Abstract
Objectives
Candida albicans cells form biofilms on polymeric surfaces of dentures and other prostheses introduced into the oral cavity. Many biofilm microorganisms exhibit resistance to antimicrobial agents; C. albicans cells may also develop resistance to naturally-occurring antifungal peptides in human saliva including histatins (Hsts) and defensins (hBDs). Therefore, we evaluated Hst 5 activity on C. albicans biofilm cells compared to planktonic cells and measured whether surface treatment of denture acrylic with Hst 5, hBD-3, or chlorhexidine gluconate could inhibit in vitro biofilm development.
Methods
Acrylic disks were preconditioned with 500 μl saliva for 30 min, and inoculated with C. albicans cells (106 cells/ml) for 1 h, at 37 °C. Non-adherent cells were removed by washing and disks and were incubated in YPD growth medium for 24, 48, and 72 h at 37 °C. Candidacidal assays were performed on 48-hour-biofilms and on planktonically-grown cells using Hst 5 (15.5 μM, 31.25 μM, 62 μM). Cell adhesion was compared on disks pre-coated with 0.12% chlorhexidine gluconate, 50 μM Hst 5, or 0.6 μM hBD-3 after 24 h, 48 h, and 72 h growth.
Results
No significant difference was observed in sensitivity to Hst 5 of biofilm cells compared to planktonic cells (p > 0.05). Pre-coating disks with hBD-3 did not inhibit biofilm development; however, Hst 5 significantly inhibited biofilm development at 72 h, while 0.12% chlorhexidine significantly inhibited biofilm development at all time intervals (p < 0.05).
Conclusions
C. albicans biofilm cells grown on denture acrylic are sensitive to killing by Hst 5. Surface coating acrylic with chlorhexidine or Hst 5 effectively inhibits biofilm growth and has potential therapeutic application.
Keywords: Candida albicans, Biofilm, Denture acrylic, Histatin, Defensin, Chlorhexidine
INTRODUCTION
Candida albicans is an opportunistic pathogen, commonly affecting individuals with a compromised immune system. In the oral cavity, candidiasis is often related to denture use, leading to the development of a condition referred to as denture-induced stomatitis. Olsen (1974) identified yeasts in 78–100% of patients with denture-induced stomatitis compared to 30–60% in a non-denture-wearing population.1 Factors such as prosthesis fit, hygiene, and host susceptibility contribute to the development and progression of this condition so that the reported prevalence ranges between 10% to 67% in complete denture wearers among several populations and age groups.2,3
Dentures create an environment that favors the localization and development of potentially virulent organisms. In addition to isolating the underlying mucosa from the self-cleansing action of the oral musculature, anaerobic and acidic conditions develop at the tissue-contacting surface of a denture promoting yeast proliferation.4 C. albicans readily forms biofilms on prosthetic surfaces, including denture acrylic.5 In vivo sampling has demonstrated higher levels of Candida spp. on the denture surface compared to the palatal mucosa,6 and attachment of these microorganisms to denture acrylic may permit dentures to serve as a reservoir for continual infection.7 Biofilm cells typically exhibit increased resistance to antifungal agents and the host immune system. Chandra et al. demonstrated increased resistance of C. albicans biofilms grown on denture acrylic to fluconazole, amphotericin B, nystatin, and chlorhexidine.8 Furthermore, C. albicans cells resuspended from a biofilm typically maintain some degree of resistance to antimicrobials compared to planktonic cells.9 LaFleur et al. documented the presence of resistant C. albicans cells following resuspension of a biofilm exposed to amphotericin B and chlorhexidine.10 Although the killing activity of Hst 5 is well documented against planktonic cells of C. albicans, the sensitivity of C. albicans cells grown in a biofilm to Hst 5 has not been reported.
Efforts are ongoing to identify naturally occurring peptides with antimicrobial activity against Candida biofilms. The benefits of using salivary peptides in the treatment of candidiasis include non-toxicity to humans and effectiveness against C. albicans, including drug-resistant strains. Salivary Histatins (Hsts) are a family of histidine-rich peptides produced in acinar cells of human parotid and submandibular glands.11 Hsts contribute to the innate host defense system and have potent antifungal activity against yeast and filamentous forms of Candida.11,12 At least 50 Hst peptides have been identified in saliva. Hst 5 is the N-terminal 24 amino acid segment of Hst 3 and has been shown in vitro to be highly toxic to yeast and filamentous forms of C. albicans and other fungi at physiologic concentrations (15–50 μM).13 Hst 5 also has candidacidal activity against azole-resistant strains of C. albicans.14 Structurally, Hst 5 has an α-helical conformation with high levels of histidine, a net positive charge, and no disulfide bonds. This structure prevents direct insertion across microbial cell membranes and development of pores.13 Instead, Hst 5 binds to surface proteins on the cell membrane, followed by entry into the cytoplasm and binding to intracellular targets. The binding site on the cell wall has been identified as the Ssa family of proteins.15 Hst 5 is translocated across the cell membrane and induces an efflux of ATP and ions, leading to a noncytolytic loss of cell volume and cell death.16,17 The selectivity of Hst 5 to yeast cell wall contributes to candidacidal activity against C. albicans and non-toxicity to human cells.18
Defensins are a family of cationic peptides providing innate immune defense, including antifungal activity.19 They are divided into alpha and beta subfamilies based on sequence homology and location of six conserved cysteine residues. Human β-defensin-1 (hBD-1) through hBD-3 are expressed in epithelial tissues, including salivary glands, salivary secretions, gingival epithelium, and gingival crevicular fluid.20 Release of hBD-3 is induced by several factors, including mediators of inflammation and bacterial or candidal challenge.21 Antimicrobial activity of hBD-3 has been demonstrated across a broad spectrum, including gram-negative and gram-positive bacteria, enveloped viruses, and fungi.22 Both hBD-2 and hBD-3 have fungicidal activity against C. albicans, with hBD-3 having 10 times greater potency than hBD-2.19 The mechanism of action of hBD-3 against bacteria involves membrane depolarization and permeabilization.22 However, little is known about its mechanism of action against fungi, although the same Ssa cell wall proteins involved in the candidacidal activity of Hst 5 are also necessary for hBD-3 killing.23
Chlorhexidine is a cationic chlorophenyl bisbiguanide that binds to negatively charged surfaces. It has a broad spectrum of antimicrobial activity24 including C. albicans.25–27 Chlorhexidine is more effective than nystatin or amphotericin B in killing adherent C. albicans cells.27 Studies by McCourtie et al. and Spiechowicz et al. demonstrated that pre-treatment of acrylic with chlorhexidine reduces adherence of C. albicans.29,30 Exposure of Candida to chlorhexidine results in loss of structural integrity, diminished ability to adhere, and fragmentation of the cell wall.26 A notable feature of chlorhexidine is that it adheres to salivary glycoproteins in plaque and is slowly released over time. Following a one min rinse with chlorhexidine, 30% was retained in the oral cavity for 24 h.31 This pharmacologic feature of chlorhexidine permits extended activity following exposure in the oral environment and allows for wider spacing of doses.
Current treatment regimens for denture-induced stomatitis include the use of topical antifungal agents applied to the affected musoca and prosthesis. Despite resolution of the mucosal infection, fabrication of new dentures is generally recommended to eliminate the source for renewed infection. Treatment methods directed toward reducing initial fungal attachment and subsequent biofilm development on denture acrylic would be beneficial in reducing the incidence and severity of this condition. This study was designed to evaluate the potential for surface treatment of denture acrylic with antifungal peptides or agents to inhibit biofilm development.
METHODS
Fabrication of Acrylic Disks
Heat-cured polymethylmethacrylate (PMMA) disks (23 mm × 1.5 mm) were fabricated using two injection-molding processing techniques: 1) SR Ivocap Injection System with SR Ivocap High Impact resin (Ivoclar Vivadent, Schaan, Liechtenstein); and 2) Success Injection System with Lucitone 199 resin (Dentsply International, York, PA). PMMA disks were processed according to manufacturers’ instructions from standard aluminum disks invested in Type III dental stone. Disks were equivalent in size and surface finish. No surface modification was made to the processed disks following recovery. Disks were placed in distilled water for 24 h and stored dry until used.
Cell Preparation
C. albicans strain CAI-4 was used in all experiments. This strain was subcultured from a thawed suspension of CAI-4 stored at −70 °C. Cells were incubated on YNB agar (Difco) at room temperature for 48 h and then maintained at 4 °C until used. Prior to use in cell adhesion and candidacidal assays, cells were inoculated in 10 ml of YPD growth medium in a shaker (250 rpm) at room temperature for 14 h to an Optical Density600 of 0.8 –1.2 (Genesys 10 UV Spectrophotometer, Thermo Fischer Scientific, Waltham, MA). Cells were washed twice with 10 ml of 10 mM of sodium phosphate buffer (NaPB) (Na2HPO4/NaH2PO4, pH 7.4) by centrifugation (2125 × g, 5 min). Cells were resuspended in NaPB and quantified with phase-contrast microscopy (40× power) using a counting grid. Cells were diluted to 106 cells/ml in NaPB and held in suspension until used for the candidacidal assay or the cell adhesion assay.
Saliva Collection and Preparation
Whole unstimulated saliva was collected from one healthy volunteer into a chilled sterile vial and centrifuged (5900 × g, 10 min) at 4 °C to remove cellular debris. The supernatant was collected and stored at −70 °C. Thawed saliva was used to condition disks as described below.
Biofilm Conditions
PMMA disks were placed in a 12-well polystyrene culture plate (BD Biosciences, San Jose, CA). Disks were conditioned with 500 μl of saliva at room temperature for 30 min, then washed twice with NaPB. Disks were inoculated with 1 ml of CAI-4 cells (106 cells/ml) and placed in an incubator for 1 h at 37 °C. Non-adherent cells were then removed by washing twice with NaPB, and 1 ml of YPD growth medium was placed over the inoculated disks and incubated for 24, 48, or 72 h at 37 °C. At 24-hour intervals, the growth medium was removed and disks were washed twice with NaPB to remove non-adherent cells. For the 48 and 72 h groups, wells were replenished with 1 ml of YPD growth medium and disks were incubated at 37 °C. At the end of the incubation interval (24, 48, or 72 h), growth medium was removed and disks were washed twice with NaPB to remove non-adherent cells. Cells were recovered from the disks into NaPB suspension by using a cell scraper (BD Biosciences, San Jose, CA). Cells were quantified with phase-contrast microscopy (40× power) (Nikon Eclipse E400, Nikon Instruments, Inc., Melville, NY) using a counting grid (Petroff-Hausser Counter, Hausser Scientific, Horsham, PA). Mean cell number and standard error are reported.
Pre-coating PMMA Disks with Antifungal Agents
The effect on biofilm cell numbers was evaluated by pre-coating Lucitone disks with either of the following: 1) 0.12% chlorhexidine gluconate (PerioGard, Colgate-Palmolive, New York, NY); 2) 50 μM Hst 5 (synthesized by GeneMed Synthesis, Inc., San Antonio, TX); or 3) 0.6 μM hBD-3 (Peptides International, Osaka, Japan). Concentrations of antifungal peptides were selected based on previous studies which demonstrated potent killing at physiologic concentrations.13,23 Disks were fabricated and conditioned with saliva as above. Following washing with NaPB, 500 μl of antifungal solution was applied to test disks and 500 μl of NaPB was applied to control disks for 1 h at room temperature. Disks were washed twice with NaPB and treated as above for inoculation, biofilm development, and quantification. Experiments were conducted in triplicate. Mean cell numbers and standard error are reported.
Candidacidal Assay
Candidacidal assays were performed on cells resuspended from three independent 48-hour-biofilms using Hst 5 in concentrations of 15.5 μM, 32.25 μM, and 62 μM. Cell suspensions were diluted with NaPB and aliquots of 500 cells were spread on agar plates in duplicate and incubated for 48 h at room temperature. Cell colonies were quantified using an automated counter (aCOLyte, Synbiosis, Frederick, MD) and cell survival was expressed as a percentage of control, and loss of viability was calculated as (1 – [colonies from Hst 5-treated cells ÷ colonies from control cells]) × 100.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism v4.02 (GraphPad Software Inc., San Diego, CA). Cell death in the candidacidal assay was analyzed using a two-tailed, two-sample independent t-test. Biofilm cell numbers was analyzed using a paired one-tailed, two-sample independent t-test. A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Development of C. albicans biofilm on denture acrylic
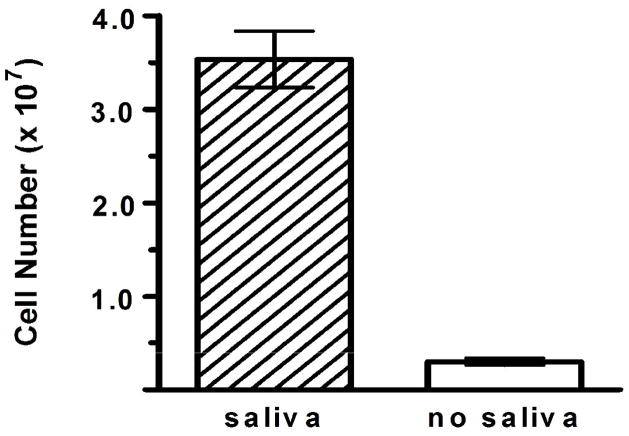
Figure 1 provides a visual representation of the biofilm formed on acrylic disks following staining with Coomassie blue. Growth times were selected to optimize the density and surface area of the candidal biofilm. Saliva was applied to the acrylic prior to inoculation with C. albicans based on the results of our initial experiments demonstrating a positive effect of saliva on increasing biofilm growth (Figure 2). Acrylic surfaces that were conditioned with saliva 30 min prior to inoculation had a significantly greater mean cell number of 3.5 ± 0.3 × 107 after 72 h of growth compared to non-conditioned disks, which had a mean cell number of 3.0 ± 0.4 × 106 (p = 0.0097).
Fig. 1.
Biofilm formation on acrylic disks (stained with Coomassie blue). The intensity and surface area of the biofilm increased over time. Biofilm growth tended to concentrate at the periphery of the disks.
Fig. 2.
Conditioning acrylic with saliva promotes C. albicans biofilm growth. Biofilms were developed on acrylic with or without saliva conditioning prior to inoculation with C. albicans (106 cells/ml). After 72 h of biofilm growth, cells were removed and quantified with phase-contrast microscopy. Acrylic conditioned with saliva had a significantly higher mean cell number (3.5 ± 0.3 × 107) compared to non-conditioned disks (3.0 ± 0.4 × 106) (p = 0.0097).
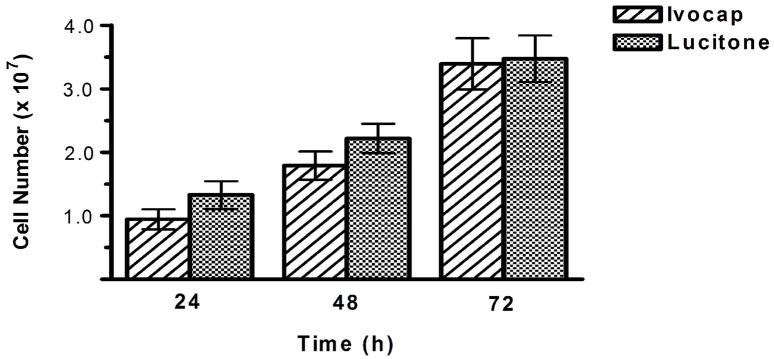
Denture acrylic from two manufacturers was initially evaluated when developing the biofilm growth model to determine whether both substrates support equivalent biofilm growth of C. albicans. Ivocap and Lucitone acrylic represent two commonly used materials used in modern injection-molding denture processing systems. Both acrylics supported equal biofilm growth at all time intervals (Figure 3). The mean cell number on Ivocap acrylic was as follows: 9.4 ± 1.6 × 106 at 24 h; 1.8 ± 0.2 × 107 at 48 h; and 3.4 ± 0.4 × 107 at 72 h. The mean cell number on Lucitone acrylic was as follows: 1.3 ± 0.2 × 107 at 24 h; 2.2 ± 0.2 × 107 at 48 h; and 3.5 ± 0.4 × 107 at 72 h. There was no difference in biofilm cell number on either acrylic at 24, 48, or 72 h (p > 0.10).
Fig. 3.
Ivocap and Lucitone acrylic substrates support equivalent C. albicans biofilm growth. Acrylic was conditioned with saliva, inoculated with C. albicans (106 cells/ml), and incubated for 24, 48, or 72 h at 37 °C. Cells were quantified with phase-contrast microscopy. Mean cell numbers and standard error are reported. There was no significant difference in biofilm cell numbers between either acrylic at any time interval (p > 0.10).
C. albicans resuspended from a biofilm on acrylic are sensitive to Hst 5 killing
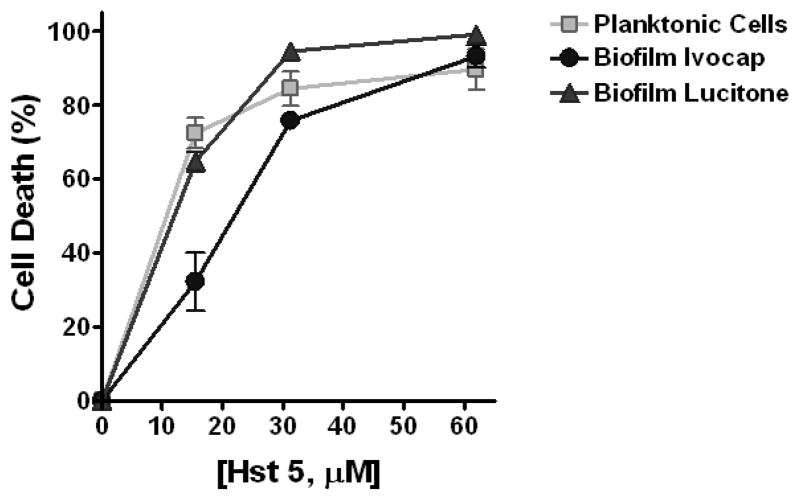
Killing activity of Hst 5 on C. albicans was evaluated by performing a candidacidal assay on two types of C. albicans cells – planktonic cells and cells obtained from a 48 h biofilm grown on denture acrylic. The candidacidal activity of Hst 5 was measured over a range of physiologic concentrations.. Hst 5 activity on C. albicans biofilm cells was similar to planktonic cells at all concentrations except one (Figure 4). The mean cell death of planktonic cells was 72.5 ± 4.1% at 15.5 μM Hst 5; 84.5 ± 4.6% at 31.25 μM Hst 5; and 89.6 ± 5.5% at 62.0 μM Hst 5; while cell death of biofilm cells from Ivocap acrylic was 32.3 ± 7.8% at 15.5 μM Hst 5; 75.8 ± 2.3% at 31.25 μM Hst 5; and 93.2 ± 3.0% at 62.0 μM Hst 5 and cells from Lucitone acrylic was 64.8 ± 3.1% at 15.5 μM Hst 5; 94.6 ± 2.4% at 31.25 μM Hst 5; and 99.1 ± 0.6% at 62.0 μM Hst 5. There was no significant difference in sensitivity to Hst 5 of resuspended C. albicans biofilm cells from either Lucitone or Ivocap acrylic compared to planktonic cells (p > 0.05) at any Hst 5 concentration, although some reduction in killing was observed in cells recovered from Ivocap acrylic biofilms compared to planktonic cells at the lowest Hst 5 concentration (p < 0.05). These results demonstrate that C. albicans biofilm cells from denture acrylic do not acquire reduced sensitivity to killing by Hst 5 at physiologic concentrations. Since there was no difference in cell growth on either acrylic, one acrylic (Lucitone) was selected as the substrate for biofilm growth studies. We next pre-coated disks with antifungal peptides to determine whether one surface treatment could decrease biofilm development.
Fig. 4.
C. albicans resuspended from a biofilm on acrylic are sensitive to Hst 5 killing. Controlled candidacidal assays were performed on C. albicans cells resuspended from three independent 48-hour-biofilms using Hst 5 in concentrations of 15.5 μM, 32.25 μM, and 62 μM. Cell survival was expressed as a percentage of control, and loss of viability was calculated as (1 – [colonies from Hst 5-treated cells ÷ colonies from control cells]) × 100. At higher concentrations of Hst 5, there was no difference in killing of planktonic cells and cells resuspended from either Lucitone or Ivocap acrylic biofilms (p > 0.05).
Pre-coating acrylic with Hst 5, hBD-3, or chlorhexidine gluconate alters C. albicans biofilm formation
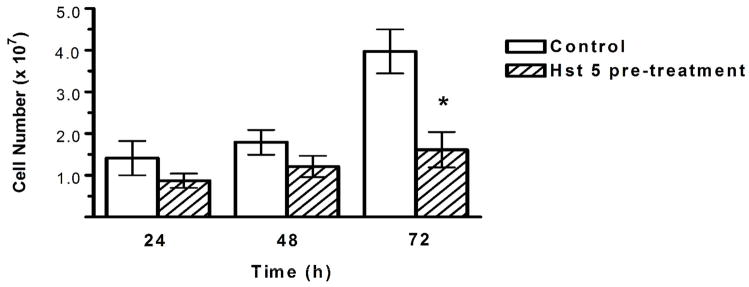
In order to evaluate the effect of denture acrylic surface treatment on C. albicans biofilm growth, assays were performed following pre-coating acrylic with various concentrations of peptides (Hst 5 or hBD-3) or chlorhexidine. Biofilm cell numbers were measured at 24, 48, and 72 h of growth and compared to a control biofilm grown on non-treated acrylic. Hst 5 inhibited biofilm development of C. albicans at later stages of growth (Figure 5). The mean cell number on control disks (without Hst 5 pre-coating) was 1.4 ± 0.4 × 107 at 24 h; 1.8 ± 0.3 × 107 at 48 h; and 4.0 ± 0.5 × 107 at 72 h, while the mean cell number on acrylic disks pre-coated with 50 μM Hst 5 was 8.7 ± 1.7 × 106 at 24 h; 1.2 ± 0.4 × 107 at 48 h; and 1.6 ± 0.4 × 107 at 72 h. This reduction in cell number was significantly lower at 72 h of growth (p = 0.011).
Fig. 5.
C. albicans biofilm cell numbers on acrylic with and without Hst 5 surface pre-treatment. Surface treatment occurred prior to inoculation of acrylic with C. albicans (106 cells/ml). Biofilms incubated for 24, 48, or 72 h at 37 °C. Cells were quantified with phase-contrast microscopy. Mean cell numbers and standard error are reported. Biofilm cell numbers were significantly lower on treated acrylic after 72 h of growth (p = 0.011).
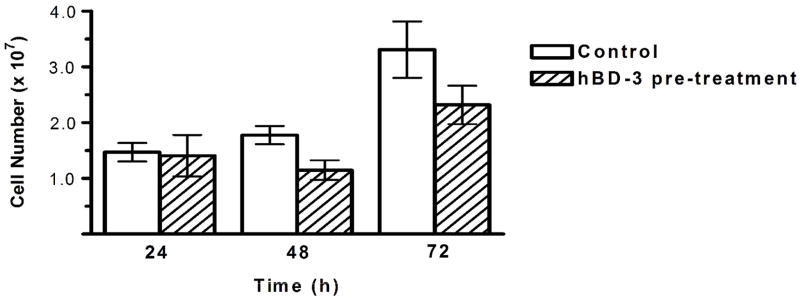
Acrylic pre-coated with hBD-3 resulted in a slight inhibition of biofilm development of C. albicans (Figure 6). The mean cell number on control disks (without hBD-3 pre-coating) was 1.5 ± 0.2 × 107 at 24 h; 1.8 ± 0.2 × 107 at 48 h; and 3.3 ± 0.5 × 107 at 72 h compared with acrylic disks pre-coated with 0.6 μM hBD-3 (1.4 ± 0.4 × 107 at 24 h; 1.1 ± 0.2 × 107 at 48 h; and 2.3 ± 0.3 × 107 at 72 h). The difference in biofilm cell number did not reach significance at any time interval (p = 0.06 at 48 h and p = 0.09 at 72 h). Increasing the concentration of hBD-3 to 1.2 μM did not result in any significant reduction in cell number (data not shown).
Fig. 6.
C. albicans biofilm cell numbers on acrylic with and without hBD-3 surface pre-treatment. Surface treatment occurred prior to inoculation of acrylic with C. albicans (106 cells/ml). Biofilms incubated for 24, 48, or 72 h at 37 °C. Cells were quantified with phase-contrast microscopy. Mean cell numbers and standard error are reported. There was no significant reduction in cell number at any time interval (p = 0.06 at 48 h and p = 0.09 at 72 h).
In contrast to defensin results, acrylic pre-coated with 0.12% chlorhexidine gluconate substantially inhibited biofilm development of C. albicans (Figure 7). The mean cell number was reduced by at least one log on Ivocap acrylic pre-coated with chlorhexidine (1.2 ± 0.2 × 106 at 24 h; 1.4 ± 0.5 × 106 at 48 h; and 2.9 ± 0.9 × 106 at 72 h) or Lucitone acrylic pre-coated with chlorhexidine (1.5 ± 0.2 × 106 at 24 h; 1.3 ± 0.4 × 106 at 48 h; and 2.2 ± 0.5 × 106 at 72 h) compared with control disks without chlorhexidine (1.1 ± 0.1 × 107 at 24 h; 2.0 ± 0.2 × 107 at 48 h; and 3.4 ± 0.3 × 107 at 72 h). The biofilm cell numbers on both Ivocap and Lucitone acrylic pre-coated with 0.12% chlorhexidine gluconate were significantly lower than uncoated acrylic at all time intervals (p < 0.05).
Fig. 7.
C. albicans biofilm cell numbers on acrylic with and without chlorhexidine surface pre-treatment. Surface treatment occurred prior to inoculation of acrylic with C. albicans (106 cells/ml). Biofilms incubated for 24, 48, or 72 h at 37 °C. Cells were quantified with phase-contrast microscopy. Mean cell numbers and standard error are reported. Biofilm cell numbers on Ivocap and Lucitone acrylic pre-treated with chlorhexidine were significantly lower than control acrylic at all time intervals (p < 0.05).
In summary, no significant reduction in C. albicans biofilm cell development was observed by pre-coating acrylic with hBD-3. However, significant reduction in biofilm cell number was achieved with 50 μM Hst 5 after 72 h and pre-coating acrylic with 0.12% chlorhexidine resulted in a significant reduction in biofilm growth over the entire 72 h time course.
DISCUSSION
In the present study, we used a simplified protocol for quantifying candidacidal activity and biofilm cell number that could readily be incorporated into a clinical investigation. The biofilm growth model simulated in vivo conditions of static biofilm growth found at the tissue-contacting surface of a denture. Static growth conditions create a relevant model for denture biofilm studies since these biofilms are well contained and protected from the rest of the oral environment by the denture. In an effort to simulate clinical conditions, acrylic disks were processed and handled using customary techniques for fabricating dentures in a modern dental laboratory. The surface finish of the acrylic disks was maintained as-processed, which simulates the tissue-contacting surface of a denture. We initially evaluated C. albicans biofilm growth on Ivocap or Lucitone denture acrylic that have different chemical structures and perhaps different manufacturing processes. We found that both Ivocap and Lucitone supported equal biofilm growth after coating with saliva, suggesting that chemical differences among acrylics are minimal once coated with saliva.
Several studies have shown that saliva promotes adhesion of C. albicans to denture acrylic.5,8,32 Like other surfaces in the oral cavity, denture acrylic becomes coated with a salivary pellicle consisting of α-amylase, high-molecular-weight mucins, lysozyme, and s-IgA.33 that may serve as receptors for the adhesion of C. albicans. Saliva may also facilitate the diffusion of nutrients into a biofilm system, promoting biofilm growth. Our studies found that disks conditioned with saliva for 30 min prior to inoculation had a significantly greater C. albicans biofilm growth than unconditioned disks, confirming the role salivary components have not only on initial adherence but also for subsequent biofilm development. In addition to promoting cell adhesion, conditioning the acrylic with saliva more closely simulates clinical conditions.
Antimicrobial resistance is a common phenomenon in cells recovered from biofilms. C. albicans cells resuspended from a mature biofilm maintained fluconazole resistance even after the biofilm had been disrupted.34 Similarly, amphotericin B resistance was observed even following resuspension and regrowth of C. albicans biofilm cells.9 In contrast to such drug resistance, we found that Hst 5 retained full candidacidal activity against C. albicans recovered from biofilm growth. Thus Hst 5 maintains high potency against biofilm phase C. albicans and suggests a potential role for this peptide as a topical therapeutic agent in the management of denture stomatitis. Interestingly, Hst 5 pretreatment of acrylic disks did not result in a significant reduction of biofilm development until 72 h, suggesting that Hst 5 is effective in reducing growth of C. albicans biofilms on denture acrylic during later stages of development, but has little effect on initial stages of growth. This finding is in agreement our previous work35 as well as that of others36 in which there was no difference in initial attachment of C. albicans to surface-modified acrylic; however, a significant decrease in colonization of C. albicans occurred at later stages of growth. This is consistent with the known functions of Hst 5 as a direct fungicidal peptide rather than a pellicle component that influences adhesion of C. albicans.
Surprisingly, disks pre-coated with hBD-3, which is a potent fungicidal peptide with about 10-fold higher activity than Hst 5, did not decrease biofilm formation at any time point. Two possible explanations for this lack of effect are that 1) adsorption to the salivary pellicle may prevent interactions with C. albicans by not favoring its release or 2) hBD-3 may adhere poorly to salivary pellicles or be degraded by candidal or salivary enzymes. Further studies are needed to determine why hBD-3 has such poor activity in this model.
In contrast, surface treatment by chlorhexidine was highly effective in reducing both C. albicans adhesion and growth when applied directly to the surface of acrylic, in agreement with previous studies.29,30 This is likely due to the ability of chlorhexidine to adhere to salivary components in pellicle (and being slowly released) in addition to its antifungal activity.25–27 The reduction in biofilm growth and adhesion supports the role of chlorhexidine as a potential therapeutic agent in the prevention and treatment of denture stomatitis, however the sensitivity of other strains of C. albicans to such surface treatment requires further evaluation.
We show in this study that pre-coating acrylic with Hst 5 and chlorhexidine has potential for reducing biofilm development of C. albicans in a model of denture stomatitis. Given that resistant strains of C. albicans are becoming increasingly problematic, there are important benefits to developing therapeutic applications of Hst 5. They are nontoxic to human cells and are effective against drug-resistant strains of C albicans. Further in vitro and in vivo research is needed to develop Hst 5 as a potential therapeutic peptide for the treatment of denture stomatitis.
Acknowledgments
This work was supported by DE10641 (to ME) from the National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen I. Denture stomatitis. Occurance and distribution of fungi. Acta Odontol Scand. 1974;32:329–33. doi: 10.3109/00016357409002556. [DOI] [PubMed] [Google Scholar]
- 2.Budtz-Jørgensen E. Denture stomatitis V. Candida agglutinins in human sera. Acta Odontol Scand. 1972;30:313–25. doi: 10.3109/00016357209004599. [DOI] [PubMed] [Google Scholar]
- 3.Corbet EF, Holmgren CJ, Philipsen HP. Oral mucosal lesions in 65–74-year-old Hong Kong Chinese. Community Dent Oral Epidemiol. 1994;22:392–5. doi: 10.1111/j.1600-0528.1994.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 4.Budtz-Jørgensen E. The significance of Candida albicans in denture stomatitis. Scand J Dent Res. 1974;82:151–90. doi: 10.1111/j.1600-0722.1974.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 5.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriology. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport JC. The oral distribution of Candida in denture stomatitis. Br Dent J. 1970;129:151–6. doi: 10.1038/sj.bdj.4802540. [DOI] [PubMed] [Google Scholar]
- 7.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–8. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 9.Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–5. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–46. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7. [PubMed] [Google Scholar]
- 12.Xu T, Levitz SM, Diamond RD, Oppenheim FG. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–54. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raj PA, Edgerton M, Levine MJ. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–905. [PubMed] [Google Scholar]
- 14.Tsai H, Bobek LA. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2224–8. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XS, Reddy MS, Baev D, Edgerton M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem. 2003;278:28553–61. doi: 10.1074/jbc.M300680200. [DOI] [PubMed] [Google Scholar]
- 16.Koshlukova SE, Lloyd TL, Araujo MW, Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J Biol Chem. 1999;274:18872–9. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 17.Baev D, Rivetta A, Vylkova S, Sun JN, Zeng GF, Slayman CL, Edgerton M. The TRK1 potassium transporter is the critical effector for killing of Candida albicans by the cationic protein, Histatin 5. J Biol Chem. 2004;279:55060–72. doi: 10.1074/jbc.M411031200. [DOI] [PubMed] [Google Scholar]
- 18.Edgerton M, Koshlukova S, Lo T, Chrzan B, Straubinger R, Raj PA. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–47. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 19.Joly S, Maze C, McCray PB, Guthmiller JM. Human β-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, Tack BF, McCray PB., Jr Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–5. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–50. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 22.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 23.Vylkova S, Li XS, Berner JC, Edgerton M. Distinct antifungal mechanisms: β-defensins require Candida albicans Ssa protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother. 2006;50:324–31. doi: 10.1128/AAC.50.1.324-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem AM, Adams D, Newman HN, Rawle LW. Antimicrobial properties of 2 aliphatic amines and chlorhexidine in vitro and in saliva. J Clin Periodontol. 1987;14:44–7. doi: 10.1111/j.1600-051x.1987.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 25.Lal K, Santarpia RP, 3rd, Pollock JJ, Renner RP. Assessment of antimicrobial treatment of denture stomatitis using an in vivo replica model system: therapeutic efficacy of an oral rinse. J Prosth Dent. 1992;67:72–7. doi: 10.1016/0022-3913(92)90053-d. [DOI] [PubMed] [Google Scholar]
- 26.MacNeill S, Rindler E, Walker A, Brown AR, Cobb CM. Effects of tetracycline hydrochloride and chlorhexidine gluconate on Candida albicans. An in vitro study. J Clin Periodontol. 1997;24:753–60. doi: 10.1111/j.1600-051x.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 27.Giuliana G, Pizzo G, Milici ME, Giangreco R. In vitro activities of antimicrobial agents against Candida species. [erratum appears in Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:524,] Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:44–9. doi: 10.1016/s1079-2104(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 28.Hamers AD, Shay K, Hahn BL, Sohnle PG. Use of a microtiter plate assay to detect the rate of killing of adherent Candida albicans by antifungal agents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:44–9. doi: 10.1016/s1079-2104(96)80146-6. [DOI] [PubMed] [Google Scholar]
- 29.McCourtie J, MacFarlane TW, Samaranayake LP. A comparison of the effects of chlorhexidine gluconate, amphotericin B and nystatin on the adherence of Candida species to denture acrylic. J Antimicrob Chemother. 1986;17:575–83. doi: 10.1093/jac/17.5.575. [DOI] [PubMed] [Google Scholar]
- 30.Spiechowicz E, Santarpia RP, 3rd, Pollock JJ, Renner RP. In vitro study on the inhibiting effect of different agents on the growth of Candida albicans on acrylic resin surfaces. Quintessence Int. 1990;2:35–40. [PubMed] [Google Scholar]
- 31.Bonesvoll P, Lokken P, Rolla G, Paus PN. Retention of chlorhexidine in the human oral cavity after mouth rinses. Arch of Oral Biol. 1974;19:209–12. doi: 10.1016/0003-9969(74)90263-5. [DOI] [PubMed] [Google Scholar]
- 32.Edgerton M, Scannapieco FA, Levine MJ. Human submandibular-sublingual saliva promotes adhesion of Candida albicans to polymethylmethacrylate. Infect Immun. 1993;61:2644–52. doi: 10.1128/iai.61.6.2644-2652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 34.Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 35.Edgerton M, Raj PA, Levine MJ. Surface modified poly methylmethacrylate enhances adsorption and retains anticandidal activities of salivary histatin 5. J Biomed Mat Res. 1995;29:1277–86. doi: 10.1002/jbm.820291015. [DOI] [PubMed] [Google Scholar]
- 36.Yoshinari M, Kato T, Matsuzaka K, Hayakawa T, Inoue T, Oda Y, Okuda K, Shimono M. Adsorption behavior of antimicrobial peptide histatin 5 on PMMA. J Biomed Mater Res Part B Appl Biomater. 2006;77:47–54. doi: 10.1002/jbm.b.30393. [DOI] [PubMed] [Google Scholar]