Abstract
The manner in which Ca2+-sensitive signaling proteins are activated in contracting cardiomyocytes is an intriguing theoretical problem given that the cytoplasm is continually bathed with systolic Ca2+ concentrations that should maximally activate most Ca2+ sensitive signaling kinases and phosphatases. Store-operated Ca2+ entry, partially attributed to transient receptor potential (TRP) proteins, can mediate activation of the Ca2+-sensitive phosphatase calcineurin in non-excitable cells. Here we investigated the gain-of-function phenotype associated with TRPC3 expression in the mouse heart using transgenesis to examine the potential role of store-operated Ca2+ entry in regulating cardiac calcineurin activation and ensuing hypertrophy/myopathy. Adult myocytes isolated from TRPC3 transgenic mice showed abundant store-operated Ca2+ entry that was inhibited with SKF96365 but not verapamil or KB-R7943. Associated with this induction in store-operated Ca2+ entry, TRPC3 transgenic mice showed increased calcineurin-nuclear factor of activated T cells (NFAT) activation in vivo, cardiomyopathy, and increased hypertrophy following neuro-endocrine agonist or pressure overload stimulation. The cardiomyopathic phenotype and increased hypertrophy following pressure overload stimulation was blocked by targeted disruption of the calcineurin Aβ gene. Thus, enhanced store-operated Ca2+ entry in the heart can regulate calcineurin-NFAT signaling in vivo, which could secondarily impact the hypertrophic response and cardiomyopathy.
Keywords: Signaling, Calcium, Store-operated, NFAT
INTRODUCTION
Calcineurin is a highly conserved serine-threonine protein phosphatase that exists as a heterotrimer of a 57−61 kD catalytic A subunit (CnA) and two small EF hand-containing Ca2+ binding proteins, calcineurin B (CnB) and calmodulin (1). CnB contains four EF-hand Ca2+-binding motifs and it tightly binds CnA at sub-micromolar concentrations of Ca2+. At low Ca2+ concentrations, calmodulin is not bound to the complex, and an autoinhibitory domain within the C-terminus of CnA sterically blocks the active site. As Ca2+ concentration elevates, it binds the EF-hand motifs of calmodulin, facilitating greater interaction with CnA and a conformational shift that displaces the autoinhibitory domain permitting calcineurin activation (1). Once activated, calcineurin regulates development and stress adaptation of multiple organ systems in mammals (2-5). While higher organisms have a number of confirmed targets for this phosphatase, many of the biological effects of calcineurin are mediated through two classes of transcriptional effectors, nuclear factor of activated T cells (NFAT) and myocyte enhancer factor 2. For example, in the heart calcineurin functions as a potent regulator of cardiac hypertrophy through activation of NFAT transcription factors to mediate the growth response (6).
Activation of calcineurin-NFAT requires a unique mechanism of Ca2+ alteration within most cells such that intracellular Ca2+ concentration is dramatically increased over a significant period of time (7-10). For example, engagement of immune cell receptors leads to sustained Ca2+ entry in the form of ICRAC, a type of store-operated Ca2+ entry, and this sustained Ca2+ entry mechanism is necessary for NFAT nuclear localization/activation and proper cytokine expression (7-13). The current known as ICRAC is hypothesized to mediate store-operated Ca2+ entry, in part, through canonical members of the transient receptor proteins (TRP) of the C class, or TRPC. Drosophila melanogaster carrying trp mutations generate only a transient photoreceptor depolarization in response to sustained light signals, suggesting a function as a store-operated Ca2+ entry channel (14). Since this initial description, a large superfamily of TRP-homologous channels has been elucidated in mammalian species (15), some of which are prominently expressed in the heart (16,17). In response to agonist stimulation resulting in the generation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3), Ca2+ is released and eventually depleted from the endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR). One hypothesis is that TRPC activity is directly activated following IP3 and DAG signals in the sensing of ER/SR Ca2+ depletion, thus inducing Ca2+ entry to replete internal stores (18). That such a mechanism exists in cardiomyocytes is uncertain given that essentially all Ca2+ exchange during each contractile cycle can be attributed to other channels and pumps associated with excitation-contraction coupling (19).
Recent studies have demonstrated the existence of a store depletion-sensitive Ca2+ entry mechanism in both neonatal and adult rat cardiomyocytes. Treatment of adult cells with IP3 or IP3-generating agonists and agents that block ER Ca2+ reuptake resulted in store depletion and induced extracellular Ca2+ influx sensitive to pharmacologic inhibitors of store-operated Ca2+ entry, but not L-type Ca2+ current (20,21). In addition, store-operated Ca2+ entry in neonatal cardiomyocytes was associated with NFAT nuclear localization and hypertrophy, while L-type channel inhibitors had no effect on these processes (21). More recently, store-operated calcium entry was shown to partially regulate SR Ca2+ homeostasis in neonatal rabbit ventricular myocytes (22). Consistent with these observations, store-operated Ca2+ entry also plays an important role in maintaining internal contractile Ca2+ levels in smooth muscle cells (23). Here we generated lines of cardiac-specific TRPC3 expressing transgenic mice to evaluate the potential association between store-operated Ca2+ entry and calcineurin-NFAT activation and cardiac hypertrophy in vivo. TRPC3 transgenic mice showed a prominent increase in cardiac NFAT transcriptional activity that was associated with, but preceded mild cardiac hypertrophy or lethal cardiomyopathy. Moreover, TRPC3 transgenic mice showed a dosage-dependent enhancement in cardiac hypertrophy following neuroendocrine, as well as enhanced hypertrophy following pressure overload stimulation, which was ameliorated by calcineurin Aβ gene targeting. Our results are the first to suggest that TRPC proteins have the ability to regulate calcineurin signaling and the hypertrophic growth of the myocardium in vivo.
MATERIALS AND METHODS
Generation of Transgenic Mice
A cDNA encoding human TRPC3 (gift from Lutz Birnbaumer, NIH, Research Triangle Park, North Carolina, USA) was subcloned into the murine α-myosin heavy chain (α-MHC) promoter expression vector (gift from Jeffrey Robbins, Children's Hospital, Cincinnati, Ohio, USA). The α-MHC-TRPC3 transgene was injected into newly fertilized oocytes to generate transgenic mice (FVB/N background). NFAT-luciferase reporter transgenic mice and calcineurin Aβ gene—targeted mice were each described previously (24,25). Experiments involving animals were approved by the Institutional Animal Care and Use Committee.
Isolation of Adult Cardiomyocytes for Ca2+ Measurements
Cardiomyocytes were isolated for analysis, and only Ca2+-tolerant cells with clear cross striations and without spontaneous contractions or significant granulation were selected for experimental studies. In brief, the heart was rapidly excised and placed in Tyrode solution containing (in mmol/l): 120 NaCl, 5.4 KCl, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, 1.6 MgCl2, 10 2,3-butanedione monoxime (BDM), and 5 taurine (buffer A), gassed with 95% O2−5% CO2. All solutions were filtered and equilibrated with 95% O2−5% CO2 for at least 20 min before use. The heart was retrogradely perfused with buffer A for 4−5 min and then with buffer A containing 1 mg/ml collagenase Type II (Worthington) and 0.08 mg/ml protease Type XIV (Sigma) at 37°C. After 2 min of enzyme perfusion, 50 μM Ca2+ was added to the enzyme solution. When the heart became “swollen and soft” after ∼5 min of digestion, the enzyme was re-circulated. The heart was perfused for an additional 8−12 min or until flow rate surpassed pre-enzyme flow rate. After perfusion, the ventricles were separated from the atria and minced. Myocytes were incubated with 2 μmol/l Fura-2 AM (Molecular Probes) for 30−35 min in buffer C (buffer A with 1 mmol/l Ca2+ without BDM) at room temperature. After being loaded, the cells were washed and resuspended in buffer C. After 3 washes (40 min), the cell suspension was placed in a plexiglas chamber, containing 500 μl of fresh buffer C. The fura-2 fluorescence ratio was determined at room temperature using a Delta Scan dual-bean spectrofluorophotometer (Photon Technology), operating at an emission wavelength of 510 nm with excitation wavelengths of 340 and 380 nm. The stimulating frequency for Ca2+ transient measurements was 0.5 Hz. Baseline, amplitude (estimated by 340/380 nm ratio), of the Ca2+ signal was acquired and the data were analyzed using software from Felix 1.1 Software and IonWizard (IonOptix). Adult myocytes were pretreated with 1 μM Ang II and the reversible sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor CPA (cyclopiazonic acid, 5 μM) for 9−10 minutes in Ca2+ free buffer to achieve more complete SR store depletion. Myocytes were then given buffer with an extracellular Ca2+ of 1.8 mM to assay for store-operated entry measured as a sustained increase in fura-2 fluorescence (measured as the 340/380 ratio) 4−5 minutes after Ca2+ addition. In some experiments verapamil (10 μM) or KB-R7943 (5 μM) was added to the 1.8 mM solution to abolish [Ca2+]i spikes and L-type calcium entry, or the possibility of Na+/Ca2+ activity, but this did not alter the sustained increase in the fura-2 ratio in a small percentage of wiltype cells. SKF 96365 was used at 5 μM, which inhibits store-operated Ca2+ entry, although its cross-reactivity with other channels has not been proven so that its true specificity is uncertain.
Generation of Purified Adult Cardiomyocytes for Western blotting
Wildtype and TRPC3 transgenic adult mouse hearts (2 months age) were dissected, washed in ice-cold cannulation buffer (10 mM 2,3-butanedione monoxime, 25 μM CaCl2 in MEM) and perfused with digestion media (1 mg/ml BSA, 90 units/ml collagenase, 10 mM 2,3-butanedione monoxime, 25 μM CaCl2 in MEM) until the myocardium began to visually dissolve. Perfused hearts were then dissociated in a dish with mechanical disruption and filtered through a 200 μM mesh into MEM containing 10 mM 2,3-butanedione monoxime and 10 μM CaCl2 until individual cells were generated. Cells were collected by centrifugation and differentially plated to remove any residual fibroblasts, after which the cardiac myocytes were collected for western blotting.
Western Blot Analysis
Ventricle samples from mouse hearts were frozen in liquid nitrogen and stored at −70 °C. Protein samples for membrane-rich fractions were prepared from heart tissue using extraction buffer (250 mM sucrose, 10 mM Tris-HCl pH 7.5, 1 mM DTT and protease inhibitors Complete EDTA-free, Roche) as described previously (26). In brief, heart samples were homogenized in extraction buffer and homogenates were centrifuged at 3000 X g for 5 min at 4°C and the resultant supernatant was centrifuged at 8000 X g for 30 min at 4°C. The pellets were resuspended in extraction buffer and stored at −70 °C until use. Western blotting conditions were described previously (24,25). Whole cell protein homogenates were extracted from adult cardiomyocytes in cell lysis buffer (20 mM sodium phosphate, pH 7.0, 150 mM NaCl, 2 mM MgCl2, 10 mM NaF, 0.1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM dithiothreitol, 1% Nonidet P-40, 10% glycerol, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, 10 μg of N-α-tosylphenylalanyl chloromethyl ketone (TPCK) per ml, 10 μg of N-α-tosyllysyl chloromethyl ketone (TLCK) per ml). Forty micrograms of protein was loaded in each lane of all western blots. Antibodies included TRPC1, 4, 5, 6, 3/6/7 (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) TRPC3 (Alomone Labs, Jerusalem, Israel), and GAPDH (Research Diagnostics Inc., Flanders, New Jersey, USA). Isoform specific calcineurin Aα and Aβ rabbit polyclonal antibodies were custom synthesized in rabbits and affinity purified by Zymed Laboratories.
Echocardiography and Surgical Models
Mice from all genotypes or treatment groups were anesthetized with isoflurane and echocardiography was performed using a Hewlett Packard 5500 instrument with a 15-MHz microprobe. Echocardiographic measurements were taken on M-mode in triplicate from at least four separate mice per group. The investigator was blinded to the identity of the mice for analysis. Two month-old non-transgenic and transgenic mice were subjected to transverse aortic constriction (TAC) as described previously (24). Alzet miniosmotic pumps (no. 2002; Alza Corp., Mountain View, California, USA) containing a mixture of phenylephrine (PE) (50 mg/kg/day) and angiotensin II (Ang II) (432 μg/kg/day), or isoproterenol (60 mg/kg/day), or phosphate buffered saline (vehicle control) were surgically inserted dorsally and subcutaneously in two-month-old mice under isoflurane anesthesia. All mice were sacrificed 2 weeks after the procedure.
Reporter assays in mouse hearts
Luciferase reporter assays from mouse hearts were performed as previously described (24). In brief, TRPC3 transgenic mice were crossbred with NFAT-luciferase reporter mice and ventricles were excised from double and single transgenic mice and stored at −70°C. The frozen hearts were homogenized in 1 ml luciferase assay buffer (100 mM KPO4, pH 7.8, 0.5% NP-40 and 1 mM DTT). Homogenates were centrifuged at 3000 X g for 10 min at 4 °C and the supernatants assayed for luciferase activity as described previously (24).
Histological and Hypertrophic Marker Gene Analyses
Hearts were collected at the indicated times, fixed in 10% formalin containing phosphate buffered saline, and embedded in paraffin. Serial 7-μm heart sections from each group were analyzed. Samples were stained with H&E, Masson's trichrome, or wheat germ agglutinin-TRITC conjugate at 50 μg/ml to accurately identify sarcolemmal membranes so that myofiber diameter could be quantified. Reverse transcriptasepolymerase chain reaction (RT-PCR) for atrial natriuretic factor (ANF) and control L7 mRNA was performed as previously described (27).
Statistical Analysis
The results are presented as means plus or minus SEM. Data analyses were performed using InStat 3.0 software (GraphPad Software for Science Inc., San Diego, California, USA) or by ANOVA.
RESULTS
Generation of TRPC3 transgenic mice
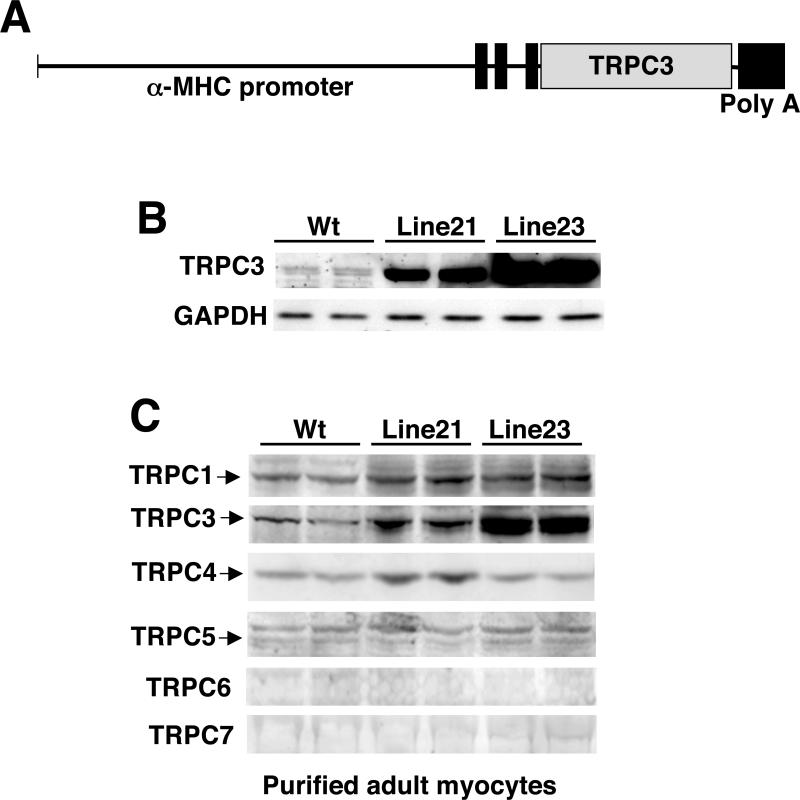
While store-operated Ca2+ entry, which is partially associated with TRPC proteins, has been identified in both adult and neonatal rat cardiomyocytes in culture, the relevance to the in tact heart is unknown. To elucidate the ability of TRPC-regulated Ca2+ entry to alter growth of the heart, or at least calcineurin-NFAT activation, a transgenic gain-of-function approach was performed. A cDNA encoding TRPC3 was fused to the mouse α-MHC promoter to generate cardiac-specific transgenic mice (Fig 1A). Two lines were generated that were characterized by low and high levels of expression by western blotting from total heart protein extracts from 2-month old mice (Fig 1B). Since the heart is comprised of many cell types, it was also of interest to survey which TRPC family members were expressed in adult ventricular myocytes, as well as to examine if overexpression of TRPC3 in cardiac myocytes might alter expression of other TRPC isoforms. To this end, ventricular myocytes were enzymatically disassociated and enriched from wildtype, line 21, and line 23 TRPC3 transgenic mice at 2-months of age for western blotting. TRPC1, 3, 4, and 5 were detectable and not altered in the TRPC3 transgenic line (expect for TRPC3 itself), while TRPC6 and 7 were not detectable (Fig 1C).
Figure 1.
Generation of TPRC3 transgenic mice. (A) Schematic of the cardiac transgene with the cardiac-specific α-MHC promoter fused to the TRPC3 cDNA. (B) Western blot for TRPC3 and GAPDH (control) protein from the hearts of wildtype (Wt) and line 21 and line 23 TRPC3 transgenic mice. (C) Western blot for the indicated proteins from enriched adult ventricular cardiac mycoytes from wildtype mice and the two TRPC3 transgenic lines. Brain extract was used as a positive migration control (not shown).
Analysis of store-operated Ca2+ entry in wildtype and TRPC3 mice
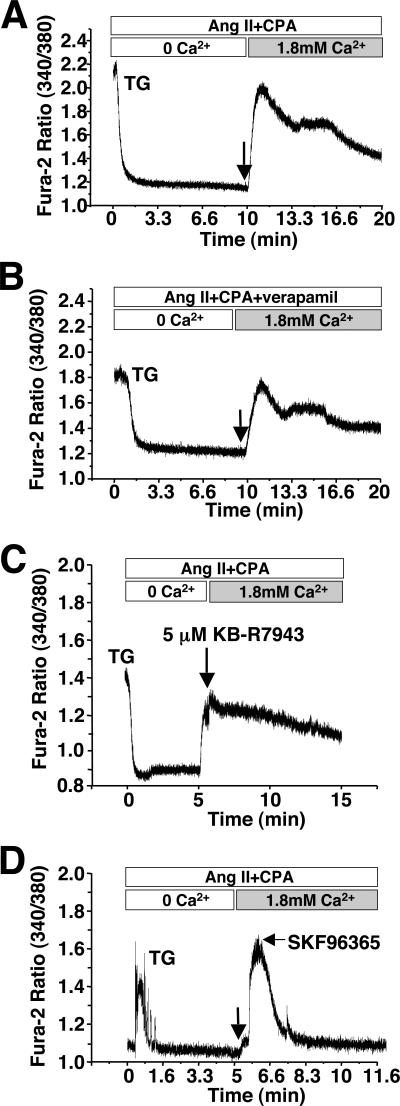
To evaluate the potential physiological relevance of TRPC3 overexpression in the mouse heart store-operated Ca2+ entry was analyzed from individual adult myocytes isolated from the hearts of 3−4 months old high-expressing transgenic and non-transgenic mice. While myocytes from adult rat hearts were previously shown to have store-operated Ca2+ entry (20), adult mouse myocytes were reported to be devoid of this activity (28). Fura-2 loaded adult myocytes from wildtype mice were prepared in Ca2+ free buffer and stimulated with Ang II in the presence of CPA, a SERCA inhibitor, to deplete internal Ca2+ stores. Ca2+ was then returned to the media and entry into the cell was monitored by a change in the fura-2 fluorescence absorbance ratio. Under these conditions, approximately 75% of adult mouse myocytes showed no store-operated Ca2+ entry, although approximately 25% of myocytes showed a very subtle but significant entry that could be blocked with SKF96365 (21,22), an inhibitor of store-operated Ca2+ entry (Fig 2A,B, and data not shown). This very subtle Ca2+ signal in a fraction of the myocytes was not blocked with the L-type Ca2+ channel inhibitor verapamil or the Na+/Ca2+ exchanger inhibitor KB-R7943 (Fig 2C, and data not shown).
Figure 2.

Analysis of store-operated Ca2+ entry in adult myocytes isolated from wiltype mice. (A) Representative fura-2 ratio fluorescence tracing from a wildtype adult cardiomyocyte that essentially showed no store-operated entry (left panel) and (B) one that showed a minimal but detectable response (right panel). The arrow indicates the timing of Ca2+ addition (1.8 mM). The baseline fura-2 fluorescence was subtracted from the peak fluorescence. Approximately 75% of the myocytes showed no Ca2+ entry while 25% showed a minimal, albeit, detectable signal. (C) Addition of the Na+/Ca2+ inhibitor KB-R7943 did not appreciably alter the ability to identify store-operated Ca2+ entry in a minor population of myocytes.
Robust store-operated Ca2+ entry was easily detectable in approximately 70% of adult myocytes isolated from high expressing TRPC3 transgenic mice (Fig 3A). The peak change in fura-2 fluorescence (340/380 ratio) averaged 0.401±0.042 in adult myocytes from TRPC3 transgenic mice (n=34 cells from 7 mice) and 0.065±0.010 in wildtype myocytes (n=44 cells from 8 mice). This store-operated Ca2+ entry observed in TRPC3 transgenic myocytes was not blocked with the L-type Ca2+ channel blocker verapamil (Fig 3B), or the Na+/Ca2+ exchanger inhibitor KB-R7943 (Fig 3C), but was completely inhibited with SKF96365 (Fig 3D). These data indicate that TRPC3 overexpression in the mouse heart substantially enhances store-operated Ca2+ entry. Finally, the potential effect of TRPC3 overexpression on the Ca2+ transient itself was also assessed in adult ventricular myocytes isolated with wildtype and TRPC3 transgenic mice. Remarkably, the peak amplitude of the Ca2+ transient was significantly increased in TRPC3 transgenic myocytes compared with wildtype myocytes, although baseline fura-2 readings, the time to 70% decay in the Ca2+ transient, and the time constant were not altered (Table 1, and data not shown). The increase in Ca2+ transient amplitude was unexpected, but may be related to greater sarcolemmal Ca2+ influx, or “membrane leak”, causing enhanced SR loading, consistent with a recent description suggesting that store-operated Ca2+ entry could influence SR Ca2+ content in neonatal rabbit ventricular myocytes (22).
Figure 3.
Analysis of store-operated Ca2+ entry in adult myocytes isolated from TRPC3 transgenic mice. (A) Representative fura-2 ratio fluorescence tracing from a TRPC3 transgenic adult cardiomyocyte. The arrow indicates the timing of Ca2+ addition (1.8 mM). The baseline fura-2 fluorescence was subtracted from the peak fluorescence. (B) Representative fura-2 ratio fluorescence tracing from a TRPC3 transgenic adult cardiomyocyte treated with verapamil or (C) KB-R7943. The arrow indicates the timing of Ca2+ addition (1.8 mM). (D) Representative fura-2 ratio fluorescence tracing from a TRPC3 transgenic adult cardiomyocyte treated with SKF96365 at the indicated time point (arrow).
Table 1.
Measurement of various indexes associated with the calcium transient in wildtype and TRPC3 transgenic myocytes.
| No. of cells/No. of mice | Diastolic Fura-2 ratio (Baseline) | Amplitude, 340/380 (Systolic-diastolic) | TRC 70%, ms | |
|---|---|---|---|---|
|
Wild-type |
75/7 |
1.51±0.022 |
0.67±0.026 |
0.69±0.010 |
| TRPC3 | 66/7 | 1.56±0.028 | 0.99±0.049** | 0.63±0.016** |
*p<0.05 vs. Wild-type
p<0.001
TRC=Time for 70% decay of the Ca2+ transient
Values are means±SEM
TRPC3 transgenic mice display cardiomyopathy
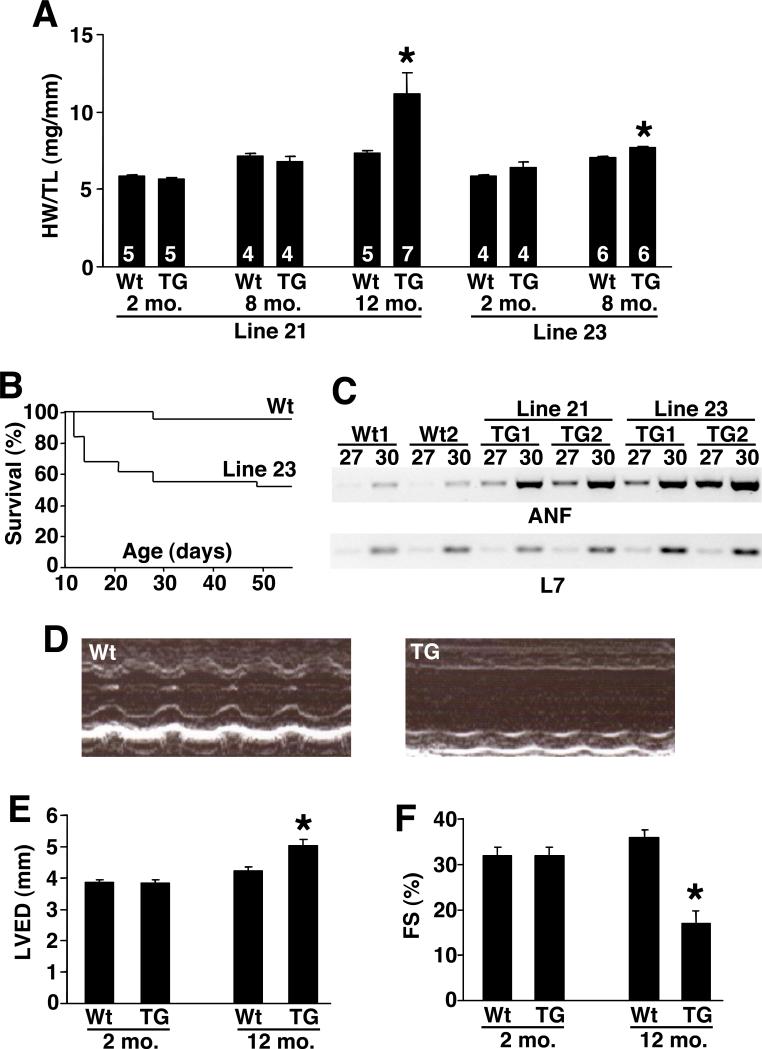
Low and high expressing TRPC3 transgenic mice were examined for signs of cardiac dysfunction or alterations in overall phenotype. Low expressing mice were essentially normal at 2 and 8 months of age with respect to overt hypertrophy as assessed by measurement of heart-weight normalized to tibial-length (Fig 4A). However, by 12 months of age low expressing TRPC3 transgenic mice showed cardiac hypertrophy. High expressing TRPC3 transgenic mice also showed almost no cardiac enlargement at 2 and 8 months of age, but nearly all succumbed to lethal cardiomyopathy by 12 months of age (Fig 4A,B). While the cause of lethality is unknown, we speculate that disturbed Ca2+ homeostasis induced by increased Ca2+ influx could cause arrhythmia and sudden death. Indeed, high expressing male transgenic mice showed nearly 50% lethality by 50 days of age (Fig 4B), while 9/34 female transgenic mice died by 8 weeks of age, suggesting a sex-dependent difference in lethality associated with TRPC3 overexpression. While both high and low TRPC3 transgenic mice had no hypertrophic enlargement at 2 and 8 months of age, cardiac ANF expression was significantly induced at 2 months of age, suggesting that the cardiac stress-response was evoked and preceded an overt hypertrophic phenotype (Fig 4C). To more carefully evaluate the affect of TRPC3 overexpression, echocardiography was performed in the low transgenic line at 12 months of age (Fig 4D,E,F). These TRPC3 transgenic mice showed a significant dilation of the left ventricle (Fig 4E) and a loss of ventricular performance as assessed by measurement of fractional shortening (Fig 4F). Thus, overexpression of TRPC3 in the heart generally leads to cardiomyopathy as mice age, but hypertrophy is only seen at later stages.
Figure 4.
Cardiac phenotype of TRPC3 transgenic mice. (A) Heart-weight normalized to tibial-length in wildtype (Wt) and lines 21 or 23 transgenic mice at the indicated times. *P<0.05 versus wt. (B) Survival analysis of high expressing line 23 TRPC3 male transgenic mice (N=31) versus wildtype male mice (N=49) over time. (C) RT-PCR for ANF and L7 (control) from the hearts of Wt or the two TRPC3 transgenic lines at 2 months of age. Either 27 or 30 cycles of amplification is shown. (D) M-mode representation of cardiac echocardiography from wildtype and low expressing TRPC3 transgenic line at 12 months of age. (E) Quantitation of left ventricular end diastolic dimension by echocardiography in wildtype and low expressing TRPC3 transgenic mice at 12 months of age. *P<0.05 versus wt. (F) Quantitation of fractional shortening (FS) by echocardiography in wildtype and low expressing TRPC3 transgenic mice at 12 months of age. *P<0.05 versus wt.
TRPC3 transgenic mice show activation of NFAT in the heart
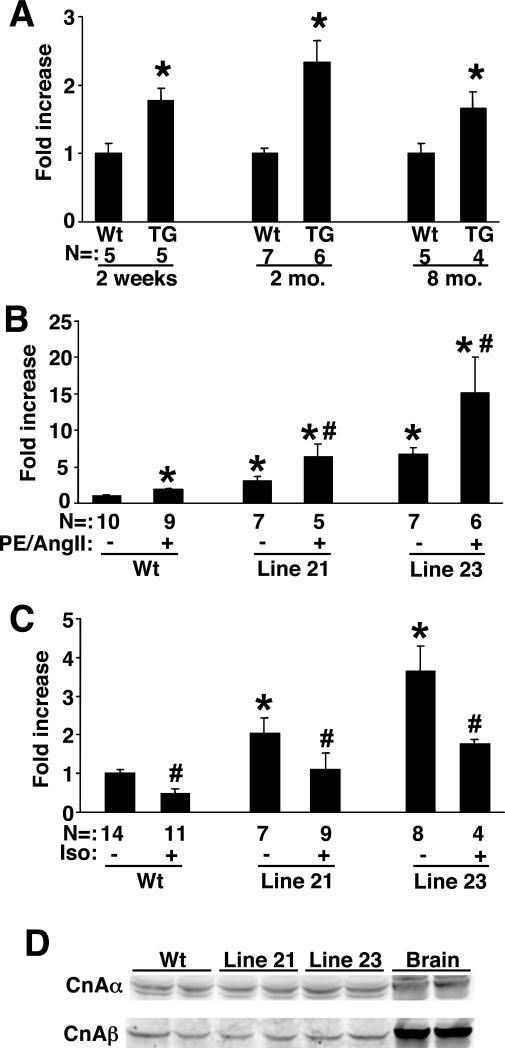
In non-excitable cells store-operated Ca2+ entry has been shown to regulate calcineurin-NFAT signaling, providing the necessary long-term increase in intracellular Ca2+ (7-13). Moreover, the source or microdomain of Ca2+ that regulates calcineurin-NFAT in the heart has not been determined, although store-operated entry has been suggested as a source (21). Here we investigated if TRPC3 overexpression would enhance calcineurin-NFAT signaling in the heart using NFAT-luciferase reporter transgenic mice (24). Low expressing TRPC3 transgenic mice showed a roughly 2-fold increase in cardiac NFAT-luciferase activity at 2 weeks, 2 months, and 8 months of age (Fig 5A). A similar profile of increased NFAT-luciferase activity was observed in high expressing TRPC3 transgenic mice. That NFAT-luciferase activity is increased as early as 2 weeks and 2 months of age, which significantly precedes any signs of cardiac pathology, suggests that TRPC3 overexpression proximally regulates NFAT activity. However, TRPC proteins are typically activated by depletion in stored Ca2+, or by phospholipase C (PLC)-inducing stimuli. Thus, we also investigated the ability of the G protein-coupled PLC agonists PE + Ang II to further upregulate NFAT-luciferase activity in the hearts of TRPC3 transgenic mice (Fig 5B). Indeed, both low and high lines of TRPC3 transgenic mice showed a greater increase in cardiac NFAT-luciferase transgene activity following 14 days of agonist stimulation in 2 month-old mice (Fig 5B). These results are consistent with the hypothesis that G protein-coupled receptor agonists that promote PLC activation and formation of IP3 and DAG, in turn promote greater TRPC channel activity and hence more NFAT activation. As a control, isoproterenol was also infused in TRPC3 transgenic mice, which functions as a β-adrenergic receptor agonist that signals independent of PLC. Remarkably, isoproterenol infusion did not augment NFAT-luciferase activity in the hearts of wildtype or TRPC3 transgenic mice (high or low line), but instead lead to significant inhibition (Fig 5C). As a final control, we also measured total calcineurin Aα and Aβ protein levels in the hearts of wildtype and TRPC3 transgenic mice, which showed no difference, suggesting that the alteration in NFAT-luciferase activity is not due to a secondary change in calcineurin A protein levels (Fig 5D).
Figure 5.
NFAT activation in TRPC3 transgenic mice. (A) Fold activation of luciferase activity from the hearts of NFAT-luciferase transgenic mice crossed with wildtype or low expressing TRPC3 transgenic mice. Hearts were collected at 2 weeks, 2 months, or 8 months. The number of mice analyzed in each group is shown in the figure. *P<0.05 versus corresponding wt. (B) Fold activation of luciferase activity from the hearts of NFAT-luciferase transgenic mice crossed with wildtype, low, or high expressing TRPC3 transgenic mice at baseline or after 14 days of PE + Ang II infusion. Hearts were collected at 2 months of age. The number of mice analyzed in each group is shown in the figure. *P<0.05 versus wt with PBS. #P<0.05 versus Wt with agonist. (C) Relative luciferase activity from hearts of NFAT-luciferase transgenic mice crossed with wildtype, low, or high expressing TRPC3 transgenic mice at baseline or after 14 days of isoproterenol (Iso) infusion. Hearts were collected at 2 months of age. The number of mice analyzed in each group is shown in the figure. *P<0.05 versus wt with PBS. #P<0.05 versus no Iso within each group. (D) Western blot for calcineurin Aα and Aβ from the hearts of the indicated mice, or brain as a control.
Agonist and pressure overload stimulation promote greater cardiac hypertrophy in TRPC3 transgenic mice
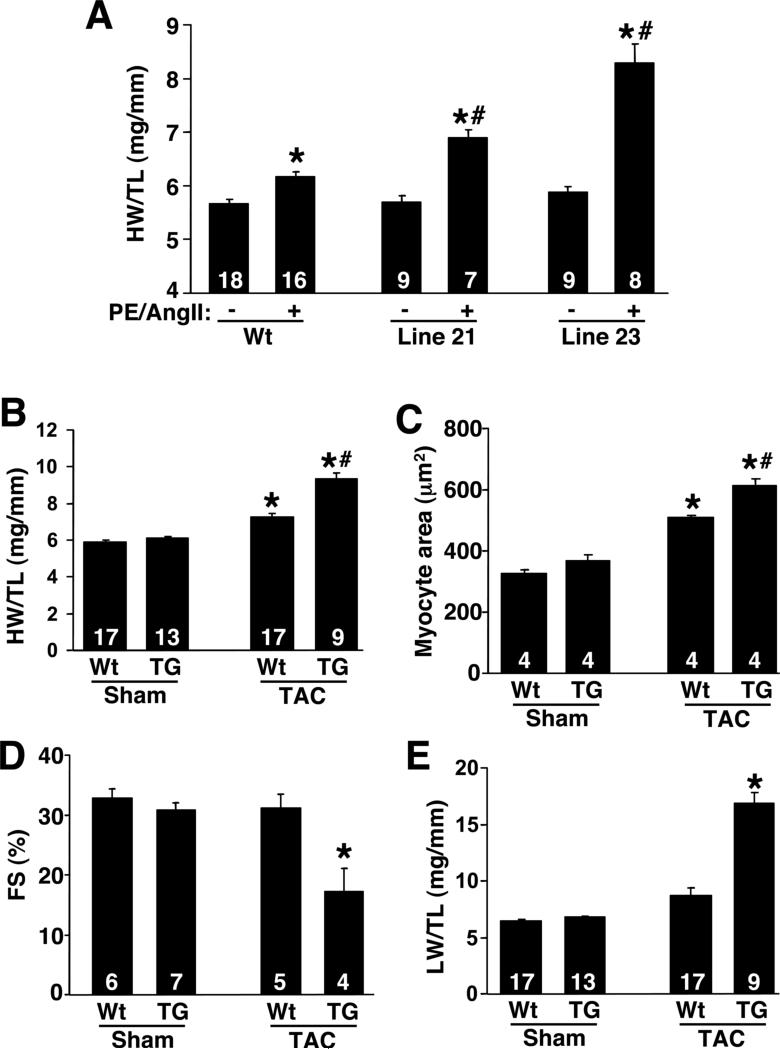
While baseline hypertrophy was only minimally altered by constitutive TRPC3 expression in the heart, it is possible that full store-operated Ca2+ entry through the overexpressed TRPC3 protein requires stimulation. Indeed, significantly greater NFAT-luciferase activity was observed in TRPC3 transgenic mice infused with PE + Ang II (Fig 5B). Associated with this greater increase in NFAT-luciferase activity was a corresponding augmentation in cardiac hypertrophy. Two month-old wildtype male mice showed a characteristic 15% increase in cardiac hypertrophy following agonist infusion (Fig 6A). However, low and high TRPC3 male transgenic mice showed significantly greater cardiac hypertrophy after 14 days of agonist infusion (Fig 6A). Thus, TRPC3 overexpression predisposed the heart to greater hypertrophy, in association with augmented NFAT-activity, suggesting a role for store-operated Ca2+ entry in this response.
Figure 6.
Analysis of propensity towards cardiac hypertrophy in TRPC3 transgenic mice. (A) Heart-weight normalized to tibial-length in wildtype and TRPC3 transgenic mice at 2 months of age subjected to 14 days of PE + Ang II infusion. The number of mice used in each cohort is shown in the bars. *P<0.05 versus no agonist, #P<0.05 versus wt with agonist. (B) Heart-weight normalized to tibial-length in 2 month-old wildtype or low expressing TRPC3 transgenic subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt sham, #P<0.05 versus wt TAC. (C) Histological analysis of cardiomyocyte cross-sectional areas in 2 month-old wildtype or low expressing TRPC3 transgenic subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt sham, #P<0.05 versus wt TAC. (D) Fractional shortening (FS) in 2 month-old wildtype or low expressing TRPC3 transgenic subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt TAC. (E) Lung-weight normalized to tibial-length in 2 month-old wildtype or low expressing TRPC3 transgenic subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt TAC.
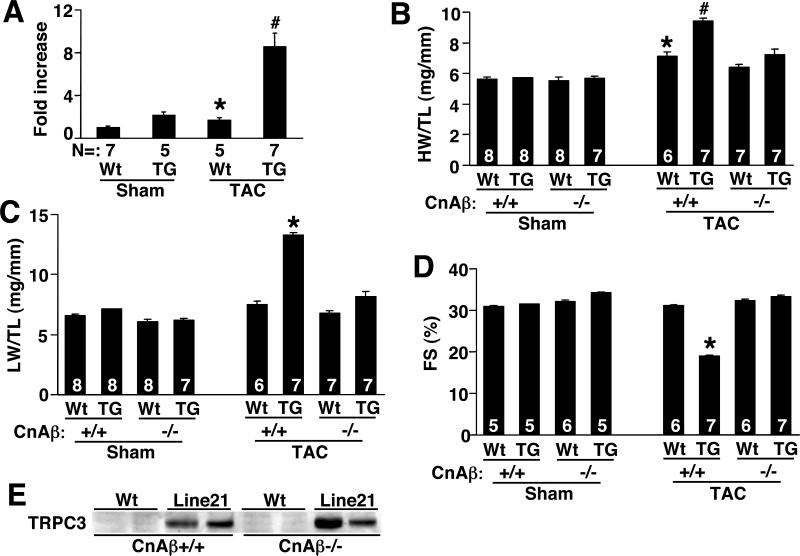
In addition to agonist infusion for 14 days, wildtype male and TPRC3 male transgenic mice were subjected to pressure overload stimulation to further assess the hypertrophic phenotype and its modulation by TRPC3 overexpression. Consistent with the agonist infusion studies, TRPC3 transgenic mice developed significantly greater cardiac hypertrophy compared with wildtype mice following 2 weeks of TAC stimulation (Fig 6B). Analysis of myocytes from heart ventricular histological sections also showed a significantly greater increase in cardiomyocyte hypertrophy in low expressing TRPC3 transgenic mice compared with wildtype mice following TAC stimulation (Fig 6C). Associated with this greater increase in cardiac hypertrophy observed in TRPC3 transgenic mice was an enhanced propensity towards heart failure. Specifically, TRPC3 transgenic mice showed a dramatic reduction in fractional shortening following 2 weeks of TAC stimulation, while wildtype mice were fully compensated during this time (Fig 6D). Moreover, low expressing TRPC3 transgenic mice showed increased lung-weight normalized to bodyweight following 2 weeks of TAC, suggesting pulmonary edema, while wildtype mice did not (Fig 6E). Thus, TRPC3 transgenic mice are predisposed to greater cardiac hypertrophy and failure following pressure overload stimulation, associated with greater calcineurin-NFAT activation. Indeed, TAC stimulation produced a synergistic increase in cardiac NFAT-luciferase activity in the hearts of low expressing TRPC3 transgenic mice (Fig 7A).
Figure 7.
Analysis of calcineurin-NFAT association with TRPC3-mediated hypertrophy. (A) Fold activation of luciferase activity from the hearts of NFAT-luciferase transgenic mice crossed with wildtype or low expressing TRPC3 transgenic mice that were subjected to a sham or TAC procedure for 14 days. The number of mice analyzed in each group is shown in the figure. *P<0.05 versus wt sham, #P<0.05 versus wt TAC. (B) Heart-weight normalized to tibial-length in 2 month-old wildtype or low expressing TRPC3 transgenic crossed into the calcineurin Aβ wildtype or null background. Mice were then subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt CnAβ+/+ sham, #P<0.05 versus wt CnAβ+/+ TAC. (C) Lung-weight normalized to tibial-length in 2 month-old wildtype or low expressing TRPC3 transgenic crossed into the calcineurin Aβ wildtype or null background. Mice were then subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt CnAβ+/+ TAC. (D) Fractional shortening (FS) in 2 month-old wildtype or low expressing TRPC3 transgenic crossed into the calcineurin Aβ wildtype or null background. Mice were then subjected to TAC or sham procedure for 14 days. The number of mice used in each cohort is shown in the bars. *P<0.05 versus Wt CnAβ+/+ TAC. (E) Control western blot for TRPC3 transgenic protein expression in the hearts of wildtype and calcineurin Aβ−/− mice.
Loss of calcineurin Aβ rescues TRPC3-dependent hypertrophy and heart failure
The data presented above suggest that TRPC3 overexpression leads to cardiomyopathy and augmented stimulus-induced hypertrophy in association with greater NFAT activity. To more carefully evaluate this association, we reasoned that loss of calcineurin Aβ might “short-circuit” the signaling process from TRPC3-to-Ca2+-to-calcineurin activation in the heart. Thus, low expressing TRPC3 transgenic mice were crossed into the calcineurin Aβ null background, or controls were generated from the same backcross that were wildtype for calcineurin Aβ (Fig 7B,C,D). As previously observed, calcineurin Aβ null mice were largely resistant to hypertrophy following 2 weeks of pressure overload stimulation (25) (Fig 7B). In contrast, wildtype mice showed a 25% increase in heart-weight normalized to tibial-length, and wildtype mice with the TRPC3 transgene showed a synergistic increase in hypertrophy (Fig 7B). However, loss of calcineurin Aβ blocked pressure overload-induced hypertrophy, and more importantly, essentially blocked the affect of the TRPC3 transgene on the hypertrophic response (Fig 7B). Loss of calcineurin Aβ also obviated heart failure in TPRC3 transgenic mice following 2 weeks of TAC, as assessed by measurement of lung-weight normalized to tibia-length and measurement of ventricular fractional shortening (Fig 7C,D). As a control, we also verified that loss of calcineurin Aβ did not alter the level of TRPC3 overexpression in the heart (Fig 7E). In conclusion, these results suggest that calcineurin could be involved in facilitating TRPC3-dependent hypertrophy and heart failure, although loss of calcineurin could also more globally disrupt the hypertrophic potential of the heart independent of a store-operated Ca2+ entry mechanism.
DISCUSSION
That store-operated Ca2+ entry and TRPC proteins might play a role in reactive Ca2+-signaling in the heart is controversial given the ability to account for nearly all Ca2+ cycling through established channels and pumps that mediate excitation-contraction coupling. For example, in response to depolarization Ca2+ first enters the sarcolemma through the voltage-dependent L-type Ca2+ channel, which directly stimulates underlying ryanodine receptors embedded within the SR to collectively increase intracellular Ca2+ concentration by more than 10-fold. During diastole, Ca2+ is removed from the cytoplasm by resequestration in the SR through the action of SERCA, as well as extrusion from the cell itself through the action of the Na+/Ca2+ exchanger within the sarcolemma. Thus, excitation-contraction-mediated Ca2+ cycling accounts for nearly all the Ca2+ alterations that occur within a cardiomyocyte, making it difficult to explain how Ca2+-activated signaling proteins function in this background. Indeed, it would make little sense to regulate calcineurin (or other Ca2+ sensitive signaling proteins) through changes in Ca2+ cycling associated with excitation-contraction coupling, since such a mechanism would not afford a disconnection between inotropy and signaling. For example, continuous maximal Ca2+ cycling due to phospholamban deletion in mice does not induce cardiac hypertrophy or otherwise predispose the heart to dysfunction, indicating that inotrophic drive associated with contractility regulation is not the source of Ca2+ for calcineurin activation (29). Despite this observation, contractile Ca2+ can regulate calcineruin-NFATc1 activation and calmodulin-dependent protein kinase (CaMKII) — histone deacetylase 4 (HDAC4) signaling in skeletal muscle myotubes, at least establishing the possibility that such a mechanism could also occur in the heart (30,31). This last point not with standing, specialized pools of Ca2+, which are region specific or buffered from cytoplasmic Ca2+, could still be required to regulate select Ca2+-sensitive signaling proteins in the heart. However, demonstrating microdomains or region-specific buffering of Ca2+ has been largely beyond routinely employed Ca2+-imaging techniques and thus has yet to be definitively described in cardiac myocytes. Despite the general lack of supportive data, Brochet et al recently described region specific depletion of Ca2+ in the junctional cisternae of the SR within nanometer-sized stores (32). Such region specific depletion may require local mechanisms for store-operated Ca2+ influx, such as through TRPC proteins, which could explain the relatively large array of expressed TRPC proteins found in the heart (Fig 1C, and ref 33). These results are also of interest given the recent description of store-operated Ca2+ entry serving to regulate SR Ca2+ homeostasis in neonatal rabbit ventricular cardiac myocytes (22). Alternatively, expression of TRPC proteins in the heart could also regulate local pools of Ca2+ within lipid-raft domains that are coupled with PLC-dependent signaling circuits, as is characteristic of most other non-excitable celltypes (18). Finally, in agreement with the general concept of region-specific control of Ca2+, Wu et al recently inferred a mechanism whereby changes in nuclear envelope Ca2+, as mediated by the IP3 receptor, serves as a means of activating CaMK-HDAC5 in ventricular cardiac myocytes, although no alterations in global Ca2+ were observed (34).
Here we showed that graded overexpression of TRPC3 in the heart produced a dosage-dependent manifestation of cardiomyopathy. The rationale for overexpressing TRPC3 was simply to examine the gain-of-function phenotype associated with TRPC proteins in the heart, and to examine the potential downstream signaling mechanisms. TRPC3 was selected for overexpression in the heart since TRPC3 is the most highly characterized and is regulated by both store-depletion and by PLC-coupled signaling events (18). There is also good evidence that TRPC3 is functional on its own when overexpressed, while TRPC1 appears to be unstable due to aberrant intracellular targeting (18). A final consideration is that the function of TRPC proteins is not entirely consistent with biophysical measures of store-operated Ca2+ entry in whole-cell configuration (18). While TRPC3 can elicit a store-operated Ca2+ entry event in most cells assayed, it is likely that other components are required to generate a properly regulated and store-operated channel with the proper conductance characteristics. Despite this concern, overexpression of TRPC3 in our transgenic mice did evoke a characteristic store-operated Ca2+ entry event in isolated adult myocytes, although it remains possible that TRPC3-dependent influx is somewhat distinct from an endogenous store-operated Ca2+ entry mechanism in the duration of a response, the localization of the channel to select microdomains, its activation by select agonists, or its regulation by co-factors.
Previous work has established that both neonatal and adult rat cardiomyocytes are capable of store-operated Ca2+ entry once depleted with PLC-dependent agonists (20,21), as well as neonatal rabbit ventricular myocytes (22). Moreover, Marchase and colleagues showed that general inhibitors of store-operated Ca2+ entry could reduce NFAT activation in cardiomyocytes, further supporting the hypothesis that specialized pools of Ca2+ are uniquely coupled to Ca2+-sensitive signaling proteins such as calcineurin (21). Indeed, in non-myocytes, calcineurin-NFAT is prominently regulated by store-operated Ca2+ entry (7-13). In support of such a mechanism, Uehara et al similarly showed that embryonic and neonatal mouse cardiomyocytes displayed store-operated Ca2+ entry using a similar experimental approach (28). However, Uehara et al failed to identify store-operated Ca2+ entry in adult mouse cardiomyocytes in culture, consistent with our inability to detect such entry in ≈75% of non-transgenic adult mouse cardiomyocytes (Fig 2A).
In conclusion, it remains uncertain if store-operated Ca2+ entry plays a physiological role in regulating reactive signaling in the adult mouse heart. Targeted deletion of specific TRPC family members may provide significant insight into this important issue. However, the observation that overexpression of TRPC3 in the heart promoted greater NFAT activation, cardiomyopathy, and enhanced stimulus-induced cardiac hypertrophy at least suggests that store-operated Ca2+ entry can serve in such a capacity, although definitive proof of the requirement of this signaling circuit as a required mediator of hypertrophy and/or myopathy awaits an in vivo loss-of-function approach.
Acknowledgement
This work was supported by the National Institutes of Health (to J.D.M.) H.N. was supported by a Post-Doctoral Fellowship from the Ohio Valley Affiliate branch of the American Heart Association (0425386B). J.D.M. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 2.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr. Opin. Genet. Dev. 2001;11:505–512. doi: 10.1016/s0959-437x(00)00225-2. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 4.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell. Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 6.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 8.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 9.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 10.Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 11.Bikah G, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Regulating T helper cell immunity through antigen responsiveness and calcium entry. Nat. Immunol. 2000;1:402–412. doi: 10.1038/80841. [DOI] [PubMed] [Google Scholar]
- 12.Mignen O, Thompson JL, Shuttleworth TJ. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc. Natl. Acad. Sci. USA. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 15.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 16.Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem. Biophys. Res. Commun. 1997;239:279–283. doi: 10.1006/bbrc.1997.7458. [DOI] [PubMed] [Google Scholar]
- 17.Ohki G, Miyoshi T, Murata M, Ishibashi K, Imai M, Suzuki M. A calcium-activated cation current by an alternatively spliced form of Trp3 in the heart. J. Biol. Chem. 2000;275:39055–39060. doi: 10.1074/jbc.M003606200. [DOI] [PubMed] [Google Scholar]
- 18.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 19.Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ. Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 20.Hunton DL, Zou L, Pang Y, Marchase RB. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1124–1132. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 21.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J. Biol. Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, van Breemen C, Kuo KH, Hove-Madsen L, Tibbits GF. Store-operated Ca2+ entry modulates SR Ca2+ loading in neonatal rabbit cardiac ventricular myocytes. Am. J. Physiol. Cell Physiol. 2006 Jan 18; doi: 10.1152/ajpcell.00226.2005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 25.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc. Natl. Acad. Sci. USA. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J. Biol. Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J. Biol. Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- 28.Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell. Calcium. 2002;31:89–96. doi: 10.1054/ceca.2001.0257. [DOI] [PubMed] [Google Scholar]
- 29.Kiriazis H, Sato Y, Kadambi VJ, Schmidt AG, Gerst MJ, Hoit BD, Kranias EG. Hypertrophy and functional alterations in hyperdynamic phospholamban-knockout mouse hearts under chronic aortic stenosis. Cardiovasc. Res. 2002;53:372–381. doi: 10.1016/s0008-6363(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brochet DX, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freichel M, Schweig U, Stauffenberger S, Freise D, Schorb W, Flockerzi V. Store-operated cation channels in the heart and cells of the cardiovascular system. Cell. Physiol. Biochem. 1999;9:270–283. doi: 10.1159/000016321. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Zhang T, Bossuyt J, Li X, McKinsey T, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin Invest. 2006:xxxx. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]