Abstract
Analytical ultracentrifugation is a widely used method for characterizing the solution behavior of macromolecules. However, the two commonly used detectors (absorbance and interference) impose some fundamental restrictions on the concentrations and complexity of the solutions that can be analyzed. The recent addition of a fluorescence detector for the XL-I analytical ultracentrifuge (AU-FDS) enables two different types of sedimentation experiments. First, the AU-FDS can detect picomolar concentrations of labeled solutes allowing the characterization of very dilute solutions of macromolecules, applications we call Normal Use Tracer Sedimentation (NUTS). The great sensitivity of NUTS analysis allows the characterization of small quantities of materials and high affinity interactions. Second, AU-FDS allows characterization of trace quantities of labeled molecules in solutions containing high concentrations and complex mixtures of unlabeled molecules, applications we call Biological On Line Tracer Sedimentation (BOLTS). The discrimination of BOLTS enables the size distribution of a labeled macromolecule to be determined in biological milieu such as cell lysates and serum. Examples are presented that embody features of both NUTS and BOLTS applications, along with our observations on these applications.
Introduction
The analytical ultracentrifuge (AUC) is a useful instrument for characterizing the solution behavior of macromolecules. Sedimentation equilibrium analysis provides first principle thermodynamic information, while sedimentation velocity analysis provides first principle hydrodynamic information [1, 2]. For both equilibrium and velocity sedimentation, the principle measurement is the radial concentration distribution as a function of time. Concentration distributions can be determined using either the UV absorbance or the Rayleigh interference detectors in the Beckman Coulter, Inc. (Fullerton, CA) XL-I AUC [3], or using an XL-I retrofitted with the Aviv Biomedical fluorescence detector (AU-FDS, Aviv Biomedical, Inc., Lakewood, NJ) [4, 5].
The AU-FDS detector uses a confocal optical configuration comprising a 10 mW, 488 nm solid-state laser for excitation and a pair of long-pass filters (> 505 nm) for emission detection [4], making it suitable for use with fluorescein-like dyes (e.g. Alexa488, Oregon green, FITC; Invitrogen, Carlsbad, CA) or 488 nm-excited variants of green fluorescent protein (GFP). The AU-FDS complements the existing absorbance and interference detectors with its high sensitivity (< 100 pM for a single fluorescein label), broad dynamic range (from pM to μM) and selectivity [4].
While a custom-built fluorescence detector was described for the Model E analytical ultracentrifuge [6], its use was restricted to applications where low concentration detection was necessary [7]. The AU-FDS is the first commercially available fluorescence detection system for the XL-I AUC, and its confocal design extends the useable concentration range by reducing inner filter effects and minimizing artifacts resulting from steep refractive index gradients [4]. Consequently, the AU-FDS dynamic range spans 4–5 decades of concentration, compared to the absorbance and interference detectors which typically cover 2–3 decades of concentration.
The sensitivity and dynamic range of the AU-FDS enables the characterization of high affinity interactions, Kd ~ 10 nM or less, that might not be amenable to analysis with absorbance or interference systems [8]. These dilute-solution applications are direct analogs of ordinary AUC methods. Consequently, we call them Normal Use Tracer Sedimentation (NUTS).
While the sensitivity and dynamic range of the AU-FDS allows the analysis of more dilute samples, the selectivity of fluorescence detection allows AUC analysis of trace quantities of material in more complex and concentrated samples (e.g. plasma, serum, sputum, urine, cerebral spinal fluid, cell lysates, etc.). Elegant sedimentation analyses of trace quantities of proteins in biological fluids have been conducted employing a variety of detection methods and the use of preparative centrifuges [9, 10]. However, since sample fractionation is a separate step that follows sedimentation a limited amount of data can be obtained on any given sample. In addition, centrifugation followed by fractionation methods are not well-suited to sedimentation velocity analysis. The AU-FDS can acquire several hundred radial scans of each sample during an experiment, thus taking advantage of the increased sensitivity and resolution available from sedimentation velocity analysis [11, 12, 13]. While current sedimentation analysis programs cannot provide detailed quantitative information about the samples, qualitative information is available from the tracer sedimentation coefficient distributions. We call these more qualitative applications Biological On-Line Tracer Sedimentation (BOLTS).
During the development of the AU-FDS we tested a variety of systems to assess its capabilities. Presented here are a number of AU-FDS examples that demonstrate both NUTS and BOLTS applications. While the AU-FDS may be used for equilibrium or velocity sedimentation [14], the examples described here focus on the use of velocity sedimentation and analysis. Several observations are made on the advantages, limitations and caveats of using the AU-FDS for NUTS and BOLTS applications.
Materials and Methods
Chemicals
Reagent grade TrisHCl, KCl, NaCl, and EDTA were purchased from Sigma.
Proteins
Soybean Trypsin Inhibitor Type 1-S (T-9003, Lot 074K7009, E280 = 16,960 cm M−1, MW = 20,095 g mole−1) was purchased from Sigma. Concentrations were determined from the A280 nm using the listed molar extinction coefficient.
Expression and Purification of Green Fluorescent Protein (GFP)
The GFP clone was a kind gift from Dr. Eric Schaller (Dartmouth). This clone of GFP contains multiple mutations (S65T, Q80R, F99S, M153T, V163A) and a hexa-histidine tag followed by a thrombin cleavage site for removal of the HIS-tag after purification. A T7 tag follows the thrombin cleavage site, and cannot be removed. The clone is inserted in a pET28a plasmid at the SacI and BamHI restriction sites. A single colony was selected and used to inoculate a 25 ml culture of LB media containing 100 μg ml−1 of Kanamycin (Bristol Laboratories) and grown at 37 °C overnight. A 2 liter flask containing 500 mls of LB media (3002–075, BIO101, Inc), with 100 μg ml−1 of Kanamycin was inoculated with 1 ml of the 25 ml culture and placed in a shaker at 37 °C. The OD600 of the culture was monitored and at 0.5 OD the culture was induced with 1 mM Isopropyl β-D-Thiogalacto Pyranoside (IPTG) (I-6758, Sigma). The bacteria were harvested 5 hours after induction and stored at −20 °C.
The GFP was purified in batch mode using Qiagen nickel resin using the method outlined by the manufacturer. After elution from the resin the protein was exhaustively dialyzed against a pH 7.5 buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA. The concentration of GFP solutions was determined using the molar extinction coefficient E280 = 21,890 cm M−1. The sequence molecular weight of GFP is 30,838 g mole−1. The partial specific volume of this variant of GFP was measured by densitometry to be 0.738 ml g−1 [15]. For the work presented here, the HIS-tag was not removed.
Recombinant soluble endothelial Protein C receptor (rsEPCR)
rsEPCR was a kind gift from Eli Lilly. The molar extinction coefficient of rsEPCR is E280 = 29,910 cm M−1. The molecular weight of rsEPCR is 32,490 g mole−1.
Antibodies
Monoclonal rat anti-GFP IgG D153-3 was purchased from MBL International (Woburn, MA), and used without further purification. Alexa488-goat-anti-rabbit-IgG (A11034), Alexa488 goat anti-mouse IgG (A11001) were purchased from Molecular Probes (now Invitrogen, Carlsbad, CA). Unlabeled murine IgG1 (MMS-156P) was purchased from Covance (Emeryville, CA).
Fluorescently labeled Lipids and Proteins
1-Palmitoyl-2[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl) amino] dodecanolyl]-sn-Glycero-3-phosphocholine (DPPC-NBD) catalog number 810131 and 1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine-N-(Carboxyfluorescein (F-DPPE) catalog number 810332 were purchased from Avanti-polar lipids. Alexa488-labeled BSA (A13100) was purchased from Molecular Probes (now Invitrogen, Carlsbad, CA) and used without further purification.
Isolation of Human Serum and Plasma
Human blood (5 × 8mls) was collected into clot-activator tubes. The tubes were centrifuged in a clinical centrifuge to pellet the red blood cells and approximately 20 mls of serum were recovered.
Methods
Fluorescence Detection System (FDS) Instrumentation
A prototype version of the AU-FDS was used for these studies. The detector optics uses a confocal configuration [4] with ~20–50 μm radial resolution (using a 100 μm pinhole). The excitation light source is a Picarro, Inc. (Sunnyvale, CA) Cyan (10 mW, 488 ± 0.5 nm) laser. For some earlier experiments, a Novalux, Inc. (Sunnyvale, CA) Protera laser (15 mW, 486–490 ± 0.5 nm) was used [14]. Emitted light is filtered through two long-pass (>505 nm) dichroic filters, and focused on a 100 μm pinhole in front of the detector [4]. For a few of the earlier experiments, a > 490 nm long-pass filter and 600 μm pinhole was used [14]. The 100 μm pinhole and the > 505 nm long-pass filter reduced the background signal and greatly improved the quality of data collected, and are identical to the components used in the Aviv AU-FDS. The detector is a Hamamatsu H6779-04 photomultiplier tube with a cathode luminous sensitivity of ~120 μA/lm, an anode luminous sensitivity of ~75 μA/lm and dark noise of ~0.7 nA.
The prototype AU-FDS optics will detect < 100 pM fluorescein (extinction coefficient ~60,000, quantum yield > 0.95, Invitrogen, Carlsbad, CA) provide useable sedimentation velocity data from fluorescein from 10−10 to 10−5 M, and provide a linear intensity reading with concentration over each decade in this range [4].
Data collection and XL-I operation used version 1 AOS operating software (available at www.rasmb.bbri.org). Data were collected at a radial increment 20 μm, averaging between 50 and 150 independent intensity readings acquired from 5 rotor revolutions at each radial position [5]. Using these settings, a radial scan (5.8 – 7.3 cm) of all of the samples requires ~90 seconds. Typically, 300–500 scans were acquired for each sample. Using a large number of scans taken at short intervals over the entire experiment provides an abundant supply of data sets for the analysis of portions of an experiment. A subset of about 100 scans typically was used for analysis.
Sedimentation Velocity (absorbance optics)
GFP
Sedimentation velocity data at 280 nm was collected for 16μM GFP at 20°C in a pH 7.5 buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA. Data was collected at 60,000 rpm using a 4-hole An60 Ti rotor and Beckman charcoal-epon centerpieces with quartz windows. Data were collected at 30 μm intervals using the continuous scan mode of the Beckman software (version 4.5). The data were fit using Sedfit version 8.9 software [11, available online at www.analyticalultracentrifugation.com] using the non-interacting discrete species model fitting for Sapp, Dapp, Capp, time-independent (TI) and radially invariant (RI) noise. For each fit 68.3% confidence intervals were calculated for both Sapp and Dapp fit values. S20,w values were calculated using measured viscosity (1.0051) and density (1.00362 g ml−1) values and the experimentally determined partial specific volume. Viscosity measurements were made using an Anton Paar microviscometer and density measurements were made using a Mettle/Paar Precision density meter DMA O2D. The GFP data were also fit using the continuous c(M) distribution model fitting for TI and RI noise.
rsEPCR
Sedimentation velocity data was collected for 14 μM rsEPCR at 20°C in a pH 7.5 buffer containing 20 mM Tris, 100 mM NaCl and 3 mM CaCl2. Experiments were conducted at 60,000 rpm using an 4-hole An60 Ti rotor, SedVel60K (Spin Analytical, Durham, NH) centerpieces and quartz windows. Data were collected at 280 nm, at 30 μm intervals using the continuous scan mode of the Beckman software (version 4.5). These data were fit using the continuous c(s) distribution model in Sedfit fitting for TI and RI noise and allowing the f/fo ratio to float.
AU-FDS Sedimentation Velocity
GFP and Anti-GFP
Antibody was dialyzed against a pH 7.5 buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA at 4 °C to remove glycerol (50%) from the stock solution. After dialysis the antibody was diluted serially 1:3 into buffer covering a concentration range of 540 to 0.003 nM, including a zero concentration control. GFP was added to a final concentration of 40 nM in each dilution. Both Beckman and SedVel60K double-sector, charcoal-epon centerpieces were used with either quartz or sapphire windows. No difference in data quality was observed for any arrangement of centerpieces and windows. Samples were loaded into both the reference and sample channels. Data were collected at 20 °C and 50,000 rpm in an 8-hole An50 TI rotor. The data were fit using the continuous c(s) distribution model of Sedfit, fitting for TI and RI noise. Monte Carlo analysis using 100 simulations over an integrated weight average range of Sapp values was performed to estimate the error on the sedimentation coefficients. The 40 nM GFP data also were fit using the non-interacting discrete species model fitting for Sapp, Dapp, Capp, TI and RI noise. For each fit 68.3% confidence intervals were calculated for both Sapp and Dapp fit values. These data were also fit using the c(M) distribution model fitting for TI and RI noise.
AU-FDS of goat anti-mouse Alexa488-IgG with mouse IgG
Sedimentation was conducted at 30,000 rpm, 20 °C using an 8-hole An50 rotor, SedVel60K double-sector, charcoal-epon centerpieces with samples loaded into both the sample and reference sectors. Two hundred scans at ~90 second intervals were acquired at a step size of 20 μm, averaging intensities from 5 rotor revolutions (100–150 intensities) at each radial position. Data analysis was performed using the c(s) model in Sedfit, fitting for RI and TI noise, with 95% regularization, and holding f/fo at 1.2.
AU-FDS of DPPC-NBD in the presence of rsEPCR
Fluorescence detected sedimentation velocity studies were carried out at 20°C in a pH 7.5 buffer containing 20 mM Tris, 100 mM NaCl and 3 mM CaCl2. Data were collected for 400 nM DPPC-NBD in 10 μM rsEPCR at 60,000 rpm using a 4-hole An60 Ti rotor. Samples were loaded into both the sample and reference sides of SedVel60K double sector, charcoal-epon centerpieces with sapphire windows. The data were fit using Sedfit using both the continuous c(s) distribution and non-interacting discrete species models fitting for TI and RI noise. Error analysis was performed on the discrete species fits calculating the 68.3% confidence intervals for the Sapp fit value.
AU-FDS of GFP in the presence of varying concentrations of soybean trypsin inhibitor (STI)
Fluorescence detected sedimentation velocity studies were carried out at 20 °C in a pH 7.5 buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA. Data were collected at 50,000 rpm using an 8-hole An50 Ti rotor with samples loaded into both the sample and reference sides of either SedVel60K or Beckman double-sector, charcoal-filled epon centerpieces and either quartz or sapphire windows. For the STI experiments, data were collected for 40 nM GFP in background concentrations of STI ranging from 0.75 to 50 mg ml−1. The STI was dissolved in buffer and exhaustively dialyzed prior to the addition of GFP. The data were fit using Sedfit using the continuous c(s) distribution model fitting for TI and RI noise. The Sapp values of STI measured as a function of concentration were fit using the non-interacting discrete species model.
AU-FDS of GFP in E. coli lysates
Bacteria were lysed in a pH 7.9 buffer containing 20 mM Tris, 500 mM NaCl and 10 mM imidazole. After sonication, the lysate was dialyzed overnight at 4 °C into a buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA. After dialysis, the total protein concentration of the lysate was measured using a Biorad protein assay (catalog # 500–0002) and found to be 6.8 mg ml−1. GFP was added to a final concentration of 20 nM in dilutions of the E. Coli lysate ranging from 6.8 to 0.1 mg ml−1 total protein concentration. Data were acquired using Beckman double-sector, charcoal-epon centerpieces and quartz windows. Samples were loaded into both the sample and references channels and data collected at 20 °C and 50,000 rpm in an 8-hole An50 Ti rotor. The data were fit using Sedfit using the continuous c(s) distribution fitting for TI and RI noise.
AU-FDS of Alexa488 BSA and Alexa488-IgG
Alexa488-BSA was re-suspended in a pH 7.5 buffer containing 10 mM Tris, 100 mM KCl and 0.5 mM EDTA. Data were collected at 20 °C and 50,000 rpm in a 8-hole An50 Ti rotor for 500 nM Alexa488-BSA in buffer, 100 nM Alexa488-IgG in buffer, 100 nM Alexa488-BSA in human serum and 100 nM Alexa488-IgG in human serum. Cells were assembled with quartz windows using either Beckman or SedVel60K double sector charcoal epon centerpieces. The data were fit using Sedfit version 8.9 using the continuous c(s) distribution fitting for TI and RI noise. Monte Carlo analysis using 100 simulations over an integrated weight average range of Sapp values was performed to estimate the error on the fit sedimentation coefficients from these fits.
AU-FDS of F-DPPE in serum
Human serum was spiked with 2 μM fluorescein-labeled DPPE (F-DPPE). Data were collected at 20 °C and 50,000 rpm in a 8-hole An50 Ti rotor, using Beckman double-sector, charcoal-epon centerpieces and quartz windows. The data were fit using Sedfit using the ls-g*(s) model fitting for TI and RI noise.
AU-FDS of GFP in serum
GFP was added to undiluted human serum at concentrations ranging from 540 to 4 nM. Data were collected at 20 °C and 50,000 rpm in a 8-hole An50 Ti rotor, using Beckman double-sector, charcoal-epon centerpieces and quartz windows. The 4 nM GFP data were fit using Sedfit using the continuous c(s) distribution model fitting for TI and RI noise.
AU-FDS of GFP and anti-GFP in serum
Equal concentrations of GFP and anti-GFP were added to human serum to a final concentration of 100 nM. Data were collected at 20 °C and 50,000 rpm in a 8-hole An50 Ti rotor, using Beckman double-sector, charcoal-epon centerpieces and quartz windows. The data were fit using Sedfit using the continuous c(s) distribution model fitting for TI and RI noise.
Results and Discussion
The applications of the AU-FDS may be divided into two categories: 1) Normal Use Tracer Sedimentation (NUTS), which are those applications that simply replace absorbance or interference detection with fluorescence detection, and 2) Biological On Line Tracer Sedimentation (BOLTS), which are those applications that take advantage of the selectivity of fluorescence detection to monitor labeled material in the presence of complex or concentrated solutions of unlabeled molecules. Examples of both NUTS and BOLTS applications are presented here.
NUTS applications
The AU-FDS may be used to characterize dilute solutions of fluorescently labeled solutes. The same types of information are sought in NUTS applications as with absorbance or interference optics: solution molecular weights, demonstration of macromolecular interactions, sedimentation coefficient distributions, etc. Due to the sensitivity of the AU-FDS, these determinations may be made at lower solute concentrations than for the other optical systems. AU-FDS data collected for low concentration solutions may be interpreted using present day analysis programs and models. Consequently, we will use NUTS applications to describe aspects of data acquisition and analysis that are unique to FDS.
Solution molecular weight determination
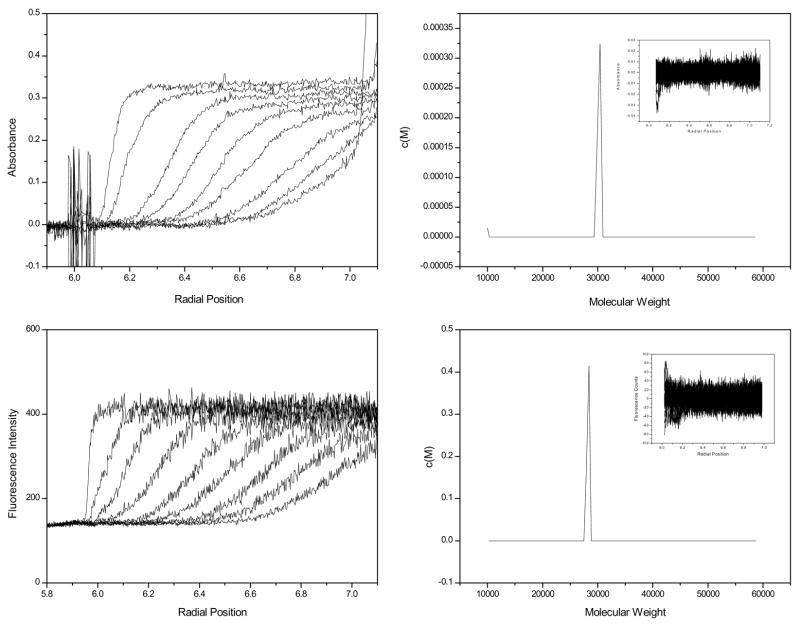
One common AUC application is the determination of solution molecular weight, e.g. to determine a protein’s quaternary structure. Sedimentation velocity data was collected for solutions of GFP using both the absorbance and the fluorescence optics. The raw data collected using the absorbance optics for a 16 μM solution is shown in Figure 1, panel A and the raw data collected using the fluorescence optics for 40 nM solution is shown in Figure 1, panel C.
Figure 1.
Raw absorbance data at 280 nm for 16 μM GFP, one out of every 10 scans is displayed (panel A). c(M) of absorbance data (panel B), 100 scans were used for the c(M) analysis. Raw AU-FDS data for 40 nM GFP (panel C). One out of every 50 scans is displayed. One out of every 5 scans (100 total) were used for the data analysis shown in panel D. c(M) of AU-FDS data (panel D).
Using the measured partial specific volume, the apparent molecular weight distributions were determined for the data in Figure 1 using the c(M) model in Sedfit [11]. The c(M) distribution for the absorbance data is shown in Figure1, panel B, and for the AU-FDS data in Figure 1, panel D. The c(M) peak position is 30,427 Da for absorbance data and is 28,396 Da for the AU-FDS data. The value for the AU-FDS is slightly lower (8%) than the sequence molecular weight, 30,840 Da. Random residuals are obtained for both the AU-FDS and absorbance data (insets). Better agreement of measured and expected molecular weights were obtained when these data were fit to the discrete, non-interacting component model in Sedfit. The absorbance data fit with S20,w 2.83 (2.73, 2.89), rmsd = 0.004457 and molecular weight of 29,520 (25520, 33720) Da, rmsd = 0.00446. The AU-FDS data fit to a S20,w of 2.71 (2.63, 2.90), rmsd = 12.31 and a molecular weight of 27,297 (21715, 32119) Da, rmsd = 12.29. The residuals from these fits were indistinguishable from those for the c(M) analysis. We are uncertain about what causes the slight discrepancy between the maximum in the c(M) distribution and M calculated from the discrete model.
The peak width for the FDS data (Figure 1D) is slightly larger than that for the absorbance data (Figure 1B), (width at half height- Absorbance data = 5384, FDS data = 5526). Since both analyses produce random error distributions, the broader peak width for the FDS data suggests there is more noise in the AU-FDS data than in the absorbance data. It is worthwhile examining the sources of noise for the AU-FDS system. These are conveniently divided into stochastic and systematic sources.
Stochastic noise
The stochastic and systematic noise characteristics of the AU-FDS are similar to those described for a prototype fluorescence apparatus [4]. Our experience is that over a wide range of intensities the magnitude of the stochastic noise is typically about 1% of the total fluorescence signal. We have observed higher stochastic noise with small solutes than large, and lower stochastic noise with more viscous samples, suggesting that the signal level is affected by diffusion of labeled material into and out of the excitation beam. Because of the wide range of intensities (60 to 4095) that may be encountered using the AU-FDS, a convenient way to express the signal quality is the signal to noise ratio, S/N, expressed in decibels (dB) and calculated as 20*log(S/N). Thus, the typical signal to noise ratio for the AU-FDS is about 40 – 50 dB. At much lower signals, the S/N decreases. Experience shows that reasonable c(s) distributions may be obtained for a reversibly interacting system if the S/N > 15, whereas data interpretation is problematic for S/N < 5 [8]. The operating software for the AU-FDS (called AU-AOS, available free from www.rasmb.bbri.org) provides the signal to noise ratio to help guide the user when setting up an experiment.
Systematic noise
The fluorescence intensity signal is relatively unprocessed compared to the absorbance signal where the ratio of sample to reference intensities automatically cancels out some sources of systematic noise. Consequently, the extent, nature and cause of systematic noise may vary from one sample to the next. We have identified what we believe are the three major sources of systematic noise in AU-FDS data. The primary source of systematic noise originates from fluorescent material adhering to the windows. In our experience this type of systematic noise is constant over the course of the experiment and may be treated as time-invariant noise in fitting programs. Non-specific binding to surfaces may be minimized by using adding an excipient (carrier) protein to the buffer. Experiments using surface coatings (PEG, silane) reveal that they do not work as well as excipient proteins. In general, the excipient protein should carry the same sign charge as the protein of interest to minimize the chances for non-specific protein binding. Ideally the labeled component will sediment faster than the excipient protein so that it travels in a relatively constant concentration of excipient protein. However, in our experience using a faster sedimenting excipient protein has posed no problems. The effects of different excipient proteins and other surface treatments should be evaluated empirically to determine what works best at reducing adsorption while not interacting with solution components. Excipient proteins that have been used successfully include 0.1 mg/ml bovine serum albumin, hen egg white lysozyme, ovalbumin and kappa casein.
The excipient protein should have no effect on the sedimentation of the labeled component. This can be checked by varying both the concentration and the identity of the excipient protein. We have observed only one case where an excipient protein (ovalbumin, anionic) bound to a cationic labeled protein, leading to an extraneous sedimenting boundary. In that case, switching to serum albumin as the excipient protein eliminated the extraneous boundary. Typically, we include 0.1 mg/ml ovalbumin or serum albumin, especially when working with low label concentrations.
A second source of systematic noise may arise from photobleaching. Noise generated due to photobleaching will cause the plateau signal to decrease faster than expected from sedimentation alone. Fortunately, the small, high-intensity illuminated spot size (~10 microns in diameter) and low duty cycle (the sample is in the beam < 0.1% of the time) tend to keep photobleaching to a minimum. Our experience is that this noise source tends to be relatively small, and may contribute to a “hook” at low values of s or M in c(s) analysis. Care must be exercised in this interpretation, though, since unbound dye also will contribute to the analysis. These two possibilities (free dye and photobleaching) may be distinguished from one another by continuing data acquisition for a sufficiently long enough time (> 12 hours at 60,000 rpm) to resolve the peak in c(s) analysis corresponding to the sedimentation of free dye (s ~ 0.1 – 0.2 for fluorescein, and ~0.3 – 0.4 for Alexa488 in PBS). Typically, photobleaching results in either no peak at low s (just a hook), or a poorly defined peak < 0.1 s (i.e. by poorly-defined we mean its presence and position are very sensitive to fitting parameters). We have encountered more cases where there are small amounts of free dye than cases where photobleaching is detectable. The practical consequence is that c(s) or c(M) analyses for extrinsically-labeled materials typically have a hook at s < 0.5. Such hooks are less-commonly encountered when analyzing GFP-labeled samples, presumably because sGFP ~ 2.7.
Third, small errors in the tracking of the excitation beam may result in systematic intensity variations. For example, if the sample is tilted slightly with respect to the radial travel of the optics, then the focal point of the excitation beam will shift vertically during the radial scan, leading to sloped plateau intensity. A second possible source of a sloped baseline is improper synchronization of the data acquisition system as the sample passes beneath the detector [5]. Small errors in the timing algorithm (on the order of 0.1 degree) of the AU-AOS software may result in the capture of intensities taken too close to the sector walls. Similar effects may be observed if the sample cell is not aligned accurately in the rotor (< 0.1 degree offset). Both positive and negative slopes may be observed. Small quantities of aggregates in the sample also will produce a sloping plateau. In this case, though, the plateau slope is always positive (for sedimenting material, negative for floating aggregates), diminishes with time (e.g. becoming flat later in the run) and may resolve as one or more peaks in a c(s) analysis. Our experience is that mechanically-derived intensity slopes remain constant during an experiment and are treated adequately as time-invariant noise.
If one acquires scans to the base of the cell (e.g. > 7.2 cm for a double-sector cell), the intensities will “roll off” as the cell base is approached. The roll off is caused by cell components (particularly the window holder and screw ring) blocking an increasing fraction of the cone-shaped excitation beam as it moves towards the cell bottom. For samples where diffusion results in significant back flow from the cell bottom, the roll off will disappear during the experiment, and be replaced by the intensity increase anticipated for the accumulating material. The user has two options for handling the intensity roll off. First, if the backflow does not need to be taken into account, one simply excludes data at the base of the cell from analysis. If it is important to follow the sedimentation at the cell bottom, one may use ~50 μL of a fluorocarbon base fluid (e.g. FC43) to shift the sample away from the cell base.
The early FDS system used for a portion of this work used a fixed-position objective lens which was difficult to focus correctly [4]. Inappropriate focusing results in an artifactual intensity peak at the meniscus that disappears as the boundary pulls away or broadens (e.g. Figures 5b, 7a, 9a and 10a). When this artifact was encountered, the early scans were omitted from analysis. The artifact is abolished by proper focusing, which is accomplished the AU-FDS using a motorized lens that may be focused in situ.
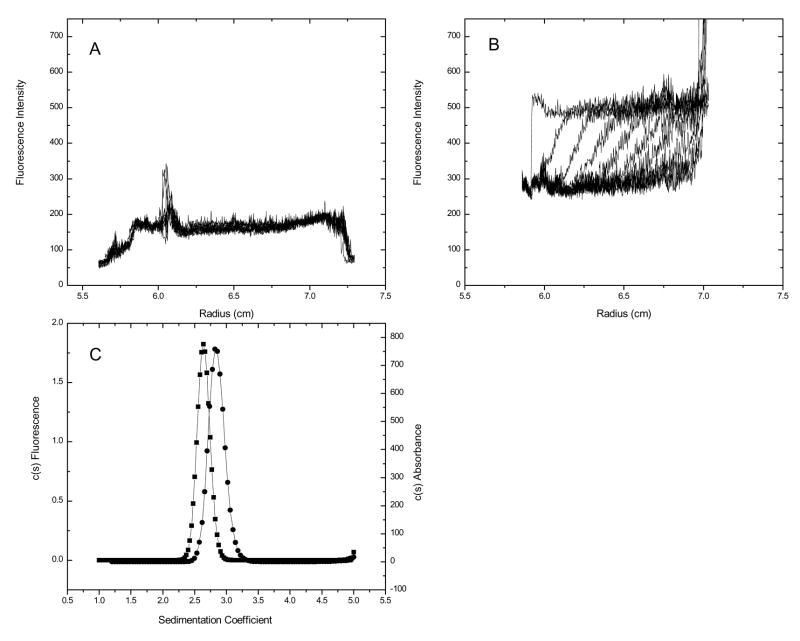
Figure 5.
Raw AU-FDS data for lipid alone (panel A). One out of every 50 scans are displayed. Raw AU-FDS data for DPPC-NBD in the presence of rsEPCR (panel B). One out of every 50 scans displayed. Overlay of c(s) distribution from absorbance (squares) and AU-FDS optics (circles).
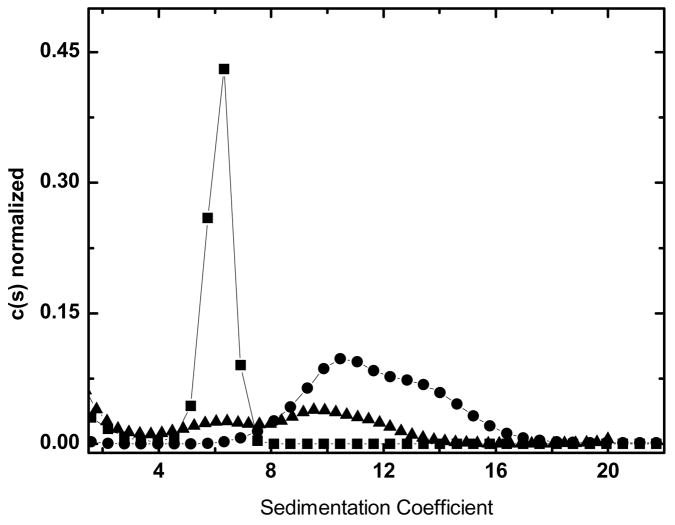
Figure 7.
Raw data of GFP sedimenting in E. Coli lysate (panel A). One out of every 50 scans are displayed. Overlay of c(s) distribution of GFP in buffer (squares) and in E. Coli lysate (circles) (panel B)
Figure 9.
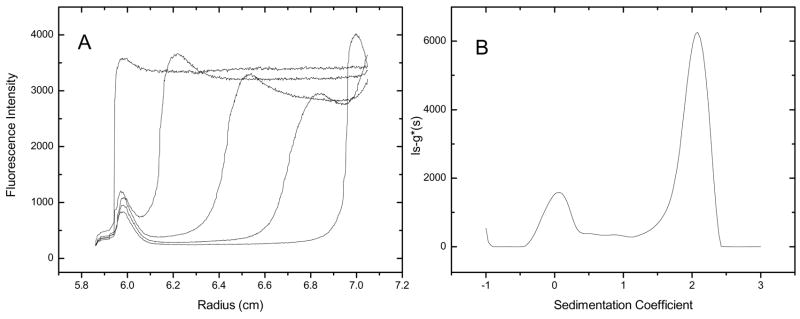
Raw AU-FDS data of 4 μM Fluorescein-DPPE sedimenting in serum (panel A). One out of every 100 scans are displayed.. ls-g*(s) distribution for Fluorescein-DPPE in serum(panel B). 100 scans were used for the ls-g*(s) analysis.
Figure 10.
Raw AU-FDS data for GFP sedimenting in serum (panel A). One out of every 100 scans is displayed. c(s) distribution for GFP sedimenting in serum (panel B). Raw AU-FDS data for GFP:anti-GFP sedimenting in serum (panel C). One out of every 100 scans is displayed. c(s) distribution for GFP:anti-GFP sedimenting in serum (panel D). 100 scans were used for each of the c(s) analyses.
Because of the nature of the systematic noise, data analysis software must be able to handle both radially independent and time independent noise. Beyond that, the analysis of AU-FDS the same data analysis protocols may be followed as would be used for absorbance and interference data. Examples of more detailed analyses for velocity and equilibrium data are available [8, 16, 17].
NUTS analysis of an interacting system
A common AUC application is to demonstrate that two solution components bind to one another and, under favorable circumstances, characterize the strength and stoichiometry of the interaction. One advantage of the AU-FDS is the ability to selectively monitor the sedimentation of a constant concentration of a labeled component of an interacting system, while varying the concentration of the other, unlabeled, components. Since the unlabeled components do not contribute to the intensity signal, it is possible to vary the concentration over a wide range while maintaining a constant signal from the labeled component.
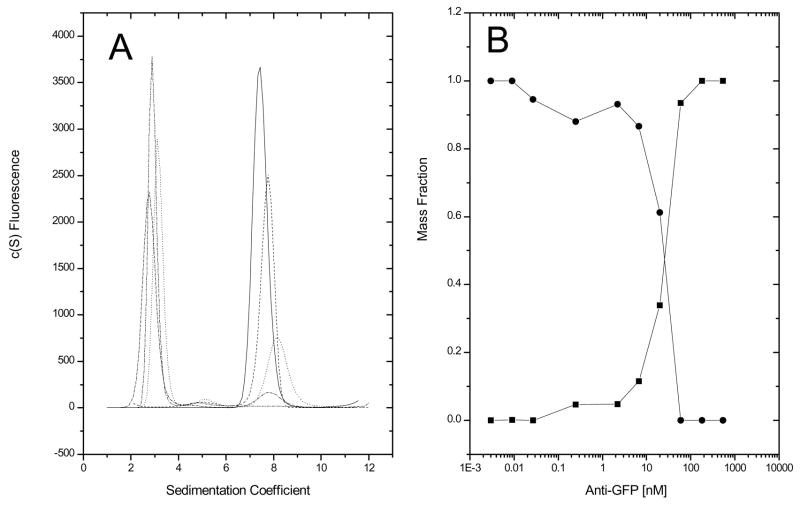
To demonstrate this application, a constant concentration (40 nM) of GFP was titrated with varying concentrations of monoclonal anti-GFP IgG. Because no reference solution is used, both channels of a double sector centerpiece may contain samples. Sedimentation velocity data were collected using the AU-FDS and fit using the c(s) model in Sedfit (Figure 2, panel A). Surprisingly, only two peaks are observed, one at approximately the same sedimentation coefficient as monomeric GFP (~2.7 s) and one at ~7.8 s that increases in area with increasing IgG concentration, but that exhibits almost no concentration-dependent shift in s. This latter observation is inconsistent with the 7.8 s peak being a consequence of a reaction boundary in reversibly associating system, where one would expect an increase in the apparent sedimentation coefficient of the faster moving boundary with increasing IgG concentration [20]. Instead, these data are consistent with constitutive IgG:GFP binding, with the 7.8 s peak corresponding to the 1:1 IgG:GFP complex. The lack of a faster moving boundary suggest that the 1:2 IgG:GFP complex does not form at these concentrations. consistent with steric hindrance of the second combining site when the first site is occupied. This result was somewhat unexpected due to the bivalent nature of IgGs. Estimates of the mass fractions of free GFP and the GFP:anti-GFP complex were determined by dividing the integrated areas of either the 2.7 sapp peak (GFP) or the 7.8 sapp peak (GFP:anti-GFP) by the total area under the c(s) distribution curve. The accuracy of these calculations will be affected by any changes in the GFP quantum yield between the free and bound state. Plotting the mass fraction of the 7.8 s species and 2.7 s species as a function of antibody concentration, a crossover point is observed at approximately 20 nM, one-half of the total GFP concentration (Figure 2, panel B), consistent with constitutive binding. A separate fluorescence polarization experiment determined the GFP:anti-GFP dissociation constant to be 1 nM [14; data not shown], consistent with this conclusion. Sedimentation experiments at much lower GFP concentrations would be necessary to analyze this as a reversible system. An example of quantitative NUTS-analysis of reversibly associating system is presented in the accompanying paper.
Figure 2.
Overlay of c(s) plots of GFP:anti-GFP titration (panel A). Antibody was serially diluted 1:3 over a concentration range of 540 nM to 0.003 nM in a constant concentration of 40 nM GFP. Mass fraction of GFP (circles) and anti-GFP/GFP (squares) as a function of antibody concentration (panel B).
Extracting qualitative information from NUTS titration data
Changes in a label’s quantum yield due to physical, chemical or experimental conditions other than molecular association confound the relationship between label intensity and concentration. Independent measurements of the quantum yield are needed for quantitative analysis. Nonetheless, NUTS titrations are useful for addressing several qualitative questions: Is a complex formed? Is an interaction reversible? Over what concentration range does an interaction appear reversible? What is the size of irreversible complexes? Are complexes mono-disperse or poly-disperse? The dynamic range of AU-FDS allows interacting systems to be studied over a broad concentration range. Since many samples may be analyzed simultaneously, data spanning several orders of magnitude in concentration may be acquired in a single experiment.
NUTS immuno-sedimentation
One type of NUTS titration is immuno-sedimentation. Immuno-sedimentation is analogous to immuno-precipitation, and may used to detect antigen presence and estimate antigen concentration [21]. In an immuno-precipitation reaction two solutions are used, with one solution (containing either the antigen or the antibody) titrated against the second. The conditions for a precipitin reaction are simple: 1) both the antigen and the antibody must be multi-valent so that an insoluble network can form, and 2) the antibody and antigen concentrations must be high enough for the precipitate to be visible. Soluble complexes form if there is either excess antigen or excess antibody present. Precipitation occurs at concentrations near the “equivalence point,” i.e. where the concentration of antibody combining sites, CAb (for IgG CAb 2x the antibody concentration), roughly equals the concentration of antigen combining sites, CAg [19,20]. From the concentration where precipitation first appears it is possible to estimate the antigen concentration (as CAg) if the antibody concentration is known or, conversely, estimate the antibody concentration (as CAb) if the concentration of antigen is known. Thus, immuno-precipitation assays provide a simple means to learn useful qualitative information about an antigen antibody reaction.
Here we demonstrate that the analog of immuno-precipitation reactions may be conducted using FDS. Because there is no need for a precipitate to form, more dilute samples may be examined and less reagent used. To conduct an immuno-sedimentation experiment, either the antibody or the antigen needs to be labeled. There is no requirement on sample purity beyond the labeling specificity. The only requirement for immuno-sedimentation to be a NUTS application is that the total solute concentration be kept low enough (< ~5 mg/ml) to prevent hydrodynamic nonideality from affecting the sedimentation coefficient distributions (e.g. a 1:20 dilution of serum would be suitable for NUTS immuno-sedimentation analysis). Samples that contain significant quantities of asymmetric or highly charged materials (e.g. sputum, nuclear extract) will need to be diluted further to minimize nonideality, and some experimentation may be needed to determine an acceptable dilution (e.g. as a rule of thumb, a 2-fold dilution should result in less than 5% change in s of a labeled antibody).
To demonstrate immuno-sedimentation, a constant concentration (133 nM) of unlabeled mouse IgG was titrated using a concentration range (0.7 to 140 nM) of Alexa488-labeled goat anti mouse IgG (Figure 3). Since both the antibody and antigen are bivalent, it is anticipated that larger and larger complexes will be formed as the system shifts from antigen excess towards the equivalence point. The largest complexes are formed when the antibody and antigen concentrations at the equivalence point (Figure 3). Note that if the total concentration of antigen, CT, is known the stoichiometry of its assembly, NAg, may be estimated from the equivalence point using a monoclonal IgG as NAg = CAb/CT, where CAb is twice the total antibody concentration.
Figure 3.
Overlay of normalized c(s) plots of 140 nM Alexa488-goat-anti-mouse-IgG (squares), 140 nM Alexa488-goat-anti-mouse-IgG + 130 nM murine IgG (circles) and 14 nM Alexa488-goat-anti-murine IgG + 130 nM murine IgG (triangles).
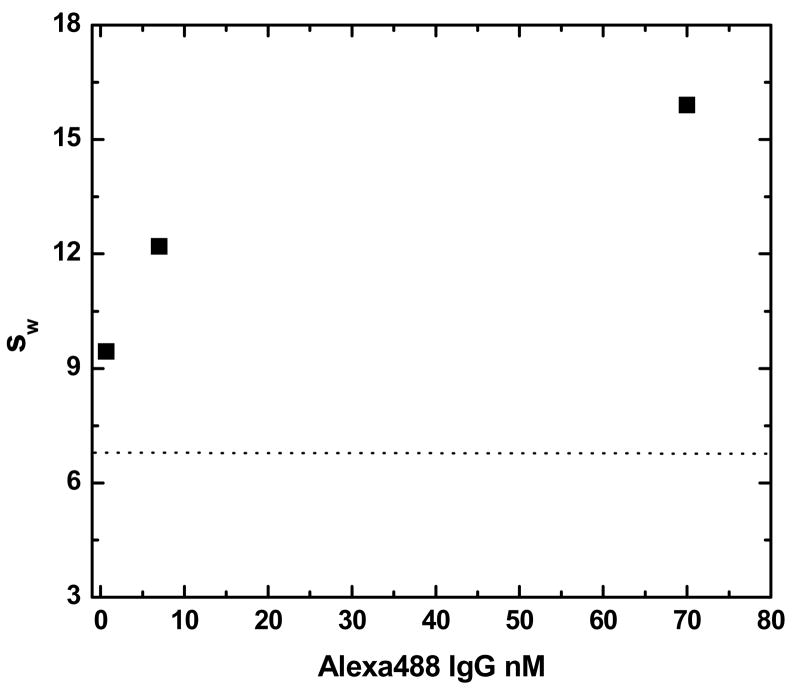
While there may be useful information about the relative sizes and concentrations of the complexes, it may be argued that it is inappropriate to use the c(s) analysis of Sedfit for a system that may exhibit both reversible and irreversible interactions. However, similar conclusions will be reached if the weight average sedimentation coefficient, sw, is determined (Figure 4). So long as the solution is at equilibrium at the start of sedimentation, the weight-average sedimentation coefficient offers a model-free way to assess the average size of the immune complexes [22].
Figure 4.
The concentration dependence of the weight average sedimentation coefficient (sw) for varying concentrations of Alexa488-goat-anti-mouse-IgG in the presence of 133 nM antigen (unlabeled murine IgG). The dashed line shows the sedimentation coefficient for the labeled antibody in the absence of antigen.
It is important to recognize that the accuracy of the sw calculation depends or the constancy of quantum yield (or knowledge of the relative quantum yield in the free and bound states). To this end, preliminary experiments can be conducted to compare the total intensity of constant concentrations of labeled material in samples containing only free material with the total intensity for samples containing saturating concentrations of unlabeled titrant.
NUTS pull down assays
A process related to immuno-precipitation is the pull down assay, in which co-precipitation of a molecule as part of an antigen-antibody complex provides evidence for binding to the antigen. We anticipate that immuno-sedimentation will provide a useful analog to pull down assays. For this application the antibody, the antigen or the co-precipitant being tested may be labeled. Titrations performed by varying the concentrations of the unlabeled components. Data analysis would proceed as shown for the simple system presented for immuno-precipitation. Shifts in the sedimentation coefficient distribution patterns when the co-precipitant is included in the solution indicate binding. Notice that either higher s (indicating binding without displacement of the labeled component) or lower s (indicating displacement of labeled material on binding) may occur. A full discussion of this application will be presented separately.
NUTS detection of small molecule binding
Selectively monitoring of labeled small molecules (drug, lipids) may be used to: 1) detect the presence of the macromolecule in a dilute fluid, 2) assess the size distribution of a macromolecule and 3) estimate the affinity of the macromolecule for the ligand. To demonstrate this application, fluorescently labeled phospholipid (DPPC-NBD) was added to a solution containing a soluble recombinant form of the endothelial protein C receptor (rsEPCR). Figure 5, panel C shows the c(s) distribution for 14 μM rsEPCR collected using the absorbance optics. These data fit to a Sapp of 2.63 (2.61, 2.66) and a Mapp of 31,524 g mol−1 (28,124; 33,224). In order to determine whether DPPC can bind to rsEPCR, AU-FDS sedimentation velocity experiments were performed selectively following a synthetic DPPC labeled with nitro-benzoxadiazol (NBD) in the presence and absence of rsEPCR. The excitation (453 nm maximum) and emission (544 nm maximum)spectra of the DPPC-NBD were measured in buffer using an SLM-AB2 fluorimeter. Since the AU-FDS light source operates at a wavelength of 488 nm, a relatively high concentration of fluorophore (400 nM) was used in these experiments. This concentration is still 250-fold less than would be necessary to obtain an 0.1 OD453 for NBD.
No sedimentation was observed in a cell containing DPPC-NBD alone (Figure 5, panel A), whereas a sedimenting boundary is apparent when 10 μM rsEPCR is included in the sample (Figure 5, panel B). Analysis of the fluorescence data yield Sapp = 2.83 (2.81, 2.85), which is in accord with DPPC-NBD binding to rsEPCR.
Sedimentation velocity analysis of fluorescently tagged lipids provides a means of studying lipid-protein interactions at concentrations below the lipid critical micelle concentration, an area of study previously inaccessible by AUC. The study of lipid-protein interactions by AU-FDS may be valuable for the study of membrane associated or membrane bound proteins.
BOLTS applications
Tracer sedimentation has been used successfully by Rivas, Minton and others to characterize the thermodynamics of labeled material (radioactivity, enzymatic activity, fluorescence, SDS gels) in solutions containing complex and concentrated solutions of unlabeled material [21]. Because a preparative centrifuge is used, only a single concentration distribution, corresponding approximately to the end of the run, is obtained per sample. This largely limits traditional tracer sedimentation methods to equilibrium analysis and, therefore, does not take advantage of the advances in sedimentation velocity analyses. The sensitivity and selectivity of the AU-FDS, as well as its ability to acquire velocity sedimentation data on-line, expands the applications of tracer sedimentation. Described below are a number of applications in which a small quantity of labeled material is added to a biological fluid, such as cell lysate or serum, and the status of the labeled material is monitored using sedimentation velocity.
It is important to recognize that at present there are no suitable analysis programs based on Lamm equation solutions to describe sedimentation in complex, concentrated fluids rigorously and quantitatively. For the present, we must be content with a qualitative understanding of the results. To differentiate these applications from NUTS applications outlined above, we call them BOLTS, short for Biological On-Line Tracer Sedimentation. Many of the NUTS applications described above may be used in BOLTS settings.
BOLTS analysis in high concentrations of background macromolecules
The selectivity of fluorescence detection allows the study of labeled molecules in concentrated solutions of same or dissimilar unlabeled macromolecules. Applications where this capability may be useful include the analysis of high-concentration protein formulations found in the biopharmaceutical and food industries. With appropriate control experiments, self- and hetero-associations may be detected and characterized by monitoring the sedimentation of a trace quantity of labeled material in these formulations.
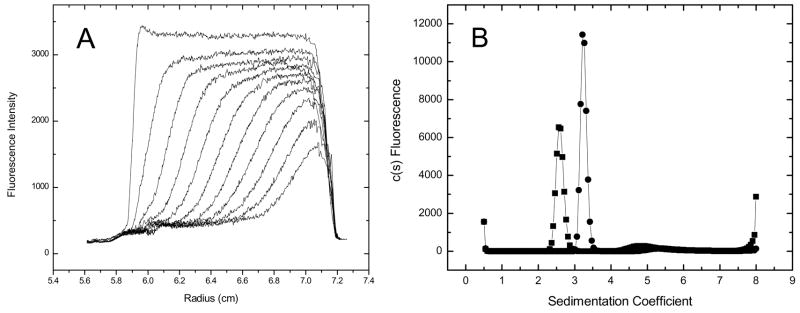
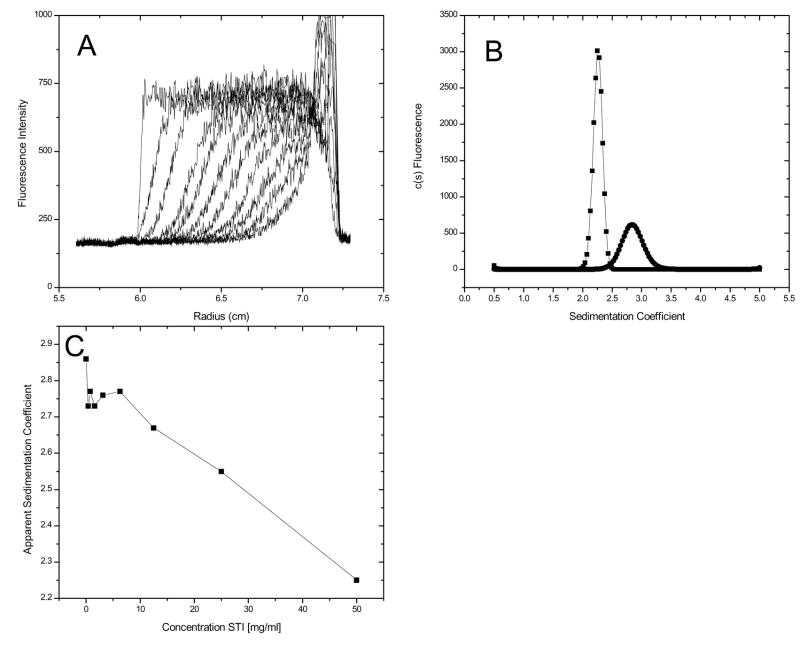
To demonstrate this application, the raw fluorescence data and the c(s) distributions for 40 nM GFP in the absence and presence of 50 mg ml−1 soybean trypsin inhibitor (STI) are presented in Figure 6. The single peak in the c(s) distribution, as well as the lack of any additional boundaries in these or any intermediate STI concentration (data not shown), suggests that this form of GFP does not undergo aggregation or self association in the presence of high concentrations of STI. Since the sedimentation coefficient of GFP alone (s20,w 2.6 – 2.7) is greater than that of STI (s20,w 2.2), the GFP sediments in a solution that is at the plateau concentration of STI. Thus, the sedimentation of GFP is slowed in a concentration-dependent manner in the presence of STI (Figure 6, panel C). The coefficient, ks, for sapp = so − kscSTI (Δs/ΔcSTI) is 0.011 s/mg/ml, which is in accord with the value anticipated for a globular protein [22]. However, in this context ks includes both the effects of STI sedimentation on solvent backflow (relative to the observer frame of reference), as well as the effects of any STI:GFP interactions. A more detailed discussion of these results and related experiments will be discussed elsewhere (manuscript in preparation).
Figure 6.
Raw data of GFP in 50 mg ml−1 STI (panel A). One out of every 50 scans are displayed. Overlay of c(s) distributions for GFP in buffer (circles) and in 50 mg ml−1 STI (squares) (panel B). Apparent sedimentation coefficient of GFP as a function of concentration of STI (panel C).
BOLTS analysis in a complex solution
Complex solutions do not necessarily contain a high concentration of any specific type of macromolecule, but instead contain a diverse array of macromolecules, each at low to moderate concentration. For example, many protocols for preparing cell lysates and sub-cellular fractions result in fairly dilute solutions (< 5 mg ml−1 total protein) containing many types of molecules. The selectivity of the AU-FDS allows tracer sedimentation to be followed in complex solutions. Labeled material may be added exogenously (as demonstrated here) or may be endogenous, such as a GFP-tagged gene product (manuscript in preparation).
To demonstrate the feasibility of monitoring a fluorescent tracer in cell lysate, sedimentation velocity experiments were conducted with a constant GFP concentration (40 nM) added to different concentrations of E. coli lysate (Figure 7). The naïve expectation for these experiments is that the sapp for GFP will decrease with increasing lysate concentration due to the increasing viscosity. Surprisingly, the sapp increases with increasing lysate concentration (inset).
There is no evidence of GFP aggregation in these samples since the shape of the c(s) distribution is unchanged in the lysate. While it cannot be ruled out entirely, it is unlikely the increase in Sapp is the result of simple mass-action self-association since saturation occurs over a narrow lysate concentration range, and there is no shoulder on the c(s) distribution as one would expect were there a reversible association. Instead, these data are consistent with a model in which the GFP is “solvated” with macromolecules. A better understanding of these data requires further investigation.
BOLTS analysis in serum
Immuno-sedimentation (described above) may find utility in proteomics, diagnostics and the pharmaceutical industry as a means to detect trace quantities of specific molecules in serum. Human serum is both a complex and high concentration fluid. The major serum protein components include serum albumin (34 – 70 mg ml−1), IgG (5 – 20 mg ml−1), transferrin (3 – 7 mg ml−1), α-1 anti-trypsin (2 – 4 mg ml−1) and serum lipoproteins (1–10 mg ml−1, which can be hundreds of nm in size and some of which float rather than sediment). In addition to these proteins, thousands of other serum components exist at concentrations less than 1 mg ml−1. The viscosity of normal human serum is ~1.8-fold greater than water [23]. Furthermore, as a result of the high concentrations and complexity, both density and viscosity gradients and boundaries are formed during sedimentation. At present, there is no analysis software capable of modeling this complexity. Nonetheless, qualitative analysis of AU-FDS data may be used to monitor the sedimentation of labeled molecules in serum, and may find use for immuno-sedimentation.
To demonstrate that AU-FDS may be used with serum, Alexa488-bovine serum albumin (Alexa488-BSA) was characterized in buffer and in human serum (Figure 8, Panel A). In buffer, the c(s) distribution for Alexa488-BSA exhibits a major peak (90% of the total signal) at 4.9 s corresponding to labeled monomer, and a minor peak (10% of the total signal) at 7.31 s. There is no change in the relative abundance of these two peaks as the total concentration of material is varied (data not shown), indicating that these two species are not linked by a reversible mass action equilibrium. Most likely the minor peak is labeled BSA dimer or some other purification byproduct.
Figure 8.
Overlay of c(s) distribution for Alexa488-BSA in buffer (circles) and in serum (squares) (panel A), overlay of c(s) distribution for Alexa488-goat-anti-rabbit-IgG in buffer (circles) and in serum (triangles) (panel B).
The sedimentation coefficient of the major peak decreases from 4.9 s in buffer to 2.9 s in serum (Δs = 2.0). Similarly, the minor peak decreases from 7.3 s to 5.3 s (Δs = 2.0). The c(s) distributions qualitatively look the same in buffer and serum, and the relative abundance of the materials in the two peaks is the same suggesting, but not proving, that any effects of serum on quantum yield are similar for the labeled components. Furthermore, the similarity of the two sedimentation coefficient distributions suggests that the labeled BSA does not interact with itself or other components in human serum.
A somewhat different picture emerges for labeled goat anti-mouse IgG (Figure 8, panel B). The c(s) distribution fit for 100 nM Alexa488-IgG (~500nM dye since each IgG has up to 5 labels attached) in buffer has a major peak (96% of the signal between 2 s and 12 s) at 6.4 s and a minor peak (4% of the signal between 2 and 12 s) at 3.4 s. In serum, the distribution for 100 nM Alexa488-IgG exhibits a major peak (74% of the total signal between 2 and 12 s) at 4.7 s and a slower minor peak (5% of the total signal) at 2.8. However, unlike dilute solution, these data also exhibit a faster, broad peak (21% of the total signal) with a maximum at 7.5 s. The appearance of this faster moving material indicates that larger complexes containing the Alexa488-IgG are formed in human serum. The concentration of IgG in human serum is estimated to be between 5–20 mg ml−1 [23]. It is possible that there is some cross-reactivity of the goat anti-mouse IgG with human IgG leading to the larger complexes. Alternatively, the Alexa488-IgG may be binding to some other serum component or it may aggregate in serum. It is impossible to distinguish between these possibilities from this experiment.
The sedimentation coefficient of the major peak is slowed from 6.4 to 4.6 s (Δs = 1.8) in going from buffer to serum, which is similar to the decrease in s observed for Alexa488-BSA (above). The slower boundary is decreased from 3.4 to 2.8 s (Δs = 0.6). Presumably the reason sapp is less affected for this 3.4 s material than 6.4 s material is because the 3.4 s material sediments slower than serum albumin in serum, and does not experience the same viscosity as material ahead of the albumin boundary. In cases where the dilute solution sedimentation coefficient of a labeled material (S°label) is close to the sedimentation coefficient of albumin in serum (SHSAserum), the labeled material may become concentrated at and form a peak behind the albumin boundary [24]. While these concentration distributions look unusual compared to ordinary sedimentation, such Johnston-Ogston effects are not unexpected and conform to solutions of the Lamm equation [25]. It remains to be seen how much quantitative information may be extracted from velocity sedimentation in undiluted serum once proper solutions to the Lamm equation are available as fitting functions. Should Johnston-Ogston effects preclude certain analyses, they may be eliminated by dilution of the serum.
BOLTS analysis of a small molecule in serum
One of the NUTS applications (above) showed that it was possible to monitor the binding of labeled small molecule to macromolecules in dilute solution. Similar experiments can be performed in a BOLTS setting. For example, changes in the sedimentation distribution function for a labeled peptide (or other antigen fragment) might be used to detect and estimate the quantity of a specific antibody.
To demonstrate this application, AU-FDS data were collected for 4 μM F-DPPE added to human serum (Figure 9). The raw data show at least one sedimenting boundary and possibly a floating boundary (Figure 9, panel A). The peak in the fluorescence intensity near the major boundary most likely results from the Johnston-Ogston effect (above). An estimate of the apparent sedimentation coefficient for the primary sedimenting boundary can be calculated using equation 1:
| Equation 1 |
where r is the radial location of the midpoint of the sedimenting boundary, rm is the meniscus position and w2t is the reduced sedimentation time. Using this method, the major boundary sediments at ~2 s. This sedimentation coefficient is significantly smaller than what we measured for Alexa488-BSA in serum (2.9 s). As a result, it is doubtful that the F-DPPE is binding to HSA. It is possible, however, that the F-DPPE is binding to some other serum component. Further experiments would be needed to identify what serum components bind F-DPPE.
When analyzed using the ls-g(s) method in Sedfit (Figure 9, panel B) a peak corresponding to the 2 s boundary is observed, along with material that is nearly neutrally buoyant. The ls-g(s) distribution does not resolve to baseline between these two peaks. It is unclear whether the lack baseline resolution is a consequence of material leaching from the 2 s boundary or due to some other phenomenon (e.g. the Johnston Ogston effect). The neutrally-buoyant material may correspond to free F-DPPE, or F-DPPE bound to some other low-density material. Further experimentation will be necessary to clearly identify all the phenomena occurring in this system. Nonetheless, these data do show the potential for studying small molecule interactions in serum.
BOLTS analysis of an interacting system in human serum
Immuno-sedimentation in dilute solutions (Figure 3) may be extended to a BOLTS application. Figure 10, panel A shows the raw data for 4 nM GFP in human serum, and panel B shows the corresponding c(s) distribution for these data. The GFP distribution in serum has a single maximum at Sapp = 2.4, whereas GFP in buffer sediments at 2.6 – 2.7 s (Figure 1). The relatively small Δs (~0.2 s) for GFP in serum is due to it sedimenting slower than the serum albumin boundary. When a 1:1 mixture of GFP and anti-GFP, each at 100 nM is added to human serum, two boundaries are observed (Figure 10, panel C) and the c(s) distribution has two peaks (Panel D). The slower material (42% of the total signal) at 2.4 s is assumed to be free GFP based on the data for GFP (Panel B). The peak (58% of the total signal) at 4.1 s moves more slowly than IgG alone (~4.6 s) and most likely is not due to a stable 1:1 complex between GFP and anti-GFP. More likely, the 4.1 s peak results from a reaction boundary indicating that the GFP:anti-GFP complex is dissociating in serum [18]. This result is surprising since the concentrations of GFP and anti-GFP in this experiment are 100-fold greater than the Kd (1 nM, above). The naïve expectation is that nearly all of the GFP would be bound with the anti-GFP. The observation of a boundary corresponding to free GFP, therefore, is a surprise. The presence of nearly half of the GFP as free material is consistent with a Kd in serum closer to 50 nM. However, it is not clear that a weakened interaction is the only possible basis for these results.
It is likely that both thermodynamic and hydrodynamic non-ideality contribute to the appearance of the free GFP peak, and the apparent reduced affinity. It is interesting to note that the peaks in the c(s) distribution are nearly symmetrical (no skewing) and the apparent distribution resolves to baseline between the peaks. However, the assumptions made in the c(s) analysis (especially that the boundaries are the consequence of independent components) are thoroughly violated in this experiment and the fitting residuals indicate significant systematic deviation from the model. Therefore, it is not appropriate to attempt to interpret these data any further at this time. We present the data, though, to show that BOLTS can detect antibody-antigen interactions in serum. BOLTS-based immuno-sedimentation in serum has both research and diagnostic applications. Consequently, the development of adequate data analysis methods is warranted.
Notes and observations on AU-FDS
In general, there are two categories of difficulties that arise when using the AU-FDS. The first category encompasses the standard set of problems associated with fluorescence detection. These include photobleaching, inner-filter effects, quenching, and variation in the quantum yield, all of which complicate the correspondence between concentration and fluorescence intensity. The second category of challenges relates to the sedimentation behavior of molecules in complex and high concentration solutions.
Inner-filter effect
The inner-filter effect results from the absorbance of either the excitation (primary) or emission (secondary) photons by the solution in portions of the optical path that are not sensed by the detector, which leads to a decreasing slope in the signal as a function of concentration [4]. Our experience has been that the inner-filter effect is negligible at concentrations below 300 nM (fluorescein) in the AU-FDS. The best way to check for the presence of the inner-filter effect is to make a dilution series of the sample and check that the fluorescence intensity is proportional to concentration. Whenever possible, experiments should be designed to keep the concentration of fluorophore low enough to avoid the inner-filter effect.
Quenching
Certain salts, oxygen or the presence of other contaminating molecules may result in collisional quenching. If the quencher concentration is uniform throughout the cell then sedimentation and diffusion coefficients may be determined accurately, and only those quantities that depend on an accurate knowledge of the concentration (e.g. affinity constants, non-ideality coefficient) will be affected. However, a gradient in the quencher concentration can distort boundary shapes, leading to inaccurate diffusion coefficient determinations and, to a lesser extent, inaccurate sedimentation coefficient determinations. The concentration of quenchers must be minimized in order to optimize sensitivity. To this end, solvents containing heavy metals, iodide, acrylamide and other known collisional quenchers should be avoided. In addition, sparging with Ar or N2, or degassing in a vacuum, should be performed prior to sedimentation to remove molecular oxygen. Our experience is that O2 contamination may reduce the fluorescence signal significantly. To date, we have not encountered systems containing a quencher concentration gradient.
The analysis of BOLTS data
The second general challenge to application of AU-FDS relates to the interpretation of sedimentation data for molecules in complex and high concentration solutions, where at a minimum it is anticipated that the viscosity and density will as a function of both radial position and time. In addition, it is anticipated that data from complex solutions may exhibit the effects of flow coupling between different macromolecular components. While these effects have been noted before (e.g. Johnston-Ogston effects), they have remained under-explored primarily due to the limited availability of instrumentation and computational methods. Because BOLTS enables exploration of biologically and medically relevant significant systems, it will be worthwhile developing models, however approximate, to extract more information from BOLTS data.
Summary
The AU-FDS provides a powerful means of characterizing the thermodynamics and hydrodynamics of trace materials in complex and concentrated solutions. The examples presented here reveal some of the new types of experiments available with fluorescence detection. They also highlight some of the caveats and complexities that arise when studying sedimentation in these solutions. At present, it is unclear how much quantitative information may be extracted from AU-FDS data. However, valuable qualitative insights are obtained from NUTS and BOLTS analyses.
Acknowledgments
The development of the AU-FDS was supported by NSF BIR-9876582, NIH 1R01GM6283601, and the Biomolecular Interaction Technologies Center. The authors wish to thank Jeff Hansen, Edward Eisenstein, Borries Demeler, Brett Austin and Jack Aviv for their help with its development.
Abbreviations
- AUC
analytical ultracentrifuge or analytical ultracentrifugation
- FDS
fluorescence detected sedimentation
- GFP
green fluorescent protein
- NUTS
Normal use tracer sedimentation
- BOLTS
Biological on-line tracer sedimentation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole JL, Lary JW, Moody TP, Laue TM. Analytical Ultracentrifugation: Sedimentation Velocity and Sedimentation Equilibrium. In: Correia JJ, Detrich H, editors. Methods in Cell Biology. Chapter 6. Vol. 84. Elsevier; 2007. pp. 143–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlett GJ, Minton AP, Rivas G. Analytical ultracentrifugation for the study of protein association and assembly. Curr Opin Chem Biol. 2006;10:430–6. doi: 10.1016/j.cbpa.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Laue, T.M. “Optical Systems of the XLA Ultracentrifuge” (1996) Applications Data Note for Spinco Division of Beckman Instruments, Inc. P.O.Box 10200, Palo Alto, California.
- 4.MacGregor IK, Anderson AL, Laue TM. Fluorescence Detection for the XLI Ultracentrifuge. Biophys Chem. 2004;108:165–185. doi: 10.1016/j.bpc.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Laue TM, Austin JB, Rau DA. A Light Intensity Measurement System for the Analytical Ultracentrifuge. Progr Colloid Polym Sci. 2006;131:1–8. [Google Scholar]
- 6.Crepeau RH, Conrad RH, Edelstein SJ. UV Laser Scanning and Fluorescence Monitoring of Analytical Ultracentrifugation with an On-Line Computer System. Biophys Chem. 1976;5:27–39. doi: 10.1016/0301-4622(76)80024-5. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, Rappold W, Rosenbaum V, Fischer R, Riesner D. A fluorescence detection system for the analytical ultracentrifuge and its application to proteins, nucleic acids, and viruses. Colloid & Polymer Science. 1989;268:45–54. [Google Scholar]
- 8.Kingsbury JS, Laue TM, Klimtchuk ES, Theberge R, Costello CE, Connors LH. The modulation of transthyretin tetramer stability by cysteine 10 adducts and the drug diflunisal: direct analysis by fluorescence-detected analytical ultracentrifugation. J Biol Chem. 2008;283:11887–11896. doi: 10.1074/jbc.M709638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bothwell MA, Howlett GJ, Schachman HK. A sedimentation equilibrium method for determining molecular weights of proteins with a tabletop high speed air turbine centrifuge. J Biol Chem. 1978;253:2073–2077. [PubMed] [Google Scholar]
- 10.Darawshe S, Rivas G, Minton AP. Rapid and accurate microfractionation of the contents of small centrifuge tubes: application in the measurement of molecular weight of proteins via sedimentation equilibrium. Anal Biochem. 1993;209:130–135. doi: 10.1006/abio.1993.1092. [DOI] [PubMed] [Google Scholar]
- 11.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stafford WF, Sherwood PJ. Analysis of heterologous interacting systems by sedimentation velocity: curve fitting algorithms for estimation of sedimentation coefficients, equilibrium and kinetic constants. Biophys Chem. 2004;108:231–243. doi: 10.1016/j.bpc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Philo JS. Improved methods for fitting sedimentation coefficient distributions derived by time-derivative techniques. Anal Biochem. 2006;354:238–246. doi: 10.1016/j.ab.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 14.Kroe R. PhD Thesis. University of New Hampshire; Durham NH: 2005. Applications of Fluorescence Detected Sedimentation. [Google Scholar]
- 15.Bean S. Senior Thesis. University of New Hampshire; Durham NH: 2006. Determination of Partial Specific Volume: Using Polyethylene Glycol, Ovalbumin, Lysozyme, Dextran and Green Fluorescent Protein. [Google Scholar]
- 16.Ryan TM, Howlett GJ, Bailey MF. Fluorescence Detection Of A Lipid Induced Tetrameric Intermediate In Amyloid Fibril Formation By Apolipoprotein C-II. J Biol Chem. doi: 10.1074/jbc.M804004200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess BR, Dobson RCJ, Bailey MF, Atkinson SC, Griffin MDW, Jameson GB, Parker MW, Gerrard JA, Perugini MA. Structure and Evolution of a Novel Dimeric Enzyme from a Clinically Important Bacterial Pathogen. J Biol Chem. 283:27598–27603. doi: 10.1074/jbc.M804231200. [DOI] [PubMed] [Google Scholar]
- 18.Walter F, Stafford . Analysis of reversibly interacting macromolecular systems by time derivative sedimentation velocity. In: Johnson ML, Ackers GK, editors. Methods in Enzymology Volume 323, Part C: Energetics of Biological Macromolecules. Academic Press; 2000. pp. 302–325. [DOI] [PubMed] [Google Scholar]
- 19.Heidelberger M, Kendall FE. A quantitative theory of the precipitin reaction : II. A study of an azoprotein-antibody system. J Exp Med. 1935;62:467–483. doi: 10.1084/jem.62.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauling L, Pressman D, Campbell DH. An experimental test of the framework theory of antigen-antibody precipitation. Science. 1943;98:263–264. doi: 10.1126/science.98.2542.263. [DOI] [PubMed] [Google Scholar]
- 21.Rivas G, Minton AP. Tracer sedimentation equilibrium: a powerful tool for the quantitative characterization of macromolecular self and hetero-association in solution. Bioch Soc Trans. 2003;31:1015–1019. doi: 10.1042/bst0311015. [DOI] [PubMed] [Google Scholar]
- 22.Rowe AJ. The Concentration Dependence of Transport Processes: A General Description Applicable to the Sedimentation, Translational Diffusion, and Viscosity Coefficients of Macromolecular Solutes. Biopolymers. 1977;16:2595–2611. [Google Scholar]
- 23.Altman PL. Blood and other body fluids, ASD Technical Report 61–199. Federation of American Societies for Experimental Biology; Washington, DC: 1961. [Google Scholar]
- 24.Johnston JP, Ogston AG. Trans Faraday Soc. 1946;42:789–799. [Google Scholar]
- 25.Correia JJ, Johnson ML, Weiss GH, Yphantis DA. Numerical Study of the Johnston-Ogston Effect in Two-component Systems. Biophy Chem. 1976;5:255–264. doi: 10.1016/0301-4622(76)80038-5. [DOI] [PubMed] [Google Scholar]