysis (PCA) was carried out for each region. Using PCA, a data set of orthogonal waveforms was determined for each region. PCA is a multivariate technique that replaces the measured variables by a set of principal components arranged in the order of decreasing standard deviation (SD) or energy distribution. For this study, the first two components accounted for more than 75% of the SD for each ROI and were therefore used. The resulting time series for each region was correlated with every other regional time series to obtain a pair-wise correlation matrix. Averages across 20 animals (initial 100 μg/kg/hr infusion rate), 5 animals (secondary 100 μg/kg/hr infusion rate), and 5 animals (secondary 300 μg/kg/hr infusion rate) were tabulated. The PCA was run independently for each region.
Results
A bench study was performed to determine if a constant infusion of medetomidine alone was sufficient to anesthetize a rat for the duration of a normal scanning session (4 hrs). The results from three representative animals are plotted in Fig. 2. One animal received a constant infusion of medetomidine at 100 μg/kg/hr, which is the previously published dosage (Cho et al., 2007; Sommers et al., 2002; Van Camp et al., 2006; Weber et al., 2006; Zhao et al., 2008). A second animal received a 300 μg/kg/hr constant infusion. The third animal was started at the 100 μg/kg/hr infusion and then was switched to a higher 300 μg/kg/hr infusion rate. Heart rate and nociceptive response to tail pinch (arrows) were used as sedation indicators. All animals that received a constant infusion of 100 μg/kg/hr (n=4) or 300 μg/kg/hr (n=4) awoke from the sedation 3½ to 4 hrs into the experiment. Response to tail pinch occurred at 2½ hrs for all animals in the two constant infusion groups, either 100 μg/kg/hr or 300 μg/kg/hr. Under the protocol of infusion of 100 μg/kg/hr with increase of the dosage to 300 μg/kg/hr 1 hr and 15 min into the experiment, the rats (n=4) remained sedated for a much longer period (> 6 hrs). Resistance to tail pinch was prolonged to around 5½ hrs in all animals that received a stepped increase in infusion dosage. Initial heart rates under medetomidine anesthesia varied by ± 50 beats per min between animals; however, the trends were consistent for all animals, and the heart rate trend was a good indicator of sedation.
Fig. 2.
Rat sedation under two different levels of medetomidine anesthesia. The heart rates of three representative animals as a function of time are plotted. The small arrows indicate tail movement in response to tail pinch, which was used as a measure of sedation. One animal received a constant infusion of 100 μg/kg/hr, the second 300 μg/kg/hr, and the third started initially with an infusion of 100 μg/kg/hr and was then switched at 1¼ hours to a 300 μg/kg/hr infusion rate. (Dose increase indicated on graph.)
EEG measurement of sedation level
The time dependence of medetomidine sedation was further explored using resting EEG measurements. The relationship of the resting EEG to the time course and infused medetomidine dose is shown in Fig. 3. By the end of the initial 100 μg/kg/hr infusion at ∼1.5 hours, the beta/delta ratio increased in a statistically significantly manner, suggestive of preliminary indications of wakening. In both groups receiving constant infusion of either 100 μg/kg/hr or 200 μg/kg/hr, the trend toward a more awakened state persisted to the end of the experiment. The waking trend was reversed when the second dose was 300 μg/kg/hr. These EEG results are in agreement with the tail-pinch bench study.
Fig. 3.
The EEG effect (ratio of the square root of the powers in the beta (12–20 Hz) and delta (1–4Hz) frequency bands) versus time and medetomidine dose administration. The average β/Δ ratio from the initial 100 μg/kg/hr medetomidine dose (open triangles; n=8) increased during the time of infusion, becoming significantly different from previous ratios at 80 min (*, P > 0.5). When the second medetomidine dose was 100 μg/kg/hr (open circles; n=2) or 200 μg/kg/hr (gray circles; n=3), the increasing trend of the β/Δ ratio continued. However, when the second medetomidine dose was 300 μg/kg/hr (black circles; n=3), the β/Δ ratio decreased, suggesting that the dose-increase prevented the tendency for awakening.
Physiological measurements and cortical signals during fMRI
Table 1 summarizes the physiological data recorded during the fMRI experiments. The table includes percent oxygen saturation (SpO2), mean arterial pressure (MAP), and temperature (°C) for all animals in the study. The parameters were averaged across animals for each primary and each secondary acquisition period for four infusion protocols of medetomidine dosages indentified in Fig. 1. No significant differences in physiology were found between the dosages, and the animals were maintained within normal ranges for the duration of the experiments.
Table 1.
Physiological Parameters
| Medetomidine Infusion Dosage | ||
|---|---|---|
| Start | End | |
| ← 100 μg/kg/hr → | ||
| SPO2 (%) | 98±1 | 97±1 |
| MAP (mmHg) | 131±9 | 138±3 |
| Temperature (°C) | 37±1 | 37±1 |
| 100 μg/kg/hr → 150 μg/kg/hr | ||
| SPO2 (%) | 97±1 | 98±1 |
| MAP (mmHg) | 139±10 | 132±5 |
| Temperature (°C) | 36±1 | 37±1 |
| 100 μg/kg/hr → 200 μg/kg/hr | ||
| SPO2 (%) | 98±1 | 97±1 |
| MAP (mmHg) | 135±18 | 137±14 |
| Temperature (°C) | 38±1 | 37±1 |
| 100 μg/kg/hr → 300 μg/kg/hr | ||
| SPO2 (%) | 99±1 | 97±1 |
| MAP (mmHg) | 129±14 | 138±9 |
| Temperature (°C) | 37±1 | 37±1 |
An activation map is shown in Fig. 4 for 7 Hz frequency, 3 ms pulse width, and 2 mA forepaw stimulation at the 100 μg/kg/hr dosage. Five acquired slices centered 1.64 mm through -2.36 mm from bregma are displayed. Each is an average across five animals. An F-test with correlation between the voxel time courses and the stimulation block design was used for fMRI analysis. Only voxels that passed the p-value threshold of 0.005 were considered active and used in further investigation and display. Robust positive BOLD activation occurred in the cortical somatosensory forelimb region (S1FL), located 1.64 mm through -1.36 mm from bregma. A diffuse negative BOLD response was observed in the caudate putamen. This negative BOLD response is consistent with our earlier study with direct forepaw nerve stimulation (Cho et al., 2007), although with decreased intensity and area. A positive BOLD response was also apparent in the thalamus and S2 regions at this dosage. Responses in regions other than S1FL were not used in further analysis.
Fig. 4.
BOLD activation maps for electrical forepaw stimulation at 7 Hz, 3 ms, 2 mA. Five slices centered 1.64 mm to -2.36 mm from bregma are plotted. Areas activated include the primary forelimb somatosensory (S1FL) region, caudate putamen (CP), thalamus, and secondary sensory (S2) region. Voxels with a p-value < 0.005 were plotted. Averages across five rats are displayed.
Frequency-dependent response to forelimb stimulation
The area of activation in S1FL induced by forepaw stimulation at the initial dosage of 100 μg/kg/hr is displayed in Fig. 5a. The average across all 20 rats is shown. The number of activated voxels increased with increasing frequency. Statistically, significant differences were found between the stimulation frequencies (3, 5, 7, 10 Hz) using a one-way repeated measures ANOVA with a Tukey comparison (p < 0.05, 95% confidence intervals). As indicated in Fig. 5a, the number of activated voxels increases linearly with frequency. The percent signal change for the activated voxels is shown in Fig. 5b for the 100 μg/kg/hr infusion. Again, the average across 20 rats is plotted. As with the area of activation, the intensity of activation increases with stimulation frequency in a stepwise fashion. This was determined using an area-under-the-curve analysis with the results shown in Fig. 5c. The largest percent signal change occurs at 10 Hz, with close to 2% maximal change at peak. The hemodynamic delay (defined as onset of stimulus to 90% of peak height) is about 6 s. Response to stimulation persists for 4–6 s after stimulus cessation, and a post-stimulus undershoot occurs around 6 s after the stimulus is turned off (most pronounced at 3 Hz). These results are consistent with other studies with rats under medetomidine anesthesia (Zhao et al., 2008).
Fig. 5.
Frequency-dependence of intensity and volume of BOLD fMRI activation at the initial 100 μg/kg/hr infusion rate. (a) Number of activated voxels as a function of frequency. (An asterisk [*] indicates statistical significance P < 0.05.) A linear regression of the frequency dependence is included. (b) Percent signal change as a function of time for activated voxels. (c) Area-under-the-curve analysis from activated voxel time-courses. All voxels in S1FL with a p-value < 0.005 were used in the analysis. Averages across 20 rats are displayed.
Dose-dependence of response to somatosensory stimulation
Figure 6 shows the frequency dependence of the area of BOLD activation in the S1FL region as a function of varying secondary dosages of medetomidine. Five rats were used in each of the four experiments. All animals were started at an initial infusion dosage of 100 μg/kg/hr, and both resting-state and functional experiments were performed followed by an increase in infusion dosage. A 30-min rest period was allowed, and the resting and functional experiments were then repeated under the new secondary infusion rate. For one experiment, the secondary dosage was maintained at the 100 μg/kg/hr initial rate as a control. The secondary infusion rate dosages were 100 (Fig. 6a), 150 (Fig. 6b), 200 (Fig. 6c), and 300 μg/kg/hr (Fig. 6d). Only the areas of activation from the secondary dosages are plotted. The results for the area of activation for the initial infusion dosage (100 μg/kg/hr) are displayed in Fig. 5a.
Fig. 6.
Frequency-dependence of volume of activation for the secondary infusion rates. The numbers of activated voxels are plotted as a function of frequency for the (a) 100, (b) 150, (c) 200, and (d) 300 μg/kg/hr second infusion rates. Averages across five animals are plotted for each experiment. Linear regressions for the secondary 100/150/200/300 μg/kg/hr infusion rates are plotted. The slopes for the lower 100/150 μg/kg/hr infusion rates are statistically different (p < 0.05) than the initial 100 μg/kg/hr and higher 200/300 μg/kg/hr infusion rates.
To compare the frequency dependence of the area of activation among different doses, the number of activated voxels as a function of frequency was fit with a linear regression model. These slopes are displayed in Figs. 5 and 6. The slopes from the second infusion dosages (Fig. 6) were statistically compared to the initial infusion dosage (Fig. 5). The slopes from the secondary 100/150 μg/kg/hr dosages significantly differ from the initial 100 μg/kg/hr dosage (p < 0.05); the slopes from the 200/300 μg/kg/hr secondary dosages do not significantly differ from the initial 100 μg/kg/hr dosage (p < 0.05). The slopes of all initial 100 μg/kg/hr dosages were not statistically significant (p < 0.05) (data not shown). This attenuation of the activated volume at the higher stimulation frequencies at the two low dosages indicates a time-dependent attenuation of the BOLD signal under constant infusion of medetomidine.
The corresponding activation intensities are plotted in Fig. 7. The largest percent signal change occurred at 10 Hz for all dosages. An area-under-the-curve analysis was performed. No statistical differences in the areas-under-the-curve were found between the dosages. These experiments establish that for the number of animals used, the number of activated voxels, Fig. 6, provides a reliable metric for changes in depth of sedation, but the BOLD signal intensity, Fig. 7, does not.
Fig. 7.
Percent signal change at varying infusion rates. The four different experiments are plotted from top to bottom and labeled (a)–(d). The initial 100 μg/kg/hr rate for each of the experiments is plotted on the left side of the figure, and the right side of the figure displays the second infusion rate in matched animals. Averages across five animals are plotted for each experiment.
Resting-state results
An additional method of assessing changes in depth of sedation using resting-state functional connectivity was developed using regional pairwise correlation coefficient (RPCC) matrices. Average filtered time-series were selected from regions in the sensorimotor system. The RPCC matrix is mirrored about the main diagonal. The lower triangular part of the matrix displays a color plot based on the color bar to the right of the matrix. The upper triangular part of the matrix contains the corresponding correlation coefficients. Along the main diagonal, each region is correlated with itself, and, therefore, the entries have a correlation coefficient value of 1.0. A region located in the visual system was chosen as a control: the superior colliculus (SC), which was expected to exhibit no functional connectivity to the sensorimotor system.
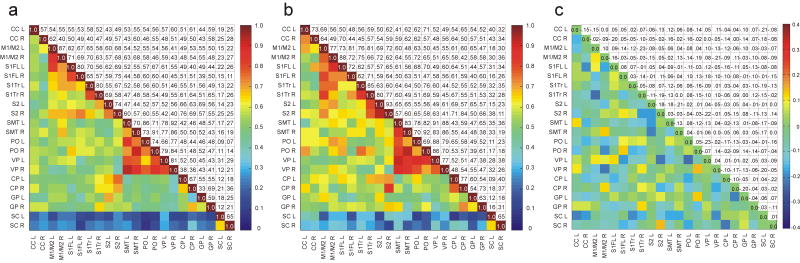
Figures 8a and 9a are the RPCC matrices averaged from all the resting-state acquisitions during the initial 100 μg/kg/hr infusion rate (n=20). Figure 8b is the matrix average from the resting-state scans acquired from rats that received a secondary 100 μg/kg/hr infusion period (n=5). Figure 9b is the matrix average from the resting-state scans acquired from animals that had a secondary 300 μg/kg/hr infusion rate. Figures 8c and 9c are difference maps taken from the subtraction of Figs. 8a–b and 9a–b, respectively. The SC region demonstrates low correlation (< 0.30) to the other regions in all of the matrices.
Fig. 8.
(a) RPCC matrix of average across 20 animals of the resting-state BOLD time courses in the rat sensorimotor system at 100 μg/kg/hr initial infusion. (b) RPCC matrix of average across five animals of the resting-state BOLD time courses in the rat sensorimotor system at 100 μg/kg/hr secondary infusion. (c) Difference matrix tabulated from the subtraction of Fig. 8b from Fig. 8a. Both the right and left sides of each region were tabulated. The SC from the visual region was included as a control. The lower triangular part of the graph displays the coefficient values based on the color bar on the right. The upper triangular part (mirror image) of the graph tabulates the corresponding coefficient values. In Fig. 8c a difference value of 0.17 corresponds to a equivalent p-value of 0.05 tested with an unpaired t-test. See Materials and methods for abbreviations of regions.
Fig. 9.
(a) RPCC matrix of average across 20 animals of the resting-state BOLD time courses in the rat sensorimotor system at 100 μg/kg/hr initial infusion. (b) RPCC matrix of average across five animals of the resting-state BOLD time courses in the rat sensorimotor system at 300 μg/kg/hr secondary infusion. (c) Difference matrix tabulated from the subtraction of Fig. 9b from Fig. 9a. Both the right and left sides of each region were tabulated. The SC from the visual region was included as a control. The lower triangular part of the graph displays the coefficient values based on the color bar on the right. The upper triangular part (mirror image) of the graph tabulates the corresponding coefficient values. In Fig. 9c a difference value of 0.17 corresponds to a equivalent p-value of 0.05 tested with an unpaired t-test. See Materials and methods for abbreviations of regions.
Rats sedated under the secondary constant infusion of medetomidine (100 μg/kg/hr) (Fig. 8b) demonstrate a decrease in the correlation coefficients between regions when compared to the initial 100 μg/kg/hr infusion (Fig. 8a). Most correlation differences between the initial 100 μg/kg/hr and secondary 100 μg/kg/hr dosage are positive. These positive differences are colored in yellow/orange/red hues and are highlighted in the difference matrix (Fig. 8c). An unpaired t-test with a 95% confidence interval was performed on the individual correlation coefficient values between regions for both the initial and secondary 100 μg/kg/hr dosages. A difference of 0.17 in the difference matrix corresponded to a equivalent p-value of 0.05. The trends for the resting-state data are consistent with the task activated BOLD data and demonstrate attenuation of the connectivities between regions under a constant infusion of medetomidine.
In contrast to the rats that received a constant infusion of 100 μg/kg/hr, rats switched to a higher secondary dose of 300 μg/kg/hr (Fig. 9b) show an increase in the correlation coefficients between regions when compared to the initial infusion rate of 100 μg/kg/hr (Fig. 9a). These negative differences are highlighted in the difference matrix (Fig. 9c) and are colored in blue hues. An unpaired t-test with a 95% confidence interval was performed on the individual correlation coefficient values between regions for both the initial 100 μg/kg/hr and secondary 300 μg/kg/hr dosages. Again a difference of 0.17 in the difference matrix corresponded to an equivalent p-value of 0.05. Figure 9c is predominately green indicating that functional connectivity during the period of secondary infusion remains unchanged from that of the period of primary infusion. However, the matrix shades overall towards blue, indicating some increase in functional connectivity during the secondary infusion period.
Discussion
The intent of this work was to find a dosage where a steady state of sedation could be safely maintained throughout a > 3 hr protocol. We find that constant infusion of medetomidine at 100 μg/kg/hr, which is a customary dosage found in the literature, is not sufficient to maintain sedation beyond 3 hrs. Moreover, a threefold increase in dosage failed to increase the duration of sedation. Bench tests of tail pinch, EEG, fMRI, and fcMRI studies are in agreement. In this study, we demonstrated that use of an initial infusion dose of 100 μg/kg/hr followed by a step increase to 300 μg/kg/hr after 90 min maintains sedation for durations as long as 6 hrs.
The use of fMRI to study anesthesia depth
Comparisons between different anesthetics (Austin et al., 2005; Huttunen et al., 2008; Willis et al., 2001), as well as between awake and anesthetized animals (Duong, 2007; Martin et al., 2006; Peeters et al., 2001), are important to the understanding of the linkage between neuronal activity and the BOLD signal. There have been several studies demonstrating that anesthetic agent and dosage have a direct effect on baseline BOLD signal (Hyder et al., 2002; Maandag et al., 2007). Isoflurane has been shown to reduce both the intensity and the area of BOLD somatosensory response in a dose-dependent manner for both rats (Masamoto et al., 2007) and cats (Zhao et al., 2007). However, in isoflurane-anesthetized rats, the neural evoked response to sensory stimulation does not attenuate with increasing concentration of the anesthetic (Imas et al., 2005). Isoflurane causes vasodilatation in the cerebral vasculature, which results in uncoupling between flow and neuronal activation (Lee et al., 1995). A hyperemic dose-dependent response to sensory stimulation was also shown for halothane-anesthetized rats using laser Doppler flowometry (Schulte and Hudetz, 2006).
Functional MRI experiments using stepwise BOLD response to variation in rat forepaw electrical stimulation frequency have been reported by several investigators (Huttunen et al., 2008; Keilholz et al., 2004; Liu et al., 2004; Martindale et al., 2003; Masamoto et al., 2007; Van Camp et al., 2005; Zhao et al., 2008). Various responses were found, which seem to depend on the anesthetic used in the study. We find for medetomidine that the response increases linearly up to a frequency of 10 Hz when the animal is sedated but that the linear increase is disrupted as the level of sedation decreases. Step-up in dosage of medetomidine at 90 min, as used here, preserves the linearity of frequency dependence up to a total scan session as long as 6 hrs. A study under medetomidine anesthesia in rats using 100 μg/kg/hr infusion has shown a similar frequency response curve to the one obtained in this study during the initial dosage period (Zhao et al., 2008). We have previously shown tight neurovascular coupling between SEPs and BOLD response to visual stimulation under medetomidine sedation (Pawela et al., 2008b). As demonstrated by Huttunen et al. (Huttunen et al., 2008), each anesthetic affects SEPs in response to frequency of forepaw stimulation in a unique manner, but the correlation between SEPs and BOLD response is maintained. Other studies with different anesthetic agents have not shown the same stepwise increase in BOLD response with stimulation frequency (Huttunen et al., 2008; Keilholz et al., 2004; Martindale et al., 2003; Masamoto et al., 2007), which leads to the suggestion that fMRI studies with stepwise stimulation frequency increase may be a useful technique for probing not only the mechanisms of anesthesia but also the BOLD-sensitive coupling between local blood flow and local neuronal activity.
Infusion dose dependence of resting-state connectivity
The discovery of spatial correlations of physiological fluctuations in the awake human sensorimotor resting-state BOLD signal (Biswal et al., 1995) led to questions about the effect of anesthetics on functional connectivity in humans and other primates. Recently, it was discovered that the extent of functional connectivity in monkeys under isoflurane anesthesia is reduced in a dose-dependent manner (Vincent et al., 2007). The uncoupling of flow by vasodilatation was implicated in this decline in the resting-state correlation. The reduction in resting-state connectivity has also been demonstrated in humans for another vasodilator, sevoflurane (Peltier et al., 2005). In contrast to these agents, dexmedetomidine has been shown to have a vasoconstrictive effect on the cerebral vasculature (Ganjoo et al., 1998), and also to reverse and inhibit the dilation induced by isoflurane and sevoflurane in dog brain (Ohata et al., 1999). Both thiopental (Kiviniemi et al., 2000) and midazolam (Kiviniemi et al., 2005), which are vasoconstrictors, have been shown to enhance resting-state BOLD fluctuations in children. It has been hypothesized that the vascular action of these drugs is responsible for this enhancement. Thus, anesthetics can either increase or decrease the level of BOLD LFFs. In the experiments described here, administration of the secondary dose of medetomidine results in an increase in correlation coefficients relative to data acquired during the primary dose period, as seen in the difference regional connectivity matrix (Figs. 8c and 9c).
General physiology
We observed an immediate hypertensive response with the initial infusion of medetomidine. This initial hypertension (10 min) has been shown to be caused by an increase in systemic vascular resistance and decreased sympathetic tone (Sinclair, 2003). A feedback response to the initial hypertension occurs in the baroreceptors causing a reflex physiologic bradycardia (Murrell and Hellebrekers, 2005). Over time, the cardiac effects subside and both heart rate and blood pressure return to normal (Murrell and Hellebrekers, 2005). We waited an initial 30 min to limit blood pressure fluctuations. The stability of the MAPs is reflected in Table 1.
Stress- and fear-management play fundamental roles in the success of medetomidine sedation. Preexisting stress can cause increases in endogenous catecholamine levels and interfere with α2-agonist-induced reductions in excitatory neurotransmitter release (Sinclair, 2003). Avoidance of adrenergic response is achievable with proper care and protocols. It has been our experience that pre-fMRI procedures must be carried out under another anesthetic that is tapered off after the placement of the animal in the scanner environment (Cho et al., 2007). Preexisting stress may be the reason for the differences in initial heart rates under medetomidine sedation in our bench tail-pinch study. Since the pre-protocol surgery was carried out under isoflurane anesthesia, initial heart rates may have been affected by isoflurane anesthesia depth.
Medetomidine Anesthetic Action
Medetomidine, like all α2-agonists, binds with the presynaptic membrane of the G-coupled protein receptor and controls the release of norepinephrine. Many of these receptors are located in the pons and locus coeruleus neurons of the lower brainstem. Medetomidine acts within the brain regions associated with the sleep pathway (Nelson et al., 2003) and causes a sleep-like state of sedation (Huupponen et al., 2008). This sedation can be quickly reversed with the administration of an α2-antagonist. The distribution of α2-receptors in rat brain has been studied using immunohistochemistry (Rosin et al., 1998). The concentration of α2-receptors in the rat brain has been shown to be low in the cortex and thalamus and high in the lower brain regions. It can be hypothesized that the vascular properties of medetomidine arise from the anatomical distribution of α2 receptors.
The effects of graded steady-state concentrations of medetomidine on behavior and plasma concentration have not been investigated, although the data are available for dexmedetomidine, the active enantiomer. Bol et al. (Bol et al., 2000) found a plasma concentration of 7.0 ng/ml dexmedetomidine at a constant infusion rate of 60 μg/kg/hr and a plasma concentration of 16.0 ng/ml dexmedetomidine with a constant infusion rate of 120 μg/kg/hr. Complete loss of righting reflex occurred at concentrations around 5.0 ng/ml and complete loss of response to tail pinch around 10 ng/ml (Bol et al., 1999). Because a constant infusion rate results in a time-dependent loss of sedation, we hypothesize that long-term infusion of medetomidine causes downregulation of α2-receptors. This results in the loss of frequency dependence of the BOLD response at the secondary 100 and 150 μg/kg/hr dosages. Preservation of the frequency dependence occurs with higher 200 and 300 μg/kg/hr secondary dosages. It is advisable to increase the dosage, stepwise, with long fMRI experiments (> 1 hr).
Targeted controlled infusion
It is very common to change anesthesia infusion dosage during any long procedure in an operating room environment. There is very little data on tolerance to constant infusion of medetomidine in rats, but data are available for another infused anesthetic agent with a different target of action—propofol. Ihmsen et al. (Ihmsen et al., 2002) found acute tolerance to the hypnotic effect of propofol, with a 90-min constant rate infusion period in rats as measured by a change in the median frequency of the EEG (Ihmsen et al., 2002). That is, to maintain the effect of propofol, as measured by EEG, the plasma concentration must be increased over time. This pharmacodynamic tolerance was coupled with nonlinear pharmacokinetics indicating a decrease in drug clearance over time. Because of the compensating effects of reduced drug clearance and receptor desensitization, the hypnotic effect of propofol could not be maintained at constant drug infusion rates. Densensitization to the behavioral effects of dexmedetomidine has been demonstrated in rat experiments with bolus injections carried out over the course of several days (Hayashi et al., 1995). Our method of increasing the infusion dose during the course of the experiment is analogous to targeted controlled infusion methods. We hypothesize that the pharmacokinetics of the drug change over time in a manner that maintains sedation for a longer time period.
Conclusion
This work has demonstrated that it is necessary to change infusion dosage during any long (> 2 hrs) fMRI protocol under medetomidine sedation. It has also been found that the loss of sedation is independent of initial constant infusion dosage. Finally, there is a time dependent reduction in BOLD fMRI and fcMRI response during constant medetomidine infusion. This reduction can also be prevented by increasing the infusion dosage during the course of the experiment.
Acknowledgments
This work was supported by grants EB000215, EB000215-S1, and GM56398 from the National Institutes of Health, and DABK39-03-C-0058 from the Counterdrug Technology Assessment Center, Office of National Drug Control Policy, White House. The authors thank Hanbing Lu and B. Douglas Ward for their helpful comments. The authors would also like to thank Abbie Amadio and Karen Hyde for manuscript editing and figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: A study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bol C, Danhof M, Stanski DR, Mandema JW. Pharmacokinetic-pharmacodynamic characterization of the cardiovascular, hypnotic, EEG and ventilatory responses to dexmedetomidine in the rat. J Pharmacol Exp Ther. 1997;283:1051–1058. [PubMed] [Google Scholar]
- Bol CJ, Vogelaar JP, Mandema JW. Anesthetic profile of dexmedetomidine identified by stimulus-response and continuous measurements in rats. J Pharmacol Exp Ther. 1999;291:153–160. [PubMed] [Google Scholar]
- Bol CJ, Vogelaar JP, Tang JP, Mandema JW. Quantification of pharmacodynamic interactions between dexmedetomidine and midazolam in the rat. J Pharmacol Exp Ther. 2000;294:347–355. [PubMed] [Google Scholar]
- Cho YR, Pawela CP, Li R, Kao D, Schulte ML, Runquist ML, Yan JG, Matloub HS, Jaradeh SS, Hudetz AG, Hyde JS. Refining the sensory and motor ratunculus of the rat upper extremity using fMRI and direct nerve stimulation. Magn Reson Med. 2007;58:901–909. doi: 10.1002/mrm.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Duong TQ. Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anesthetized rats. Brain Res. 2007;1135:186–194. doi: 10.1016/j.brainres.2006.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganjoo P, Farber NE, Hudetz AG, Smith JJ, Samso E, Kampine JP, Schmeling WT. In vivo effects of dexmedetomidine on laser-Doppler flow and pial arteriolar diameter. Anesthesiology. 1998;88:429–439. doi: 10.1097/00000542-199802000-00022. [DOI] [PubMed] [Google Scholar]
- Goloshevsky AG, Silva AC, Dodd SJ, Koretsky AP. BOLD fMRI and somatosensory evoked potentials are well correlated over a broad range of frequency content of somatosensory stimulation of the rat forepaw. Brain Res. 2008;1195:67–76. doi: 10.1016/j.brainres.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Guo TZ, Maze M. Desensitization to the behavioral effects of alpha 2-adrenergic agonists in rats. Anesthesiology. 1995;82:954–962. doi: 10.1097/00000542-199504000-00019. [DOI] [PubMed] [Google Scholar]
- Hayton SM, Kriss A, Muller DP. Comparison of the effects of four anaesthetic agents on somatosensory evoked potentials in the rat. Lab Anim. 1999;33:243–251. doi: 10.1258/002367799780578219. [DOI] [PubMed] [Google Scholar]
- Huttunen JK, Grohn O, Penttonen M. Coupling between simultaneously recorded BOLD response and neuronal activity in the rat somatosensory cortex. Neuroimage. 2008;39:775–785. doi: 10.1016/j.neuroimage.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Huupponen E, Maksimow A, Lapinlampi P, Sarkela M, Saastamoinen A, Snapir A, Scheinin H, Scheinin M, Merilainen P, Himanen SL, Jaaskelainen S. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihmsen H, Tzabazis A, Schywalsky M, Schwilden H. Propofol in rats: Testing for nonlinear pharmacokinetics and modelling acute tolerance to EEG effects. Eur J Anaesthesiol. 2002;19:177–188. doi: 10.1017/s0265021502000327. [DOI] [PubMed] [Google Scholar]
- Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–947. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jugovac I, Imas O, Hudetz AG. Supraspinal anesthesia: Behavioral and electroencephalographic effects of intracerebroventricularly infused pentobarbital, propofol, fentanyl, and midazolam. Anesthesiology. 2006;105:764–778. doi: 10.1097/00000542-200610000-00023. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB, Kim YR, Rosen BR. Spatio-temporal characteristics of low-frequency BOLD signal fluctuations in isoflurane-anesthetized rat brain. Neuroimage. 2008;40:1738–1747. doi: 10.1016/j.neuroimage.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med. 2004;52:89–99. doi: 10.1002/mrm.20114. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44:373–378. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ, Haanpaa H, Kantola JH, Jauhiainen J, Vainionpaa V, Alahuhta S, Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23:531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lee JG, Smith JJ, Hudetz AG, Hillard CJ, Bosnjak ZJ, Kampine JP. Laser-Doppler measurement of the effects of halothane and isoflurane on the cerebrovascular CO2 response in the rat. Anesth Analg. 1995;80:696–702. doi: 10.1097/00000539-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Li BH, Lohmann JS, Schuler HG, Cronin AJ. Preservation of the cortical somatosensory-evoked potential during dexmedetomidine infusion in rats. Anesth Analg. 2003;96:1155–1160. doi: 10.1213/01.ANE.0000053239.62623.32. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Schmidt KF, Sicard KM, Duong TQ. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn Reson Med. 2004;52:277–285. doi: 10.1002/mrm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci USA. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Jones M, Martindale J, Mayhew J. Haemodynamic and neural responses to hypercapnia in the awake rat. Eur J Neurosci. 2006;24:2601–2610. doi: 10.1111/j.1460-9568.2006.05135.x. [DOI] [PubMed] [Google Scholar]
- Martindale J, Mayhew J, Berwick J, Jones M, Martin C, Johnston D, Redgrave P, Zheng Y. The hemodynamic impulse response to a single neural event. J Cereb Blood Flow Metab. 2003;23:546–555. doi: 10.1097/01.WCB.0000058871.46954.2B. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Miller A, Sleigh JW, Barnard J, Steyn-Ross DA. Does bispectral analysis of the electroencephalogram add anything but complexity? Br J Anaesth. 2004;92:8–13. doi: 10.1093/bja/aeh003. [DOI] [PubMed] [Google Scholar]
- Murrell JC, Hellebrekers LJ. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet Anaesth Analg. 2005;32:117–127. doi: 10.1111/j.1467-2995.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Ohata H, Iida H, Dohi S, Watanabe Y. Intravenous dexmedetomidine inhibits cerebrovascular dilation induced by isoflurane and sevoflurane in dogs. Anesth Analg. 1999;89:370–377. doi: 10.1097/00000539-199908000-00023. [DOI] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008a;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Hudetz AG, Ward BD, Schulte ML, Li R, Kao DS, Mauck MC, Cho YR, Neitz J, Hyde JS. Modeling of region-specific fMRI BOLD neurovascular response functions in rat brain reveals residual differences that correlate with the differences in regional evoked potentials. Neuroimage. 2008b;41:525–534. doi: 10.1016/j.neuroimage.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, W C. The Rat Brain in Stereotaxic Coordinates. 5th. Elsevier Academic Press; New York: 2005. [Google Scholar]
- Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn Reson Imaging. 2001;19:821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Salonen JS. Pharmacokinetics of medetomidine. Acta Vet Scand (Supp) 1989;85:49–54. [PubMed] [Google Scholar]
- Savola MK, MacIver MB, Doze VA, Kendig JJ, Maze M. The alpha 2-adrenoceptor agonist dexmedetomidine increases the apparent potency of the volatile anesthetic isoflurane in rats in vivo and in hippocampal slice in vitro. Brain Res. 1991;548:23–28. doi: 10.1016/0006-8993(91)91101-6. [DOI] [PubMed] [Google Scholar]
- Schulte ML, Hudetz AG. Functional hyperemic response in the rat visual cortex under halothane anesthesia. Neurosci Lett. 2006;394:63–68. doi: 10.1016/j.neulet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair MD. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can Vet J. 2003;44:885–897. [PMC free article] [PubMed] [Google Scholar]
- Sommers MG, Pikkeemaar JA, Booij LHDJ, Heerschap A. Improved anesthesia protocols for fMRI studies in rats: The use of medetomidine for stable, reversible sedation [abstract] Proc Intl Soc Mag Reson Med. 2002;10:393. [Google Scholar]
- Sommers MG, van Egmond J, Booij LH, Heerschap A. Isoflurane anesthesia is a valuable alternative for alpha-chloralose anesthesia in the forepaw stimulation model in rats. NMR Biomed. 2008 doi: 10.1002/nbm.1351. in press. [DOI] [PubMed] [Google Scholar]
- Van Camp N, Peeters RR, Van der Linden A. A comparison between blood oxygenation level-dependent and cerebral blood volume contrast in the rat cerebral and cerebellar somatosensoric cortex during electrical paw stimulation. J Magn Reson Imaging. 2005;22:483–491. doi: 10.1002/jmri.20417. [DOI] [PubMed] [Google Scholar]
- Van Camp N, Verhoye M, De Zeeuw CI, Van der Linden A. Light stimulus frequency dependence of activity in the rat visual system as studied with high-resolution BOLD fMRI. J Neurophysiol. 2006;95:3164–3170. doi: 10.1152/jn.00400.2005. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Willis CK, Quinn RP, McDonell WM, Gati J, Parent J, Nicolle D. Functional MRI as a tool to assess vision in dogs: The optimal anesthetic. Vet Ophthalmol. 2001;4:243–253. doi: 10.1046/j.1463-5216.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jin T, Wang P, Kim SG. Isoflurane anesthesia effect in functional imaging studies. Neuroimage. 2007;38:3–4. doi: 10.1016/j.neuroimage.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]