Abstract
Background
Pharmacological rescue of behavioral, cognitive and synaptic abnormalities in the animal models of fragile X syndrome (FXS) has prompted the initiation of clinical trials of targeted treatments in humans with this condition. Objective, well-validated outcome measures that are reflective of FXS deficits and can be modeled similarly in animal and human studies are urgently needed.
Methods
A protocol measuring prepulse inhibition (PPI) of the startle reflex, including measures of test-retest stability, was evaluated in 61 individuals with the fragile X full mutation (40 males and 21 females; 19.18 ± 7.18 years) and 63 age-matched normal controls (35 males and 28 females; 20.83 ± 6.96 years) across two laboratory sites with identical equipment and protocols.
Results
Relative to controls, the fragile X group had PPI impairment of 26%, 22%, and 28% for 60 ms, 120 ms, and 240 ms prepulse interval trial types, respectively, p = 0.000002. PPI test-retest reliability in 29 of the participants was excellent for the 120 ms prepulse interval trials (intraclass correlations: FXS, 0.85; controls, 0.88, 0.89 overall).
Conclusions
This study demonstrates the feasibility and reliability of PPI measurement in a developmentally disabled population and highlights its potential as an outcome measure to test the efficacy of targeted neurotherapeutic agents.
Keywords: PPI, FMR1 gene, sensorimotor gating, mGluR5, prepulse inhibition
INTRODUCTION
Fragile X syndrome (FXS), caused by a mutation in FMR1 at Xq27.3, is the most common inherited cause of mental impairment and the leading known single gene cause of autism. This mutation results from the presence of more than 200 CGG repeats within the promoter region of the FMR1 gene, which prevents normal transcription, and leads to subsequent reduction or absence of the FMR1 protein (FMRP) (Devys and others 1993; Tamanini and others 1997) and abnormal brain development, including aberrant dendritic arborization and synaptic plasticity (Comery and others 1997; Galvez and Greenough 2005; Irwin and others 2000; Irwin and others 2002; Irwin and others 2001). The behavioral phenotype is characterized by deficits in attention and inhibitory control, autistic symptoms, social anxiety and withdrawal, hyperarousal, and gaze aversion (Reiss and Dant 2003).
There are currently no empirically validated behavioral or pharmacological treatments available for individuals with FXS. However, new leads to potential targeted treatments have come from recent advances in the neurobiology of FXS. For example, the “mGluR theory” suggests that phenotypic features of the disorder arise from enhanced activity of the metabotropic glutamate receptor 5 pathway (mGluR5) (Bear 2005; Bear and others 2004). Flies lacking dfmr1, the homologue of FMR1 in humans, show altered courtship behavior, decreased memory, and dendritic abnormalities that are rescued when raised with food containing the mGluR5 antagonist MPEP (2-methyl-6-phenylethynylpyridine) (McBride and others 2005). These and additional studies demonstrating rescue of behavioral, cognitive and epilepsy phenotypes in the fmr1 knockout mouse with genetic or pharmacological down regulation of mGluR5 activity have set the stage for targeted neurotherapeutic trials in humans with FXS (Dolen and Bear 2008).
There is an urgent need to develop objective and reliable outcome measures that are reflective of FXS deficits and can be modeled similarly in animal and human studies. An excellent candidate is prepulse inhibition (PPI), a measure of sensorimotor gating. PPI is regulated by many neurotransmitter systems, including the glutamatergic receptor pathways such as the mGluR (Brody and Geyer 2004; Pietraszek and others 2005; Zou and others 2007), GABA (Arai and others 2008; Bortolato and others 2007; Fendt 1999; Kodsi and Swerdlow 1995; Sayin and others 2001), and NMDAR receptor systems (Wolf and others 2007). In addition, disruption of the GABA and mGluR systems has been documented in the fmr1 knockout mouse (Centonze and others 2007; Chang and others 2008; D'Antuono and others 2003; D'Hulst and Kooy 2007; Dolen and Bear 2008; Selby and others 2007).
PPI deficits occur in a number of neuropsychiatric conditions including schizophrenia (Braff and Light 2005), obsessive-compulsive disorder (Hoenig and others 2005), attention deficit hyperactivity disorder (Hawk and others 2003), Huntington's disease (Swerdlow and others 1995), Tourette's syndrome (Castellanos and others 1996), and autism spectrum disorders (McAlonan and others 2002; Perry and others 2007). Most of these conditions are characterized by abnormalities in inhibitory control. Furthermore, review of the animal and human literature supports the use of PPI as a cross-species measure of sensorimotor gating and an endophenotypic trait in genetic studies (Braff and others 2001).
There are several published reports of PPI response to pharmacological treatment in humans. In a randomized, double blind placebo controlled study of young boys with ADHD (Hawk and others 2003) methylphenidate improved PPI by approximately 20% over placebo, bringing patients to levels comparable to controls without ADHD. However the PPI improvement was found only when participants were asked to attend to the prepulses. Several studies have shown that PPI is significantly improved in patients with schizophrenia who are treated with antipsychotic medications (Kumari and Gray 1999; Minassian and others 2007; Oranje and others 2002; Weike and others 2000). These studies appear to support the use of PPI protocols to examine the efficacy of psychopharmacological treatment.
In a study of 10 boys with FXS and 7 age-matched controls, Frankland and colleagues (Frankland and others 2004) demonstrated significant PPI deficits in FXS that were strongly associated with a number of clinical measures including IQ, attention, and autistic symptoms. Surprisingly, within the same paper, it was shown that PPI as measured by whole body startle response was enhanced in two samples of fmr1 knockout mice. Chen and Toth (Chen and Toth 2001) also reported enhanced PPI in frm1 knockout mice. However, in the Frankland paper, the authors noted that the mice also had enhanced performance on learning tasks and reduced anxiety, which is inconsistent with previous fmr1 knockout studies and the FXS phenotype. More recently, however, two independent studies have documented impaired PPI in fmr1 knockout mice (using an eyeblink startle procedure; de Vrij and others 2008) or fmr1 knockout mice in combination with an Fxr2 deficiency (Spencer and others 2006), and in one of these studies the PPI deficit was rescued to wild-type levels by MPEP, the mGluR5 antagonist (de Vrij and others 2008). The lack of consistent PPI results in fmr1 knockout mice may be related to methodological differences as suggested by de Vrij and colleagues, or perhaps differences in background strains.
In our view, there is strong theoretical support for using PPI as a physiologic outcome measure to test the efficacy of targeted pharmacological treatments in patients with FXS. This is based on (a) the well-established science of PPI in humans and animals, (b) the involvement of mGluR5 neurobiology in both PPI regulation and fragile X mental retardation, (c) the prominent sensory processing and inhibitory deficits associated with FXS, and finally (d) the PPI deficit in patients with FXS reported by Frankland and colleagues. However, to date these published PPI findings in patients with FXS are based on a very limited sample size and only included males. Most importantly, although PPI reliability (e.g. test-retest, internal consistency) is well established in normal control groups (Abel and others 1998; Cadenhead and others 1999; Flaten 2002; Ludewig and others 2002) and some clinical populations (Ludewig and others 2002; Swerdlow and others 2007), there is no information on the reliability of PPI within the FXS population. The sensitivity of PPI as an outcome measure to gauge efficacy of treatment will depend on its reliability.
In the current study, we sought to expand upon the findings of impaired PPI in FXS by studying (a) a larger group of participants including females and adults, (b) the reliability of the PPI measure, and (c) the feasibility of multi-site data collection. Although not a primary focus, we also examined the association between PPI and cognitive ability in the group with FXS.
MATERIALS AND METHODS
Participants
Participants were 61 individuals with the fragile X full mutation (40 males and 21 females; ages 8 - 40, 19.18 ± 7.18 years) and 63 typical controls (35 males and 28 females; ages 8 - 40, 20.83 ± 6.96 years). All participants or their parents provided written consent according to a protocol approved by Institutional Review Boards at U.C. Davis and Rush University Medical Center. Individuals with FXS were recruited through the fragile X research and clinical programs at U.C. Davis and Rush University. Controls were recruited from throughout the U.C. Davis and Rush campus communities (adults) and local schools in the Sacramento and Chicago areas. Controls with prior psychiatric diagnosis or treatment, learning disability, or CNS involvement were excluded. Any individual with known hearing loss was also excluded. Fragile X status was confirmed by FMR1 DNA testing using both PCR and Southern blot analysis as previously described (Saluto and others 2005; Tassone and others 2004). In the FXS group, the ethnic distribution was 80.0% Caucasian, 3.3% African American, 3.3% Native American, 11.7% Hispanic, and 1.7% unknown. In the control group, the distribution was 78.7% Caucasian, 4.9% African American, 6.6% Asian, and 6.6% Hispanic. Forty-four participants with FXS and 39 typical controls were seen at U.C. Davis M.I.N.D. Institute and 17 with FXS and 24 typical controls were seen at Rush Medical Center. In the FXS group, 66.7% of participants were taking psychoactive medication at the time of the PPI testing session (40.3% SSRI/SNRI; 21% antipsychotic; 19.4% stimulant; 9.7% other antidepressant; 4.8% anticonvulsant; 3.2% sedative; 14.5% miscellaneous).
PPI Testing
Stimuli
The PPI protocol was administered using a stimulus presentation and physiology recording system (James Long Company; Caroga Lake, NY). Acoustic stimuli were presented binaurally through Telephonics high-impedance headphones. The startle stimuli (SS) were 50 ms 105 db SPL white noise pulses (limited to below 4 kHz), with 0 ms rise and fall times. Acoustic prepulses (PP) were 25 ms 75 db SPL 1 kHz tones with 4 ms rise and fall times. There were four types of trials: (1) SS presented alone, (2) PP 60 ms prior to the SS, (3) PP 120 ms prior to the SS, and (4) PP 240 ms prior to the SS. The 4 trial types constituted a trial block and eight such blocks were presented for a total of 32 trials. The trial order within block was random. Inter-trial intervals ranged from 25 s to 45 s. Prior to the first block, there was a series of adaptation trials consisting of 6 startle stimuli only (50 ms white noise), at increasing intensities from 80 dB to 105 dB SPL in 5 dB increments.
Recording
Obicularis oculi electromyogram (EMG) was recorded bipolarly from the right eye, with Electro-Cap International, Inc. (Eaton, OH) E21-6S 6 mm tin cup electrodes 1.0 cm apart, edge to edge, as close to the margin of the lower lid as possible, and the lateral electrode 0.6 cm medial to the exterior canthus. A ground electrode was placed behind the right ear, on the mastoid. EMGs were amplified at a fixed gain (1000, with an A/D input range of +/− 2.5 V) and with band pass of 10 Hz to 250 Hz. Electrode impedances were generally maintained below 5 kΩ and were measured prior to and following the procedure. All data were digitized at 1 kHz.
Procedure
Participants with cognitive, attention, and behavioral control deficits typically have difficulty complying with the usual conditions imposed upon individuals with typical development during reflex modulation experiments (i.e., to sit motionless, without head or eye movements, while fixating one point in their surroundings). We have developed a PPI protocol designed for these individuals that utilizes two methods for minimizing movement-related artifact - presenting a silent movie to participants to encourage consistent forward visual fixation and reduce motor activity (Frankland and others 2004; Ornitz and others 1986; Ornitz and others 1991; Ornitz and others 1999) and offline review of the synchronized video-recording to mark events that typically produce artifacts.
Prior to the experimental session each subject was familiarized with the laboratory and electrodes were applied. In addition, many participants with FXS were given electrode collars and reviewed a DVD with their parent of a model participant completing the protocol prior to the session. Participants with FXS and significant anxiety or limited language were shown a picture schedule of activities to improve understanding and compliance. All participants were asked to sit quietly and enjoy watching a cartoon movie (clips from “The Lion King” and “Finding Nemo”, chosen to be visually engaging but not over-stimulating). Participants were told that from time to time they will hear some sounds. There was no instruction either to pay attention to or ignore the sounds. A DVD recording of the participant's head and upper body during acquisition was used off-line for scoring of movement artifact. The offline coding of motor activity, large eye and head movements, and various examples of noncompliance was done from the DVD recording using the Video Coding System (James Long Company).
Test-retest reliability
Participants residing within reasonable driving distance of U.C. Davis or Rush University were asked to return for PPI retesting. Efforts were made to conduct the re-test 14 days later; however family schedules often prevented rigid adherence to the test-retest interval. The initial test and the re-test were identical in all aspects of the PPI procedure.
Data scrutiny and preparation for analysis
We followed published guidelines for human startle eye blink studies, including subject preparation, electrode placement, amplification and filtering, response quantification, and artifact analysis and removal (Blumenthal and others 2003). All analytic procedures described below were conducted blinded to participant identifying information. The orbicularis oculi EMG were scrutinized individually on each trial. Participants with no visual EMG response to more than ∼ 50% of startle only trials were deemed “non-responders” and were excluded from analysis. Raw EMG was digitally bandpass filtered at 80 Hz to 240 Hz. The data were analyzed in 75 percent overlapping 8 ms windows, yielding a time resolution of 2 ms. Baseline EMG activity was sampled 50 ms before stimulus onset to 20 ms after stimulus onset and aggregated across all trials. This aggregated baseline was used to detect confounding natural blinks exceeding baseline. Trials with baseline periods in which the threshold was exceeded (greater than 2 SD above aggregate baseline mean EMG) were rejected from analysis. The EMG peak magnitude between 20 ms and 200 ms post startle probe onset was analyzed for each trial. Trials also were rejected if video recording revealed excessive movement at time of trial, if the participant became excessively sleepy, if electrodes were manipulated or removed, or if headphones were removed. Percent PPI was calculated as follows: 100 X [(response magnitude in the startle stimulus alone trials - response magnitude in the prepulse trials)/response magnitude in the startle stimulus alone trials]. This calculation was done for the 60 ms, 120 ms, and 240 ms prepulse trials types.
Success of data acquisition
Of the 61 participants with FXS enrolled in the study, 53 (86.9%) completed the session with usable physiological data (4.9% were noncompliant, 4.9% had problematic EMG signal, and for 3.3% there were equipment problems). Of the 63 controls enrolled, 47 (74.6%) completed the session with usable data (3.2% noncompliant, 1.6% with problematic EMG signal, 4.8% with equipment problems, 9.5% were identified as EMG non-responders, and 6.3% were excluded due to history of psychiatric disorder or treatment). Startle magnitude data are reported on these individuals with complete, valid physiological data. Of the remaining participants, 49 with FXS and 42 controls had adequate EMG startle responses to each trial type (5 of 8 valid trials were required) to produce PPI results. This consisted of 18 females with FXS (mean age 20.56), 31 males with FXS (mean age 19.25 years), 18 female controls (mean age 18.21 years), and 24 male controls (mean age 19.71 years). PPI data are reported on these remaining participants. Percentages of rejected trials were as follows: EMG physiology artifact, 3.0% in the FXS group vs. 3.6% in controls; behavioral noncompliance/movement, 4.9% in FXS vs. 2.7% in controls; both artifact and noncompliance, 1.0% in FXS vs. 0.6% in controls.
Cross-site protocol replication
The PPI protocol described above was initially established at the M.I.N.D. Institute at U.C. Davis (PI D. Hessl), and was replicated at Rush Medical Center in Chicago (PI E. Berry-Kravis). The psychophysiology equipment (amplifiers, electrodes, headphones), startle stimuli (sound generating equipment, sound types and levels), video recording, electrode application procedures, and PPI procedures (trial types and timing, silent videos) were identical.
Cognitive Assessment
Cognitive ability was measured using the Wechsler Scales of Intelligence (Wechsler Adult Intelligence Scale, Third Edition; Wechsler Intelligence Scale for Children, Fourth Edition; Wechsler Abbreviated Scale of Intelligence; The Psychological Corporation, San Antonio, TX) or the Stanford Binet, Fifth Edition (Riverside Publishing, Rolling Meadows, IL). In the FXS group, 47 of the 61 participants had valid IQ results available (41 with the Wechsler Scales, 6 with the Stanford Binet V). Males with FXS had a mean IQ of 52.0 (SD = 9.9; range = 36 - 75) and females with FXS had a mean IQ of 75.3 (SD = 19.4; range = 44 - 116).
RESULTS
Group Differences in PPI
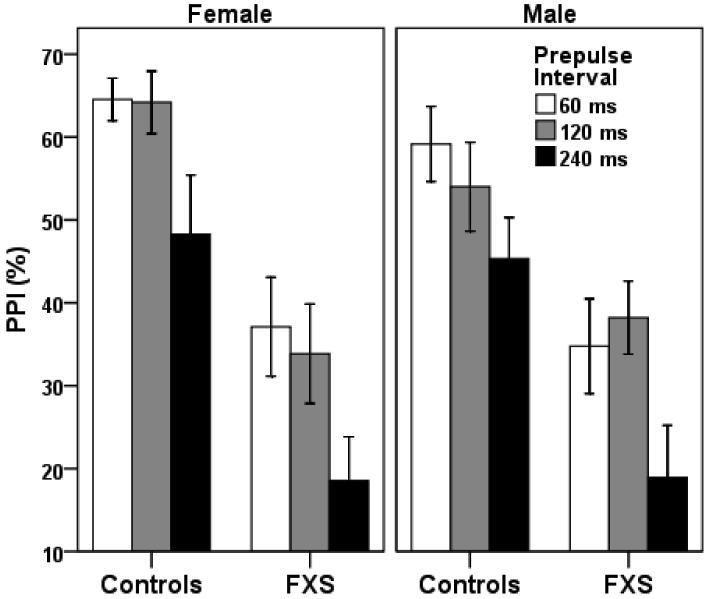
A group (FXS vs. controls) by gender by site (UCD vs. Rush) repeated measures ANOVA with prepulse interval type (60, 120, or 240 ms) as the repeating measure, revealed significant main effects for interval type, F (2, 78) = 5.16, p = 0.008, ηp2 = 0.13, group, F (1, 78) = 26.50, p = 0.000002, ηp2 = 0.27, site, F (1, 78) = 5.34, p = 0.02, ηp2 = 0.07, and a group by gender interaction that approached significance, F (1, 78) = 4.07, p = 0.05, ηp2 = 0.05. Compared to controls, participants with FXS had PPI reductions of 26%, 22%, and 28% for the 60 ms, 120 ms, and 240 ms prepulse interval trial types, respectively (Figure 1). The main effect of site showed that individuals tested at Rush had lower PPI than those tested at U.C. Davis, however this was driven by a significant effect for the fragile X group only; there was no significant main effect of site within the control group. The group by gender interaction suggested that the difference in PPI between FXS and controls was greater in females than in males. Within the FXS group, an analogous repeated measures ANOVA with prepulse interval type as the repeating measure and medication (yes vs. no), site, and gender as the independent variables yielded no significant main effect for medication, F (1, 63) = 0.11, p = 0.92. As seen in Table 1, the number of individuals with FXS taking specific types of medication does not appear to be associated with level of PPI impairment.
Figure 1.
Prepulse inhibition (PPI) in males and females with FXS and controls, by prepulse interval trial type. Repeated measures ANOVA resulted in a significant main effect for group, F (1, 78) = 25.32, p = 0.000004, ηp2 = 0.26, and a group by gender interaction that approached significance, F (1, 78) = 4.07, p = 0.05, ηp2 = 0.05. The interaction indicated that the difference in PPI between FXS and controls was greater in females than in males. Error bars show ± S.E.M.
Table 1.
Summary of the number of participants with FXS taking different types of medication at the time of testing, by PPI% quartiles
| Quartile (n) | PPI Range | No Medication | SSRI/SNRI | Antipsychotics | Stimulants | Anticonvulsants | Sedatives | Other Antidepressants |
|---|---|---|---|---|---|---|---|---|
| 1 (11) | −20.83-18.53 | 2 | 7 | 0 | 3 | 0 | 0 | 0 |
| 2 (10) | 18.54-37.52 | 4 | 4 | 1 | 2 | 0 | 0 | 1 |
| 3 (11) | 37.53-55.11 | 4 | 3 | 3 | 3 | 0 | 0 | 1 |
| 4 (10) | 55.12-74.23 | 3 | 4 | 2 | 3 | 1 | 1 | 2 |
Reliability of Startle and PPI
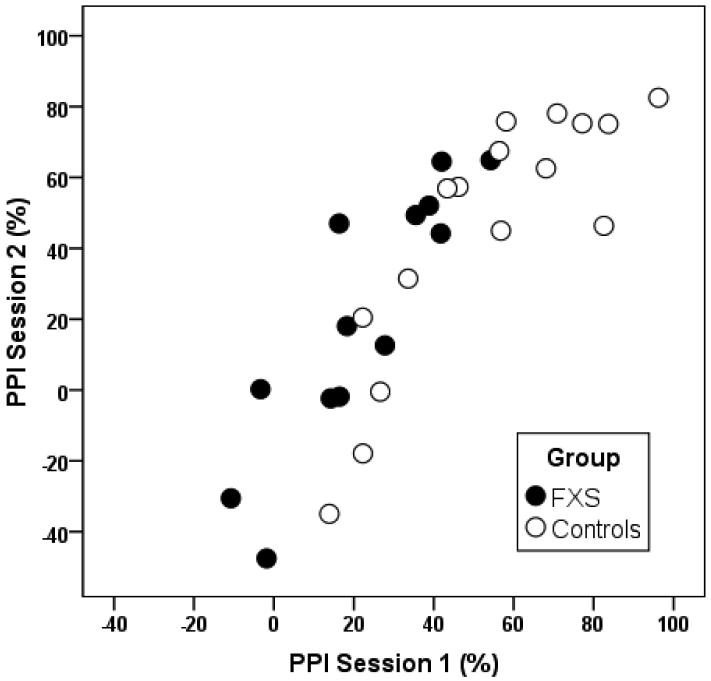
The internal consistency of startle magnitude was examined for each group using Cronbach's alpha coefficient. Alphas for startle magnitude were excellent for each group indicating very consistent startle responses across trials (Table 3). Thirteen participants with FXS (ages seven to 40 years; five female, eight male; mean IQ = 56.6) and 16 controls (ages eight to 40 years; seven female, nine male) completed PPI retesting. The test-retest interval ranged from 2 to 67 days with a mean interval of 13.1 days. Intraclass correlation coefficients demonstrated excellent test-retest reliability for the 120 ms prepulse interval for both groups (Figure 2). Reliability was adequate for the 240 ms prepulse interval in both groups and relatively weak for the 60 ms interval (Table 3). In addition, paired samples t-tests demonstrated no significant differences in PPI between session 1 and session 2 for the FXS (120 ms mean difference = −5.98, t = −0.65, p = 0.53) and control (120 ms mean difference = 8.57, t = 1.70, p = 0.11) groups.
Table 3.
Test-retest reliability (intraclass correlation) of PPI in individuals with FXS and controls, by prepulse interval
| FXS (n = 13) | Controls (n = 16) | Total (n = 29) | |
|---|---|---|---|
| Prepulse interval | |||
| 60 ms | 0.57 | 0.35 | 0.65 |
| 120 ms | 0.85 | 0.88 | 0.89 |
| 240 ms | 0.68 | 0.69 | 0.74 |
Figure 2.
Scatterplot showing the association between PPI (120 ms prepulse interval) measured at two sessions, on average 13 days apart, in participants with FXS and controls.
One adult male control was tested at the U.C. Davis site and then again at the Rush site 9 months later. The Spearman-Brown coefficient testing the correlation between trial-by-trial EMG startle amplitudes was 0.61, p < .0001. PPI for this participant was consistent between sites even with the long test-retest interval (60 ms, 66.4% vs. 68.2%; 120 ms, 83.7% vs. 75.1%; 240 ms, 58.1% vs. 58.8% for U.C. Davis and Rush, respectively).
Group Differences in EMG Startle Magnitude
Due to positively skewed values and outliers that could not be corrected with arithmetic transformation, group differences in EMG magnitude in response to the startle only trials were tested using nonparametric Mann-Whitney test. This test showed no FXS vs. control group differences for males (Z = 0.11, p = 0.91) or females (Z = 0.04, p = 0.97).
Clinical Correlates
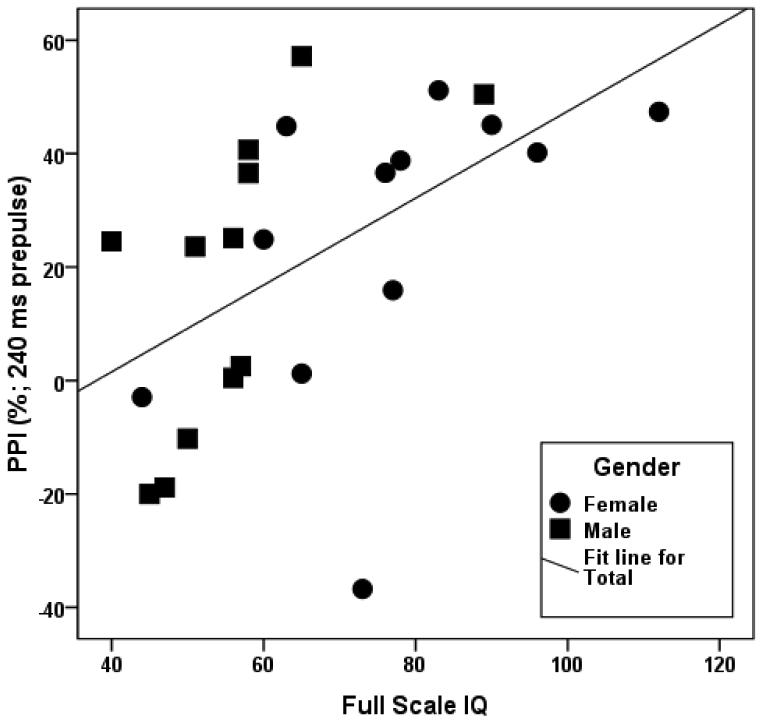
Spearman rank correlations were conducted to examine the associations between PPI, startle magnitude and full scale IQ among the participants with FXS. The PPI measurements were not significantly correlated with IQ in the overall sample, although the correlation between PPI at 60 ms and IQ approached significance, ρ = 0.29, p = 0.08 (n = 39). Frankland and colleagues reported strong correlations between the PPI deficit and clinical measures in the FXS group. However, there are differences in age and gender distributions between our sample (children and adults, males and females) and the prior study (all children, males only). To further investigate this, we conducted post-hoc correlations between PPI and IQ for children and adults separately. Although PPI at the 240 ms prepulse interval was not as reliable, we found significant positive correlations between this measure and IQ in children, especially males (Figure 3; overall, ρ = 0.66, p < 0.001, n = 24; males, ρ = 0.79, p = 0.002, n = 12; females, ρ = 0.68, p = 0.015, n = 12). The correlation of IQ with PPI at the most reliable interval, 120 ms, approached significance in this group of children, ρ = 0.42, p = 0.07, n = 20.
Figure 3.
Scatterplot showing the association between PPI (240 ms prepulse interval) and full scale IQ in boys (ρ = 0.79, p = 0.002, n = 12) and girls (ρ = 0.68, p = 0.015, n = 12) with FXS.
DISCUSSION
We have shown that prepulse inhibition (PPI) deficits can be reliably measured in children and adults with FXS and mental impairment. To our knowledge, this is the first study demonstrating the test-retest reliability of PPI measurement in a developmentally disabled population, and replicates an earlier study showing PPI deficits in boys with FXS (Frankland and others 2004). The lack of FXS group differences in baseline EMG startle magnitude suggests that the PPI deficits are not simply due to abnormality in the startle response pathway but are more specifically related to inhibitory control mechanisms.
We provide the first evidence that females with FXS have substantial sensorimotor gating deficits, despite their typically less severe phenotypic and cognitive involvement. The PPI measurement may be especially sensitive to inhibition problems that are seen even in high functioning females. Indeed, of the 9 females with FXS with IQ scores in the average range, 6 had PPI measures one standard deviation or more below the control group. These findings are surprising, given that females with FXS typically express higher levels of FMRP as a result of X chromosome inactivation; measures that are deficient in FXS are therefore typically more abnormal in males. We are conducting studies to correlate FMRP level with various clinical and physiological measures, including PPI, to better address this question.
While the participants with FXS had substantial PPI deficits, on average ranging from 22% to 28% below controls, sensorimotor gating problems are not specific to this condition. Indeed, FXS is one of many neurodevelopmental and neurodegenerative disorders having major disruptions in inhibitory control and sensorimotor gating, including schizophrenia, ADHD, autism spectrum disorder, obsessive compulsive disorder, and Huntington's disease. The deficit in FXS measured here is not as large as was estimated by the prior smaller study (47% difference; (Frankland and others 2004), but it does exceed that of schizophrenia (based on weighted means of 12 studies − 20% difference; (Hamm and others 2001). Although there is little overlap in the clinical aspects of FXS and schizophrenia, both conditions appear to have a common deficit in glutamatergic signaling, sensorimotor gating, and brain systems involved with executive function and inhibition.
Functional MRI studies of both males and females with FXS demonstrate frontostriatal dysfunction during a Go/NoGo inhibitory control task that is correlated with the degree of FMRP deficit (Hoeft and others 2007; Menon and others 2004). PPI is clearly modulated by the GABAergic and glutamatergic frontal-striatal circuits that are implicated in FXS. Therefore, PPI may offer an alternative, less expensive method for indexing CNS dysfunction from a broader range of individuals, in age and level of functioning, than is possible with functional MRI.
The PPI test-retest reliabilities reported here, at least for the 120 ms interval, were excellent and similar to other studies reporting these data from other populations. For example, Cadenhead and colleagues (Cadenhead and others 1999) studied PPI across three sessions, each one month apart, in healthy adult men, and found excellent test-retest and within-session stability, with intraclass correlations in the 0.90 range. Ludewig and colleagues (Ludewig and others 2002) conducted a study of PPI reliability, also at three time points across 3 months, in normal controls as well as patients with schizophrenia and found PPI to be highly and equally reliable in both groups, especially for the 60 ms and 120 ms prepulse trial duration, with correlations in the 0.80 range. Given the challenges associated with physiological testing of patients with FXS who demonstrate significant hyperactivity and anxiety problems, our data suggest that slight modifications to typical PPI procedures and analysis techniques, such as allowing participants to view an engaging silent movie, using an adaptation procedure (a series of startle stimuli of increasing intensities) before the PPI trials, and carefully excluding trials with movement or other artifact, can lead to reliable measures even in these challenging participants. Furthermore, the higher reliability obtained for the 120 ms prepulse interval suggests that use of this trial type may be more sensitive to changes associated with treatment, and should be more strongly considered in future intervention studies.
Our results, coupled with prior knowledge regarding inhibitory problems in patients with FXS (Cornish and others 2001; Scerif and others 2007; Sullivan and others 2007), rescue of PPI deficits in the fragile X knockout mouse (de Vrij and others 2008), and involvement of the mGluR5 pathway in FXS (Dolen and Bear 2008; McBride and others 2005) support the use of PPI measurement in novel treatment outcome studies for this disorder. Although mGluR antagonists appear to regulate PPI (Campbell and others 2004; Henry and others 2002; Kinney and others 2003; Pietraszek and others 2005) it is important to note that mutant mice lacking mGluR5 receptors demonstrate impaired PPI (Brody and others 2004), whereas the mGluR5 theory postulates that this system is up-regulated in FXS.
Frankland and colleagues (Frankland and others 2004) found strong correlations between PPI and clinical measures in young males with FXS. Within our subgroup children with FXS, we found a similar positive association between PPI and IQ, but no significant association in the larger group of patients including adults. These inconsistent results raise a concern that PPI deficiencies in FXS may not reflect clinical deficits. However, a weakness of the current study was that IQ scores were derived from several different instruments, and were not available for all participants, perhaps leading to more error and less accurate estimates of these correlations. Studies are needed that compare PPI to consistent and more sensitive cognitive testing, and more specific neuropsychological constructs associated with inhibitory control. In addition, it would be informative to compare PPI in FXS and a group of individuals with another developmental disability in order to separate the impact of the FMR1 mutation from general developmental effects. Finally, the sensory sensitivity, anxiety, and hyperarousal problems experienced by individuals with FXS are well-documented and may be associated with and influenced the PPI deficits observed.
There were several important limitations of the present study. First, we found a significant effect of laboratory site on PPI, especially within the FXS sample, with somewhat lower values from participants seen at Rush. However, we subsequently compared baseline startle magnitude data between the two sites, within each group, and found no significant differences (data not shown), and we found that one participant had very similar startle and PPI results when tested at each site. Together, these data suggest that the site effect may be more related to FXS cohort PPI effects than methodological or equipment differences, although it is also possible that differences in the testing environment may have influenced the findings. While previous studies suggest that males have increased PPI relative to females (Swerdlow and others 1999) we found no significant gender effects within the FXS or control groups. Third, unfortunately we were unable to examine the effect of specific medication types on PPI in FXS patients. Given the finding of improved PPI in children with ADHD treated with methylphenidate (Hawk and others 2003), as well as the reported success of stimulant medications in patients with FXS (Berry-Kravis and Potanos 2004), a controlled trial of the effect of stimulant treatment on PPI and ADHD symptoms in FXS could be fruitful. Fourth, we did not measure hearing threshold in the participants, although we did screen individuals for hearing loss by history. Recurrent otitis media infections are common in early childhood but they typically resolve by age 5 in children with FXS so it is unlikely that this interfered with our study. Frankland and colleagues did obtain normal audiometric data on their subjects. Future PPI studies in FXS should also check for hearing loss directly. Finally, in addition to varying the prepulse interval, it may be useful to evaluate the level of inhibition with different prepulse sound levels, and to demonstrate that participants do not respond to prepulse sounds alone.
In summary, we show that males and females with FXS have abnormal sensorimotor gating that can be reliably measured using classic prepulse inhibition methodology. We believe that PPI is an excellent candidate for an objective treatment outcome measure to be used to test the efficacy of future novel therapeutic agents such as mGluR5 antagonists aimed at normalizing brain function and behavior in patients with FXS, and perhaps other neurodevelopmental disorders characterized by executive and inhibitory dysfunction.
Table 2.
Internal consistency (Cronbach's alpha) of EMG startle magnitude in individuals with FXS and controls
| FXS | Controls | |
|---|---|---|
| Trial type | ||
| Startle alone | 0.96 | 0.96 |
| 60 ms prepulse | 0.94 | 0.86 |
| 120 ms prepulse | 0.96 | 0.96 |
| 240 ms prepulse | 0.95 | 0.95 |
ACKNOWLEDGEMENTS
We are grateful to the research participants and their families; Louise Gane for her assistance with recruitment; Elizabeth Ballinger for behavioral coding and EMG analysis; Kylee Cook for her help with data entry and management; and Alyssa Chavez for editing. Funding from the National Institutes of Health Grants MH77554 (D.H.), HD36071, AG032115, RR024922, and HD02274 (R.J.H.); a M.I.N.D. Institute Pilot Grant (D.H.); an Illinois-Eastern Iowa Kiwanis Spastic Paralysis and Related Disorders Foundation grant (E.B.K.); the Virginia Friedhofer Charitable Trust (E.O.); and a Rush University summer student fellowship (E.C.) supported this work. Drs. Hessl, Hagerman and Berry-Kravis currently receive support from Neuropharm.
REFERENCES
- Abel K, Waikar M, Pedro B, Hemsley D, Geyer M. Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. J Psychopharmacol. 1998;12(4):330–7. doi: 10.1177/026988119801200402. [DOI] [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K, Kamei H, Nabeshima T, Yamada K. Involvement of Pallidotegmental Neurons in Methamphetamine- and MK-801-Induced Impairment of Prepulse Inhibition of the Acoustic Startle Reflex in Mice: Reversal by GABA(B) Receptor Agonist Baclofen. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.41. [DOI] [PubMed] [Google Scholar]
- Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav. 2005;4(6):393–8. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–7. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome--present and future. Ment Retard Dev Disabil Res Rev. 2004;10(1):42–8. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- Blumenthal T, Cuthburt B, Filion D, Hackley S, Lipp O, Van Boxel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2003;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orru M, Piras AP, Fa M, Tuveri A, Puligheddu M, Gessa GL, Castelli MP, Mereu G. Activation of GABA(B) receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology (Berl) 2007;194(3):361–9. doi: 10.1007/s00213-007-0845-5. and others. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2-3):234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. The use of neurophysiological endophenotypes to understand the genetic basis of schizophrenia. Dialogues Clin Neurosci. 2005;7(2):125–35. doi: 10.31887/DCNS.2005.7.2/dlbraff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9(1):35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Brody SA, Geyer MA. Interactions of the mGluR5 gene with breeding and maternal factors on startle and prepulse inhibition in mice. Neurotox Res. 2004;6(1):79–90. doi: 10.1007/BF03033300. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Carasso BS, Swerdlow NR, Geyer MA, Braff DL. Prepulse inhibition and habituation of the startle response are stable neurobiological measures in a normal male population. Biol Psychiatry. 1999;45(3):360–4. doi: 10.1016/s0006-3223(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 2004;175(3):310–8. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39(1):33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, Chiara VD, Musella A, Prosperetti C, Calabresi P, Bernardi G. Abnormal Striatal GABA Transmission in the Mouse Model for the Fragile X Syndrome. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.09.008. and others. [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4(4):256–63. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–50. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94(10):5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K, Munir F, Wilding J. [A neuropsychological and behavioural profile of attention deficits in fragile X syndrome] Rev Neurol. 2001;33(Suppl 1):S24–9. [PubMed] [Google Scholar]
- D'Antuono M, Merlo D, Avoli M. Involvement of cholinergic and gabaergic systems in the fragile X knockout mice. Neuroscience. 2003;119(1):9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- D'Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30(8):425–31. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31(1):127–32. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4(4):335–40. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008 doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M. Enhancement of prepulse inhibition after blockade of GABA activity within the superior colliculus. Brain Res. 1999;833(1):81–5. doi: 10.1016/s0006-8993(99)01525-5. [DOI] [PubMed] [Google Scholar]
- Flaten MA. Test-retest reliability of the somatosensory blink reflex and its inhibition. Int J Psychophysiol. 2002;45(3):261–5. doi: 10.1016/s0167-8760(02)00034-x. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9(4):417–25. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135(2):155–60. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI, Schupp HT. The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacology (Berl) 2001;156(2-3):259–65. doi: 10.1007/s002130100827. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr., Yartz AR, Pelham WE, Jr., Lock TM. The effects of methylphenidate on prepulse inhibition during attended and ignored prestimuli among boys with attention-deficit hyperactivity disorder. Psychopharmacology (Berl) 2003;165(2):118–27. doi: 10.1007/s00213-002-1235-7. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43(8):1199–209. doi: 10.1016/s0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. Frontostriatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. 2007;28(6):543–54. doi: 10.1002/hbm.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry. 2005;57(10):1153–8. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10(10):1038–44. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet. 2002;111(2):140–6. doi: 10.1002/ajmg.10500. and others. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98(2):161–7. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. and others. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306(1):116–23. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Res. 1995;684(1):26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psychopharmacology (Berl) 1999;141(1):11–5. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res. 2002;55(1-2):129–37. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125(Pt 7):1594–606. doi: 10.1093/brain/awf150. and others. [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45(5):753–64. doi: 10.1016/j.neuron.2005.01.038. and others. [DOI] [PubMed] [Google Scholar]
- Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proc Natl Acad Sci U S A. 2004;101(10):3615–20. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Feifel D, Perry W. The relationship between sensorimotor gating and clinical improvement in acutely ill schizophrenia patients. Schizophr Res. 2007;89(1-3):225–31. doi: 10.1016/j.schres.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, Van Oel CJ, Gispen-De Wied CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J Clin Psychopharmacol. 2002;22(4):359–65. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Kaplan AR, Lane SJ, Norman RJ. Maturation of startle modulation. Psychophysiology. 1986;23:624–34. doi: 10.1111/j.1469-8986.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Sadeghpour M, Sugiyama T. Maturation of prestimulationinduced startle modulation in girls. Psychophysiology. 1991;28(1):11–20. doi: 10.1111/j.1469-8986.1991.tb03381.x. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Russell AT, Hanna GL, Gabikian P, Gehricke JG, Song D, Guthrie D. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biol Psychiatry. 1999;45(11):1455–66. doi: 10.1016/s0006-3223(98)00205-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61(4):482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pietraszek M, Gravius A, Schafer D, Weil T, Trifanova D, Danysz W. mGluR5, but not mGluR1, antagonist modifies MK-801-induced locomotor activity and deficit of prepulse inhibition. Neuropharmacology. 2005;49(1):73–85. doi: 10.1016/j.neuropharm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15(4):927–68. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Saluto A, Brussino A, Tassone F, Arduino C, Cagnoli C, Pappi P, Hagerman P, Migone N, Brusco A. An enhanced polymerase chain reaction assay to detect preand full mutation alleles of the fragile X mental retardation 1 gene. J Mol Diagn. 2005;7(5):605–12. doi: 10.1016/S1525-1578(10)60594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin U, Rutecki PA, Mellanby J, Sutula TP. Gamma-vinyl GABA reduces paired pulse inhibition in the rat dentate gyrus in vivo and in vitro. Epilepsy Res. 2001;44(2-3):109–17. doi: 10.1016/s0920-1211(01)00200-5. [DOI] [PubMed] [Google Scholar]
- Scerif G, Cornish K, Wilding J, Driver J, Karmiloff-Smith A. Delineation of early attentional control difficulties in fragile X syndrome: focus on neurocomputational changes. Neuropsychologia. 2007;45(8):1889–98. doi: 10.1016/j.neuropsychologia.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412(3):227–32. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Serysheva E, Yuva-Paylor LA, Oostra BA, Nelson DL, Paylor R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between Fragile X-related proteins. Hum Mol Genet. 2006;15(12):1984–94. doi: 10.1093/hmg/ddl121. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton DD, Hammer J, Sideris J, Hooper S, Ornstein PA, Bailey DB., Jr Sustained attention and response inhibition in boys with fragile X syndrome: measures of continuous performance. Am J Med Genet B Neuropsychiatr Genet. 2007;144(4):517–32. doi: 10.1002/ajmg.b.30504. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Hartman PL, Sprock J, Auerbach PP, Cadenhead K, Perry W, Braff DL. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacology (Berl) 1999;146(2):228–32. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry. 1995;58(2):192–200. doi: 10.1136/jnnp.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Light GA, Cadenhead K, Calkins ME, Dobie DJ, Freedman R, Green MF, Greenwood TA, Gur RE. Multi-site studies of acoustic startle and prepulse inhibition in humans: initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia. Schizophr Res. 2007;92(1-3):237–51. doi: 10.1016/j.schres.2007.01.012. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, Hoogeveen AT. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet. 1997;6(8):1315–22. doi: 10.1093/hmg/6.8.1315. [DOI] [PubMed] [Google Scholar]
- Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biology. 2004;1(2):103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Wolf R, Paelchen K, Matzke K, Dobrowolny H, Bogerts B, Schwegler H. Acute or subchronic clozapine treatment does not ameliorate prepulse inhibition (PPI) deficits in CPB-K mice with low levels of hippocampal NMDA receptor density. Psychopharmacology (Berl) 2007;194(1):93–102. doi: 10.1007/s00213-007-0824-x. [DOI] [PubMed] [Google Scholar]
- Zou D, Huang J, Wu X, Li L. Metabotropic glutamate subtype 5 receptors modulate fear-conditioning induced enhancement of prepulse inhibition in rats. Neuropharmacology. 2007;52(2):476–86. doi: 10.1016/j.neuropharm.2006.08.016. [DOI] [PubMed] [Google Scholar]