Abstract
The increasing number of population-based and epidemiological associations between oxidant pollutant exposures and cardiopulmonary disease exacerbation, decrements in pulmonary function, and mortality underscores the important detrimental effects of oxidants on public health. Because inhaled oxidants initiate a number of pathologic processes, including inflammation of the airways which may contribute to the pathogenesis and/or exacerbation of airways disease, it is critical to understand the mechanisms through which exogenous and endogenous oxidants interact with molecules in the cells, tissues, and epithelial lining fluid (ELF) of the lung. Furthermore, it is clear that inter-individual variation in response to a given exposure also exists across an individual lifetime. Because of the potential impact that oxidant exposures may have on reproductive outcomes and infant, child, and adult health, identification of the intrinsic and extrinsic factors that may influence susceptibility to oxidants remains an important issue. In this review, we discuss mechanisms of oxidant stress in the lung, the role of oxidants in lung disease pathogenesis and exacerbation (e.g. asthma, COPD, and ARDS), and the potential risk factors (e.g. age, genetics) for enhanced susceptibility to oxidant-induced disease.
Keywords: oxidative stress, antioxidant, genetics, susceptibility, infant, reproductive outcome, premature, children, elderly, asthma, chronic obstructive pulmonary disease, ozone, pollutants, particulates, PM, acute respiratory distress syndrome, hyperoxia, SNP, single nucleotide polymorphism
INTRODUCTION
As epidemiologic studies emerge from developed and developing industrialized countries, it has become clear that air pollution is associated with dramatic increases in the risk of acute and chronic diseases and death in children and adults. Many air pollutants exert their major effect by causing oxidative stress in cells and tissues that they contact. Gaseous pollutants [including ozone (O3), sulphur dioxide (SO2), and nitrogen dioxide (NO2)] and particulate matter (PM) [including ultrafine, PM2.5, PM10, and diesel exhaust particles (DEP)] are known to form reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radicals. ROS may damage proteins, lipids, and DNA directly, and form distinct products that can be used as biomarkers and help in measurement of ROS activity. ROS react with proteins to form nitrotyrosine1 and bromotyrosine2 whereas reaction with lipids leads primarily to the formation of isoprostanes3,4 and ethane5. In scenarios where DNA damage occurs, single-strand breaks and 8-hydroxyguanosine are generated.6
Health effects of air pollution can be classified into short-term and long-term effects, and a number of excellent reviews discuss these effects.7 Pollutant effects include reversible decrements in pulmonary function, airway inflammation, airways hyperreactivity, compromised immune function, enhanced responsivity to respiratory infection, increased incidence and exacerbation of lung disease (e.g. asthma), and mortality. For example, high-dose exposure of DEP can aggravate bacterial infection and induce a strong T cell mediated response.7,8 whereas O3 exposure combined with exercise is known to decrease respiratory frequency, FEV1, and FVC concurrent with an increase in airways resistance.7,9 Ozone also exacerbates allergic asthma which is characterized by increased eosinophils in induced sputum.10 Further, studies have shown that air pollutant exposures can increase susceptibility and response to bacterial and viral respiratory infections.11–13 In particular, individuals with one or more risk factors for adverse effects of oxidant exposures are of public health concern. Some of these risk factors include, but are not limited to age, gender, genetic background, nutrition, or pre-existing pulmonary disease.
Despite current regulations and an increasing awareness of the quality of the air we breathe, levels of common air pollutants remain an issue in many areas worldwide. Furthermore, the mechanisms of oxidant toxicity in the lung and other organ systems remain incompletely understood. In this review, we 1) provide a brief overview of the mechanisms through which exogenous (airborne pollutants) and endogenous oxidants may interact with bioreactive molecules (i.e., proteins, lipids, and DNA) (cigarette smoke exposures are not discussed as many reviews on this topic currently exist); 2) describe the role of oxidants in the pathogenesis of asthma, chronic obstructive airways disease (COPD), acute respiratory distress syndrome (ARDS), and cystic fibrosis; 3) discuss a number of intrinsic and extrinsic factors that may enhance susceptibility to oxidants. The review is not meant to be exhaustive, but was intended to highlight representative areas of investigation in this important field, and to identify questions that remain to be answered.
MECHANISMS OF OXIDANT-INDUCED TOXICITY
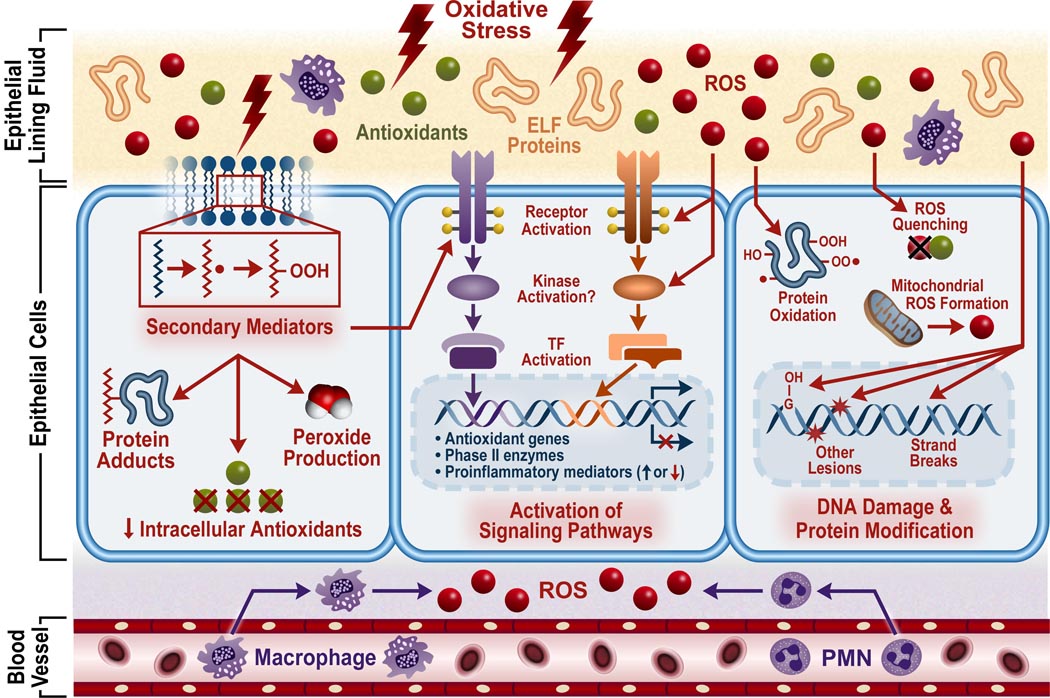
The mechanisms whereby oxidants exert their pathological effects on the lungs have been the focus of numerous studies and are still the subject of debate. Despite the diversity of these agents and the multitude of complex mechanisms that exist, several common themes have been identified which can serve as a platform for future research. Many ambient air pollutants may induce oxidative stress in the lung that arises when ROS overwhelm antioxidant defenses (Fig 1). After this imbalance is reached, ROS readily react with proteins, lipids, and DNA resulting in a number of pathological consequences.
Figure 1.
Mechanisms involved in oxidant pollutant-induced adverse health effects. Oxidant pollutants may elicit their effects through 1) the production of secondary mediators generated by reaction of pollutants or pollutant-induced ROS or free radicals with lipids in the ELF or cell membrane as well as proteins and antioxidants; 2) activation of signaling pathways by reactive oxygen species (ROS) or secondary mediators; 3) oxidation of cellular proteins; 4) damage to DNA. In addition to pollutant-induced generation of ROS, endogenous sources of ROS such as inflammatory cells, phagocytes recruited to the site of injury, as well as other cellular processes, may contribute to the oxidative stress state caused by pollutant exposure. Antioxidant molecules and enzymes mitigate the effects of ROS in the body. TF, transcription factor.
Oxidant interaction with molecules
A primary consequence of oxidative stress is lipid peroxidation, or the oxidative degeneration of lipids. Lipid peroxidation is caused by a free radical chain reaction mainly involving membrane polyunsaturated fatty acids. If not quenched, this reaction can permanently damage cell membranes, ultimately leading to cell death. Exposures to oxidant air pollutants cause lipid peroxidation in humans and rodents.15–19 Furthermore, the end products of lipid peroxidation can lead to subsequent pathological consequences. One of these end products, 4-hydroxy-2-nonenal (HNE), has numerous downstream effects. In vitro treatment of cells with HNE can cause lipid peroxidation20 and may potentiate oxidative stress through a depletion of intracellular glutathione and induction of peroxide production.21 HNE may also play a role in airway remodeling through activation of the epidermal growth factor receptor22 and induction of fibronectin production.23 Additionally, HNE-protein adducts have been found in the lungs of mice and humans after O3 exposure.24,25 Finally, HNE can induce cell death of alveolar macrophages in mice.26 These studies provide evidence for the hypothesis that secondary mediators generated by oxidant reactions with lipids, proteins, and other biomolecules contribute to toxic effects of pollutants.
Another secondary mediator can be generated by a reaction of O3 with unsaturated fatty lipids. Ozone can react directly with unsaturated fatty lipids in the epithelial lining fluid and cell membranes to produce lipid ozonation products (LOPs), which also have pathological downstream effects.27–29 These products are small, diffusible, and relatively stable, making them ideal mediators of O3 toxicity. In vitro exposure of human airway epithelial cells to different LOPs has shown that these products can activate eicosanoid metabolism similar to O3 exposure.30 Furthermore, products involved in eicosanoid metabolism are themselves highly reactive peroxides, which can contribute to the oxidative stress-induced damage. Other studies have shown that exposure of bronchial epithelial cells to LOPs caused activation of phospholipases A2, C, and D as well as the induction of inflammatory mediators such as platelet-activating factor, prostaglandin E2, interleukin (IL)-6, and IL-8.28,29 Treatment with oxidized phospholipids from O3-exposed lung surfactant reduced the viability of macrophages and epithelial cells by necrosis and apoptosis, respectively.31 This treatment also stimulated the release of IL-8 from epithelial cells. Taken together, these studies provide evidence of a direct link between LOPs produced by O3 exposure and O3-induced inflammation and cell damage.
A primary function of ELF is to protect underlying tissue from inhaled pathogens and toxins. However, current evidence suggests that antioxidants and lipids found in the ELF mediate oxidant-induced membrane oxidation. Thus, some defenses within this barrier may also contribute to the toxicity of certain agents. The capacity of O3 to oxidize cell membrane proteins and lipids in vitro was shown to be dependent on the presence of either of the antioxidants ascorbate or glutathione in the lining fluid.32 These results were corroborated by a study demonstrating that addition of ascorbate to the lining fluid increased cell injury in response to O3.33 Other studies have shown similar mechanisms for NO2. Glutathione and/or ascorbate are necessary components of the lining fluid for NO2-mediated membrane oxidation in vitro.34
Another mechanism whereby oxidant pollutants may exert their pathological effects is through the modification of proteins. ROS can act directly or indirectly on proteins to cause oxidation of the polypeptide backbone, peptide bond cleavage, protein-protein cross linking, or amino acid side chain modifications.35 Amino acid composition, particularly cysteine and methionine residues, can render proteins more susceptible to oxidation.35 For example, oxidation of methionine residues in α-1-antitrypsin by ROS in vitro results in loss of anti-neutrophil elastase activity.36 Without protection from α-1-antitrypsin, the alveolar matrix is susceptible to destruction by neutrophil elastase, which can eventually contribute to emphysema. Oxidation of multiple methionine residues by ROS impair rapid sodium channel inactivation.37 ROS also oxidize methionine residues in surfactant protein (SP)-B leading to inactivation.38 Inactivation of SP-B reduced the ability of the surfactant film to reduce lung surface tension during breathing, which can contribute to respiratory distress syndrome. Similarly, acute exposure of guinea pigs to O3 altered SP-A function contributing to the inflammatory response.39 Another study found that in vitro and in vivo O3 exposure caused oxidative modifications in SP-A that reduced ability to enhance phagocytosis of bacteria.40 Oxidative modification of surfactant proteins may also render the lung more susceptible to lipid peroxidation, inflammation, and oxidative damage since these proteins have been reported to inhibit these processes.41–43
Epidemiological and experimental studies have shown that exposure to air pollutants increases the risk of lung cancer.44 A potential mechanism for the increased cancer incidence in exposed individuals is DNA damage. Prahalad et al.45 demonstrated that PM can cause DNA damage and that this effect was inhibited by an OH scavenger and metal ion chelators, suggesting a role for PM-generated free radicals and metals adsorbed onto the particles. Further evidence also showed that PM caused increased DNA oxidative damage to human airway epithelial cells and was associated with the amount of water-soluble metals contained on these particles.46 Another group demonstrated that DEP induced DNA damage in mice and that this effect was dependent on the particle and not the organic chemicals adsorbed onto the particle surface.47 The authors proposed that alveolar macrophage generation of hydroxyl radicals during particle phagocytosis may contribute to DNA damage. These and other studies suggest that PM-induced DNA damage results from free radical formation.48 DNA damage has also been shown in lung epithelial cells exposed to O3 and this effect was reduced by pretreatment with vitamins C and E.49 It had also been reported that DNA backbone cleavages caused by O3 were dependent on hydroxyl radicals, while DNA base modifications were mainly caused by a direct effect of O3.50 Furthermore, DNA-protein cross-linking has been shown in the lungs of mice exposed to SO2.51 In addition to potential cancer etiology, DNA damage may alter gene and protein expression as well as cell death.
Oxidant-induced Cell Signaling
Activation of signaling pathways is another way in which oxidant pollutants may cause pathological responses in the lung. Air pollutants and ROS can activate MAPK signaling, which may ultimately promote inflammation. For example, inhibition of c-Jun NH2 terminal kinase (JNK) in mice attenuated O3-induced inflammation and hyper-responsiveness.52 Additionally, end products of lipid peroxidation activate extracellular signal-regulated kinase p44/42 (Erk1/2), JNK, and p38MAPK and activation can be blocked by NAC.21,23 Activation of these kinases was also accompanied by increased DNA binding activity of the transcription factor activator protein-1 (AP-1), which can lead to the transcription of stress response genes including phase II enzymes (Fig 1). Another study demonstrated that HNE could induce DNA binding of the transcription factors nuclear factor erythroid-2 related factor (NRF) 1, NRF2, JunB, c-Jun, FosB, c-Fos, Fra1, and Fra2.53 Oxidants also increase NF-κB DNA binding along with the release of the pro-inflammatory cytokine IL-8 in lung epithelial cells and this effect can be abrogated by antioxidant pretreatment.54 Other studies have also demonstrated the ability of air pollutants to activate NF-κB.55,56 Activation of stress response pathways due to oxidative stress is likely a cause of pollutant-induced NF-κB activation. PM-induced activation of NF-κB has been shown to be dependent on EGFR activation and the MAPK signaling pathway, which is involved in the stress response.57 Conversely, there is also evidence that oxidants can downregulate inflammatory pathways through an inhibitory effect on NF-κB.58
Another important transcription factor involved in the response to oxidative stress is Nrf2. Nrf2 contributes to the oxidative stress response through its binding of antioxidant response elements (AREs) leading to the induction of various genes involved in mitigating oxidative damage.63 Oxidant-induced activation of Nrf2 leads to the transcription of genes for antioxidants, DNA damage recognition, glutathione homeostasis, free radical metabolism, as well as a number of others involved in the oxidative stress response.59 Mutations in Nrf2 or a disruption of the signaling pathway would likely render individuals more susceptible to the adverse effects of pollutant exposure. Further investigation of pollutant-induced activation of Nrf2 and the consequences of Nrf2 mutation in the response to pollutant exposure is needed to fully elucidate the role of Nrf2 in mitigating the effects of oxidant pollutant exposure.
Endogenous Sources of Oxidants
Endogenous sources of ROS may have an indirect role in the toxicity induced by exposure to air pollutants. The main cellular sources of ROS in the lung include neutrophils, eosinophils, alveolar macrophages, epithelial cells, and endothelial cells. Air pollutant-induced lung inflammation involves the recruitment of inflammatory cells that release ROS, which can enhance inflammation, tissue damage, and other pathological effects. Additionally, phagocytes can be activated by PM deposition in the lung and cause ROS release contributing to the oxidative damage.47,60 Similarly, NO2 has also been shown to induce the release of ROS from macrophages.61 The predominant ROS produced by inflammatory cells and macrophages are superoxide and hydrogen peroxide, respectively. Both oxidants can react with a number of substrates and biomolecules to cause damage and generation of harmful radicals. ROS are also produced in the body during normal metabolic reactions such as aerobic respiration involving the electron transport chain within the mitochondria, and enzyme reactions involving cycloxygenases, lipoxygenase, peroxidases, and cytochrome-P450. Any alteration in these processes or decrement in the antioxidants that offset the production of ROS from them may also lead to tissue damage and other pathological consequences. Additionally, endogenously produced nitric oxide can react with oxygen to form damaging nitrogen oxides or it can react with superoxide to form peroxynitrate.62 Peroxynitrate has been shown to induce lipid peroxidation, DNA damage, and protein oxidation.63–66 Furthermore, peroxynitrate can also react with CO2 to form NO2, which can lead to further oxidant-induced damage.67 Taken together these studies demonstrate how endogenous sources of ROS can contribute to air pollutant-induced toxicity through an enhancement of the oxidative burden within the lung.
OXIDANTS AND LUNG DISEASE
Because the lung interfaces with the external environmental, it is frequently exposed to airborne oxidant gases and particulates, and thus prone to oxidant-mediated cellular damage. Enhanced levels of oxidant production and cellular injury have been implicated in many pulmonary diseases including asthma and other allergic diseases, COPD, ARDS, and cystic fibrosis. In the following section, we briefly describe investigations on exacerbation of these important pulmonary diseases due to ROS.
Asthma
It is widely agreed that a link exists between oxidants and their effect on various allergic diseases, particularly asthma pathogenesis. Oxidants can cause airway inflammation and airway hyperresponsiveness (AHR) which are major characteristics of asthma.68 Asthmatics have increased ROS production by macrophages, eosinophils and neutrophils which leads to increased hydrogen peroxide69, 8-isoprostance and CO in their breath condensates, increased pulmonary glutathione peroxidase and superoxide dismutase in lung cells,70 as well as increased pulmonary, serum and urinary peroxidation products. Because eosinophils and neutrophils are the major cells in the inflammatory infiltrate in asthma, increased levels of eosinophil peroxidase and myeloperoxidase in the peripheral blood, induced sputum and BAL fluid from patients have been documented.71,72 Other markers of oxidant activity such as malondialdehyde (MDA) and thiobarbituric acid reactive products (TBARs) have also been detected in urine, plasma, sputum and BAL fluid which relate to the severity of asthma in these patients.73–76 Elevated levels of nitrotyrosine1 and chlorotyrosine2,77 in BAL fluid from asthma patients also suggests oxidative protein damage.
External oxidant stimuli also worsen existing allergic disease. For example, O3 may evoke asthma exacerbations. Ozone inhalation was shown to increase AHR, induce higher IL-5, GM-CSF and G-CSF levels and indirectly enhancing the longevity of eosinophils via suppressing apoptosis, in a mouse model of allergic asthma.78
DEP and its components have been demonstrated to enhance AHR in a murine model of asthma.79 Human studies have also revealed that antioxidant enzymes, GSTM1 and GSTP1, can alter adjuvant function of DEP in allergic inflammation, block DEP-induced IgE and IL-4 cytokine production.80 DEP exposure leads to generation of ROS in vitro 81 and in vivo.82 DEP has also been advocated as an adjuvant in allergic sensitization mediating its effect via dendritic cells (DCs). Chan et al83 showed that DEP downregulated LPS-induced CD86 and CD54 and MHC class II maturation makers and IL-12 production by DCs thereby, interfering with DC function. The interference in DC function was attributed to the Nrf2 signaling pathway.83
Recent studies have also suggested that O3 and DEP have an additive effect on AHR and pulmonary inflammation in asthma. For example, higher PenH, increased IL-4 and reduced IFN-γ levels in BAL fluid from OVA-sensitized-challenged O3- and DEP-exposed groups were observed compared to OVA-sensitized-challenged O3-exposed groups and OVA-sensitized-challenged DEP-exposed groups.84
Chronic Obstructive Pulmonary Disease (COPD)
COPD is a slow progressive and irreversible disease state characterized by limited airflow associated with gradual decline in lung function85 with clinical manifestations such as emphysema and chronic bronchitis.86,87 Clinical and experimental investigations suggest that oxidants play a role in pathogenesis of COPD. The major contributing factors in COPD etiology are direct exogenous sources of oxidants such a cigarette smoke rich in ROS. Increased amounts of ROS are also generated endogenously by various inflammatory and epithelial cells of the airways. Further, accumulating evidence has implicated indirect local and systemic effects of oxidants in COPD pathogenesis. Locally, higher levels of oxidants have been found in exhaled breath condensates, sputum and lavage fluid of patients with COPD. Large numbers of neutrophils and macrophages migrating into the lungs of COPD patients generate ROS in excess such that these patients have higher levels of superoxide anion and hydrogen peroxide release.88–91 Recently, hydrogen peroxide in exhaled air and, IL-8 and soluble ICAM-1 in serum were found to be suitable markers in monitoring patients with exacerbated COPD.92 Impairment in gene expression of protective mechanisms (GSTP1, GSTM1, microsomal epoxide hydrolase and TIMP2) against oxidants in lung samples of COPD patients was observed along with upregulation of chemokines involved in the inflammatory process.93 Locally generated 4-HNE has been shown to modify protein levels in airway and alveolar epithelial cells, and endothelial cells in human subjects with airway obstruction.94 It also interacts with glutathione thereby reducing cells antioxidant ability.95
Systemically, oxidants cause elevation of plasma lipid peroxidation products such as malondialdehyde.96,97 Higher levels of 8-isoprostanes, products of ROS-mediated peroxidation of arachidonic acid, are also found in breath condensates as well as in the urine.98,99 Erythrocyte superoxide dismutase (SOD) which scavenges superoxide radical was significantly higher in plasma of COPD patients than in healthy non-smokers.100
Acute Respiratory Distress Syndrome (ARDS)
ARDS is a severe form of acute lung injury (ALI) and a syndrome of acute pulmonary inflammation characterized by sudden reduction in gas exchange and static compliance as well as nonhydrostatic pulmonary edema.101 The mechanisms of ARDS are an ongoing field of investigation. However, ROS have been suggested to play an important role in pulmonary vascular endothelial damage102 which is hypothesized to be responsible for clinical manifestation of ARDS. Recently, studies have suggested that pathogenesis of ARDS involves enhanced production of ROS and diminished antioxidant levels.103,104 Patients with ARDS have high levels of hydrogen peroxide in exhaled air and urine105, and high circulating levels of 4-HNE.106 Levels of antioxidant defense system including enzymes like superoxide dismutase and catalase as well as other scavengers like glutathione, vitamin E and C have been shown to drop with increasing levels of ROS.107
Iron is also known to be a mediator of oxidative stress as it can catalyze prooxidant reactions. Decreased plasma iron-binding activity leading to decreased ability to prevent iron dependent ROS formation has been detected in patients with ARDS. Transferrin receptor protein levels were found to be significantly increased in lung biopsies of patients with ARDS implicating iron a mediator of oxidative stress.108
Inflammatory mediators such as cytokines, chemokines and adhesion molecules expressed during ARDS can also indirectly mediate production of ROS109 and thereby lead to further damage. High concentrations of TNF-α and IL-1β have been detected in high concentrations of BALF of patients with ARDS. IL-6 levels in the circulation are known to be a detector of ARDS of different etiologies such a sepsis and acute pancreatitis.109
Cystic fibrosis (CF)
CF is a disease caused by an autosomal recessive mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, resulting in dysfunction of a protein involved in the transport of chloride ions across cell membranes. The deficiency in chloride transport leads to production of thickened mucus secretions in the respiratory system of CF patients who are commonly afflicted with recurrent episodes of bronchitis and pneumonia. Exhaled breath condensates (EBC) from children with cystic fibrosis expressed high levels of oxygen and carbon centered radicals in CF groups versus the healthy controls. Catalase abolished oxygen radicals in EBC whereas addition of hydrogen peroxide led to a dramatic increase.110 In a similar study, myeloperoxidase and 3-cholorotryosine levels were 10 and 5-fold elevated respectively, in the BALF of young children with CF as compared to the controls.111 Nitrotyrosine was found elevated in sputum of CF patients.112
Recent investigations have helped in understanding the important role of CFTR in CF pathogenesis. CFTR is known to regulate cellular glutathione (GSH) transport. CFTR gene expression is suppressed by oxidative stress caused by tert-butylhydroquinone, BHQ as it enhanced cellular glutathione in CFTR-expressing T84 and Calu-3 epithelial cells.113 In another recent study, mutated CFTR caused increased ROS levels and mitochondrial oxidative stress as a consequence of lower GSH levels.114 The submucosal gland serous cell is the principal site of expression of CFTR chloride ion channel which is known to dysfunction in CF.115 Cowley and Lindsell115 proposed that ROS-stimulated anion secretion from serous cells is CFTR dependent. Absence of this compensatory protective mechanism might expose lung to ROS for extended periods, which could be important in the pathogenesis of CF lung disease.
Neutrophil rich inflammation is a determinant of CF severity, and findings from a recent genetic study show that the level of myeloperoxidase gene expression which governs the microbicidal and proinflammatory activities of neutrophils may influence CF pathogenesis.116 Specifically the -463GA MPO promoter polymorphism has been shown to control the severity of CF-related pulmonary inflammation.
SUSCEPTIBLE POPULATIONS
Inter-individual differences in responses to air pollutant exposures have been well-documented.117–120 That is, in populations and clinical studies exposed similarly to air pollutants, pulmonary inflammatory and function responses are more severe in some individuals than in others. Importantly, investigators have also demonstrated high within-individual reproducibility of the responses to air pollutant exposures.120 The wide spectrum of adverse responses to the pollutants has been attributed to multiple intrinsic (e.g. age, gender, genetic) and extrinsic (e.g. nutrition, pre- or concurrent exposure, pre-existing disease) factors. In the following section, we identify and briefly discuss intrinsic (host) and extrinsic factors that may influence susceptibility to oxidant air pollutants, and the potential implications for increased risk of allergic disease. Because of space limitations, most of the discussion is focused on age and genetic background.
Age
Pre-neonatal effects
Maternal exposure to air pollutants during pregnancy may have adverse effects on the developing fetus (for comprehensive review, see 121–123). Pre-term neonates are particularly susceptible to potential injurious effects of air pollutants because exposure may disrupt normal fetal developmental processes. Adverse birth outcomes, including preterm birth, low (<2,500 g) and very low (<1,500 g) birth weight, intrauterine growth restriction, birth defects, and intrauterine and infant mortality have been associated with maternal exposure to O3, NO2, SO2, and PM (Table 1). Because low/very low birth weight and prematurity are important predictors of children’s health (e.g. mortality, cardiovascular, pulmonary, immunologic, renal, central nervous system, and neurocognitive deficits145–148), understanding of the effects of oxidants have important economic and public health implications. Interestingly, while some investigations found positive associations between maternal exposures to oxidant pollutants and adverse birth outcomes, others suggested that exposures have little or no effects.132 However, the majority of studies suggest that maternal exposures to oxidant air pollutants and the resultant effects on birth weight and other adverse birth outcomes represent an important determinant of susceptibility to lung development and diseases including allergic diseases such as asthma (Fig 2).
Table 1.
Summary of representative epidemiological investigations that have found effects of oxidant air pollutant exposures on pre- and post-neonatal outcomes in human populations.
| Age | Outcome | References |
|---|---|---|
| Pre-neonatal | Early fetal loss | Pereira124; Liu125; Sram126 |
| Pre-term delivery | Ritz127; Brauer128; Gessner129 | |
| Low birth weight (<2,500 g) | Bobak130; Maisonet131; Bell132; Slama133 | |
| Very low birth weight (<1,500 g) | Rogers134; Rogers135 | |
| Birth defects | Ritz136; Brook137; Gilboa138 | |
| Post-neonatal | Mortality (respiratory disease) | Samet139; Ha140; Gliniana141; Woodruff142 |
| Mortality (SIDS) | Dales143; Tong144; Woodruff142 |
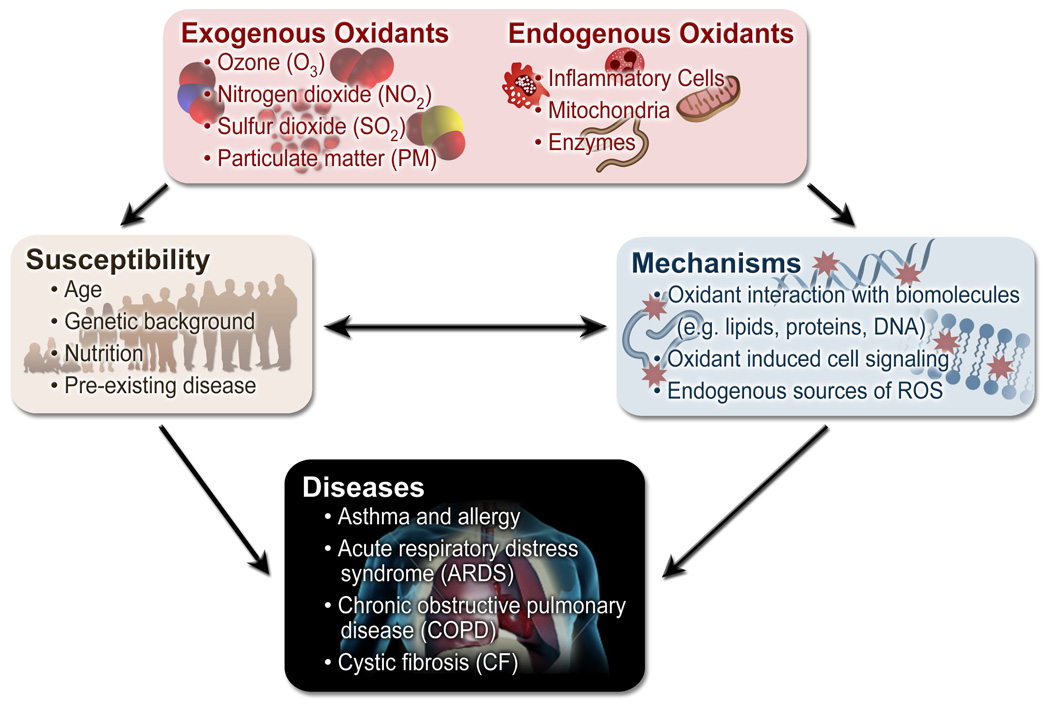
Figure 2.
Flowchart demonstrating the connection between oxidants and disease states, as well as the mechanisms and susceptibility factors involved in the development of oxidant-induced diseases.
Infants and children
Development of the lung is a complex, highly orchestrated process that begins in the embryo where the lung begins as an avascular epithelial bud and reaches maturation at approximately 6–8 years of age.149,150 Transcription factors and other molecular signals control development of respiratory bronchioles, epithelium, capillaries, and immune cell populations and processes over a number of well-defined stages.150 The lung is particularly vulnerable to adverse effects of oxidant pollutants and other toxicants during post-natal growth and development processes.121,123 However, little is known about the mechanisms through which inhaled oxidant toxicants affect the human developing lung, though animal models have provided important insight.150 The airway epithelium, in particular, is thought to be highly vulnerable, and the immune system, such as polarization of T helper cells, could also be affected by oxidants and inflammatory stimuli.151
Large cohort investigations have associated exposure to air pollutants with changes in children’s lung function.123,152–155 Children are particularly susceptible not only because the lung is developing (above), but children are also often very active outdoors and have very different ventilatory parameters compared to adults150 that facilitates deeper and greater lung deposition of particles and gas/cell membrane interactions. Gauderman et al154 reported that among over 3,600 children in southern California communities, those who lived within 500 m of a freeway had significant deficits in pulmonary function (FEV1, MMEF) compared with children who lived at least 1,500 m away from the freeway. Delfino et al155 evaluated the relationship between daily changes in FEV1 and ambient and personal air pollutant exposures in asthmatic subjects between the ages of 9 and 18 yr. These investigators found that decrements in FEV1 were significantly associated with increasing hourly peak and daily average personal PM2.5 and NO2. Interestingly, FEV1 decrements were not found with ambient PM2.5 and only weakly with ambient NO2. They concluded that pollutant associations with lung function deficits might be missed using ambient data alone, and stress the importance of using personal exposure to identify independent effects of specific pollutants.
An increasing body of population-based and epidemiological literature also indicates that asthmatic children are at great risk for asthma exacerbation with exposure to traffic-related air pollution (for review156). Asthma exacerbations have been demonstrated in many urban environments worldwide including Hong Kong157, Mexico City1158, and Los Angeles159. Air pollutants have also been associated with the development of asthma in children. For example, McConnell et al184 found that the relative risk of developing asthma was 3.3 times greater in children who played 3 or more outdoor sports in southern California communities with high O3 concentrations compared with children playing no outdoor sports. The investigators found that the number of sports had no influence on asthma incidence in low O3 communities. Further support for the hypothesis that air pollutants contribute to atopic diseases in children was provided by a prospective birth cohort study that found strong positive associations between the distance to nearest main road and asthmatic bronchitis, hay fever, eczema, and sensitization during the first 6 years of life.186
The relatively greater increases in urban asthma have been attributed to many factors, including socioeconomic status. Socioeconomic status and ethnic disparities in asthma prevalence and morbidity have been well documented162, and environmental factors may account for asthma disparities including greater traffic air pollution, disparities in treatment and access to care, housing conditions, and indoor exposure to allergens.
Elderly
Evidence has accumulated that suggests the elderly (>65 yr) may be susceptible to adverse effects of air pollutant exposures (for reviews163,164). The mechanisms for increased susceptibility in this subpopulation are not well understood, but age-related decline in lung function165,166, underlying cardiovascular and/or pulmonary disease167,168, and potential decline in antioxidant defense capacity of the respiratory tract lining fluid164 have been proposed. It is generally agreed that lung function declines with age, and oxidant stress related to smoking, chronic lung inflammation, and related diseases may increase the rate of decline.164 Furthermore, decline in antioxidant capacity has been correlated with increased risk of mortality due to multiple causes.169 Supplementation with antioxidants (for review164) or treatments with other oxidant-reducing drugs (e.g. statins170) may reduce the rate of decline in lung function. Interestingly, a recent investigation found in relatively small groups of young subjects, older current smokers, and older non-smokers that Nrf2 expression decreased in the alveolar macrophages of older current smokers and COPD patients relative to the other groups.171 However, the use of antioxidant therapies to treat diseases such as COPD that are associated with decreased lung function have been equivocal.164 The explanation for inconsistent protective effects of antioxidant therapies for these diseases is not clear, though the presence of large inter-individual variation in antioxidant levels in smokers suggest that protective response mechanisms may exist in which oxidant stress stimulates upregulation of antioxidant mechanisms.164 Understanding whether differential inducibility of antioxidant defenses due to loss-of-function polymorphisms in antioxidant enzyme genes or other mechanisms (e.g. post-translational modification of proteins) in aging normal and diseased individuals could provide important insight to development of effective strategies to prevent or reduce loss of lung function. For example, Alexeeff et al172 found that acute effects of O3 exposure were exacerbated in elderly men with polymorphisms in the antioxidant genes GSTP1 (glutathione S-transferase pi) and HMOX1 (heme oxygenease-1) relative to those with wild-type genotypes. Interestingly, antioxidant supplementation to reduce change in lung function due to O3 in asthmatic children was effective only in those with genetic deficiency in GSTM1, suggesting nutrition by gene interaction may be important in this setting173. Further investigation of interactions between antioxidant supplementation and genetic background may have implications for other subpopulations, including the elderly.
Genetic Background
A role for genetic background in pulmonary responses to the adverse effects of oxidant exposures was initially suggested based on reproducible inter-individual variation in pulmonary spirometric responses (FEV1, sRaw) by normal healthy volunteers following controlled O3 exposures.120 Similar observations were independently reported by other laboratories.119 Furthermore, investigators found that O3-induced inflammation as indicated by the number of polymorphonuclear leukocytes found in bronchoalveolar lavage fluid also varied widely between subjects.117 Because the subjects in all of these studies were otherwise healthy, non-smoking, young adults, an intrinsic factor was suggested to be an important determinant of the variation.174
To more formally evaluate whether genetic background was an important determinant of susceptibility to O3-induced lung inflammation and injury, multiple inbred strains of mice were exposed to O3.175 Significant inter-strain variation in O3-induced inflammation was found, and linkage analyses identified quantitative trait loci (QTL) that harbor candidate susceptibility genes. Proof of concept investigations have implicated tumor necrosis factor (Tnf176) and toll-like receptor 4 (Tlr4 177) as determinants of O3-induced lung inflammation and hyperpermeability, respectively. However, these susceptibility QTLs (and candidate genes) account for only approximately 20–30% of the genetic variance in O3 responsiveness which indicates that other QTLs likely interact to determine O3 response phenotype.
Evidence exists that genetic loci for inflammatory and antioxidant processes are also important in human responses to air pollutants (for review178). For example, Bergamaschi et al179 found that polymorphisms in genes for phase II xenobiotic metabolizing enzymes NAD(P)H:quinone oxidoreductase 1 (NQO1) and glutathione-S-transferase (GST) M1 (GSTM1) associated with pulmonary function and epithelial injury responses to O3 in exercising subjects. Yang et al180 found among 51 adult subjects exposed to O3 during intermittent exercise, those with the TNF -308 G/G genotype (wild type) had a significantly greater fall in FEV1 (−9% of baseline) compared to subjects with a loss of function −308 A allele (G/Aor A/A genotype). Interestingly, a similar association of TNF genotypes was found in adult asthmatic subjects exposed to 0.5 ppm SO O3 during moderate exercise.181
The role of TNF was also investigated by Li et al182 who found that children with −308 G/G TNF genotype had decreased risk of asthma and lifetime wheezing compared to children who had one or more of the mutant alleles. They also found that the protective effect of the G/G genotype on wheezing was greater in low O3 communities compared with high O3 communities. Furthermore, they found that reduction of the protective effect of the −308 G/G genotype with higher O3 exposure was most evident in children who had antioxidant GSTM1 null and GSTP1 Ile/Ile genotypes. Results suggested that the −308 G/G genotype may be important in the pathogenesis of asthma and wheezing, which in turn is dependent on airway oxidative stress. Others183,184 have also found that polymorphisms in NQO1 and GSTM1 confer differential risk to asthma in oxidant environments, thus strengthening the notion that interaction of environmental oxidants and these phase II enzyme genes (i.e. gene by environment interaction) are important in the pathogenesis of asthma.
Hyperoxic lung injury induces inflammation and noncardiogenic edema in the lung which are phenotypes of acute lung injury (ALI) and ARDS. In positional cloning studies, the nuclear transcription factor Nrf2 was identified as a potential candidate gene for susceptibility to hyperoxic lung injury in inbred mice.185 Studies in mice with targeted deletion of Nrf2 confirmed a role for this gene in the hyperoxia model.186 Interestingly, Nrf2 has also subsequently been found to be important in the pathogenesis of asthma phenotypes in a mouse model.187 Furthermore, recent investigations have also suggested a role for Nrf2 in PM-induced exacerbation of asthma.188,189
We therefore hypothesized that polymorphisms in NRF2 resulting in decreased function similarly predispose humans to ALI. Resequencing of NRF2 in four different ethnic populations and identified three new NRF2 promoter polymorphisms at positions −617 (C/A), −651 (G/A) and −653 (A/G).190 The −617 polymorphism alters the consensus recognition sequences for NRF2, and suggested that this polymorphism may affect NRF2 transcription. In vitro transcription factor binding analyses confirmed a loss-of-function effect of the −617 C/A polymorphism. Furthermore, among major trauma patients, those with the −617 A SNP had a significantly higher risk for developing ALI [OR 6.44; 95% CI 1.34, 30.8; p = 0.021] relative to patients with the wild type (−617 CC).223 These studies provided insight into the molecular mechanisms of susceptibility to ALI, and may help to identify patients who are predisposed to develop ALI under at risk conditions, such as trauma and sepsis. Furthermore, because animal models have implicated an important role for Nrf2 in asthma/allergy phenotypes, evaluation of the NRF2 promoter polymorphisms may have relevance in these and other diseases with oxidant stress etiologies.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
Oxidative stress can cause cellular damage by oxidizing nucleic acids, proteins, and membrane lipids. ROS have been implicated in the pathogenesis of many diseases and important biological processes including carcinogenesis, atherosclerosis, aging, and inflammatory disorders. Moreover, because of its interface with the environment, the lung is a major target organ for injury by exogenous oxidants such as environmental pollutants and endogenous ROS generated by inflammatory cells. Lipid peroxidation products such as isoprostanes, TBARS, and MDA can be detected in EBC, BALF, urine or plasma to give an indirect measure of oxidative stress. Levels of H2O2 in EBC can also be used to estimate oxidative stress within the lungs. Furthermore, levels of antioxidants such as GSH in EBC or BALF are another indirect measure of oxidative stress. These endpoints are indirect measures of oxidative stress, are not specific to air pollutant exposure, and considerable variability may arise from confounding factors such as collection methods and individual lifestyle habits. Markers of DNA oxidation such as 8-OHdG and and 8-oxoGuo can be detected in the urine. Protein carbonyl levels in the plasma can be used to assess protein oxidation. All of the biomarkers mentioned do not discriminate between different oxidative insults and are merely indicators of oxidative damage. Further investigation is needed to discover biomarkers that correlate well with severity of pollutant-induced injury as well as exposure to the pollutants. These markers are essential to provide a means to estimate exposure and facilitate identification of at risk individuals.
While considerable progress has been made to understand the mechanisms through which oxidants initiate and propagate cell and tissue toxicity, critical questions remain to be addressed. For example, specific signaling pathways and mechanisms of transcription factor activation by specific oxidant pollutants are not well understood. Characterization of precise cellular mechanisms may provide a means for intervention to prevent or protect against disease pathogenesis, particularly in populations that are at risk due to preexisting disease, age (very young and elderly), or other predisposing conditions such as poor nutrition.
Because of the impact that oxidants may have on lung function, a better understanding of factors that may influence individual susceptibility remains an important issue. In utero and neonatal exposures to oxidants can have profound effects on the developing lung and may impact considerably on childhood and adult health. Studies designed to investigate how early life exposures to specific oxidants affect airway morphology, immune function, as well as the epigenome are necessary if we are to understand the long-term differences in morbidity/mortality outcomes, including asthma and other allergic diseases.
Genetic background is also an important determinant of responsiveness to oxidant exposures in children and adults. Using association analyses in clinical and epidemiological investigations, functional polymorphisms in a number of candidate susceptibility genes (e.g. NQO1, GSTM1, TNF) have provided some insight to the importance of genetics in inter-individual variation in oxidant responsiveness. However, responsivity to oxidant exposures and disease pathogenesis are complex, multigenic processes, and the contribution of each gene in a complex trait is relatively minor. Therefore, it is critical to identify each of the genes that ultimately determine complex traits such as environmental lung diseases. Discovery of genes that affect physiology and pathophysiology will occur only if multi-disciplinary investigations utilize model systems to exploit the accumulating data available for comparative genetics and genomics.191 Furthermore, susceptibility genes almost certainly interact with multiple environmental exposures or stimuli that are important in the etiology of a disease, and these interactions may vary with age and from one population to another.192 It is only through investigation of the basic mechanisms and translational application that we will understand the complex interplay of gene-environment interactions and oxidant-mediated lung disease.
Acknowledgements
The authors were supported by Division of Intramural Research at the National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services. We thank Drs. Donald Cook, Michael Fessler, and Dianne Walters for reviewing the manuscript.
References
- 1.Hanazawa T, Kharitov SA, Barnes PJ. Increased nitro tyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 2.Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, et al. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 4.Dworski R, Roberts LJ, 2nd, Murray JJ, Morrow JD, Hartert TV, Sheller JR. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2alpha) Clin Exp Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 5.Paredi P, Kharitonov SA, Barnes PJ. Elevation of exhaled ethane concentration in asthma. Am J Respir Crit Care Med. 2000;162:1450–1454. doi: 10.1164/ajrccm.162.4.2003064. [DOI] [PubMed] [Google Scholar]
- 6.Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ Res. 2003;93:279–284. doi: 10.1016/s0013-9351(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, et al. Health effects of air pollution. J Allergy Clin Immunol. 2004;114:1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Yin XJ, Dong CC, Ma JY, Antonini JM, Roberts JR, Stanley CF, et al. Suppression of cell-mediated immune responses to listeria infection by repeated exposure to diesel exhaust particles in brown Norway rats. Toxicol Sci. 2004;77:263–271. doi: 10.1093/toxsci/kfh035. [DOI] [PubMed] [Google Scholar]
- 9.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 10.Peden DB. Pollutants and asthma: role of air toxics. Environ Health Perspect. 2002;110(suppl 4):565–568. doi: 10.1289/ehp.110-1241207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciencewicki J, Gowdy K, Krantz QT, Linak WP, Brighton L, Gilmour MI, et al. Diesel exhaust enhanced susceptibility to influenza infection is associated with decreased surfactant protein expression. Inhal Toxicol. 2007;19:1121–1133. doi: 10.1080/08958370701665426. [DOI] [PubMed] [Google Scholar]
- 13.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, et al. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 2007;179:4367–4375. doi: 10.4049/jimmunol.179.7.4367. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect. 2007;115:1732–1737. doi: 10.1289/ehp.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Burbure CY, Heilier JF, Neve J, Becker A, Albrecht C, Borm PJ, et al. Lung permeability, antioxidant status, and NO2 inhalation: a selenium supplementation study in rats. J Toxicol Environ Health A. 2007;70:284–294. doi: 10.1080/15287390600884875. [DOI] [PubMed] [Google Scholar]
- 16.Elsayed NM, Gorbunov NV, Mayorga MA, Kagan VE, Januszkiewicz AJ. Significant pulmonary response to a brief high-level, nose-only nitrogen dioxide exposure: an interspecies dosimetry perspective. Toxicol Appl Pharmacol. 2002;184:1–10. [PubMed] [Google Scholar]
- 17.Ergonul Z, Erdem A, Balkanci ZD, Kilinc K, Vitamin E. protects against lipid peroxidation due to cold-SO2 coexposure in mouse lung. Inhal Toxicol. 2007;19:161–168. doi: 10.1080/08958370601051883. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Meng Z. Effects of airborne fine particulate matter on antioxidant capacity and lipid peroxidation in multiple organs of rats. Inhal Toxicol. 2005;17:467–473. doi: 10.1080/08958370590964467. [DOI] [PubMed] [Google Scholar]
- 19.Yargicoglu P, Sahin E, Gumuslu S, Agar A. The effect of sulfur dioxide inhalation on active avoidance learning, antioxidant status and lipid peroxidation during aging. Neurotoxicol Teratol. 2007;29:211–218. doi: 10.1016/j.ntt.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, et al. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;806:85–96. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 21.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 22.Suc I, Meilhac O, Lajoie-Mazenc I, Vandaele J, Jurgens G, Salvayre R, et al. Activation of EGF receptor by oxidized LDL. FASEB J. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- 23.Tsukagoshi H, Kawata T, Shimizu Y, Ishizuka T, Dobashi K, Mori M. 4-Hydroxy-2-nonenal enhances fibronectin production by IMR-90 human lung fibroblasts partly via activation of epidermal growth factor receptor-linked extracellular signal-regulated kinase p44/42 pathway. Toxicol Appl Pharmacol. 2002;184:127–135. doi: 10.1006/taap.2002.9514. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton RF, Jr, Hazbun ME, Jumper CA, Eschenbacher WL, Holian A. 4-Hydroxynonenal mimics ozone-induced modulation of macrophage function ex vivo. Am J Respir Cell Mol Biol. 1996;15:275–282. doi: 10.1165/ajrcmb.15.2.8703485. [DOI] [PubMed] [Google Scholar]
- 25.Kirichenko A, Li L, Morandi MT, Holian A. 4-hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol Appl Pharmacol. 1996;141:416–424. doi: 10.1006/taap.1996.0307. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Hamilton RF, Jr, Kirichenko A, Holian A. 4-Hydroxynonenal-induced cell death in murine alveolar macrophages. Toxicol Appl Pharmacol. 1996;139:135–143. doi: 10.1006/taap.1996.0152. [DOI] [PubMed] [Google Scholar]
- 27.Pryor WA, Squadrito GL, Friedman M. The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic Biol Med. 1995;19:935–941. doi: 10.1016/0891-5849(95)02033-7. [DOI] [PubMed] [Google Scholar]
- 28.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Lipid ozonation products activate phospholipases A2, C, and D. Toxicol Appl Pharmacol. 1998;150:338–349. doi: 10.1006/taap.1998.8418. [DOI] [PubMed] [Google Scholar]
- 29.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999;160:1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- 30.Leikauf GD, Zhao Q, Zhou S, Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am J Respir Cell Mol Biol. 1993;9:594–602. doi: 10.1165/ajrcmb/9.6.594. [DOI] [PubMed] [Google Scholar]
- 31.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem Res Toxicol. 2002;15:896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 32.Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, et al. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med. 2005;38:515–526. doi: 10.1016/j.freeradbiomed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Connor LM, Ballinger CA, Albrecht TB, Postlethwait EM. Interfacial phospholipids inhibit ozone-reactive absorption-mediated cytotoxicity in vitro. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1169–L1178. doi: 10.1152/ajplung.00397.2003. [DOI] [PubMed] [Google Scholar]
- 34.Velsor LW, Postlethwait EM. NO2-induced generation of extracellular reactive oxygen is mediated by epithelial lining layer antioxidants. Am J Physiol. 1997;273:L1265–L1275. doi: 10.1152/ajplung.1997.273.6.L1265. [DOI] [PubMed] [Google Scholar]
- 35.Kelly FJ, Mudway IS. Protein oxidation at the air-lung interface. Amino Acids. 2003;25:375–396. doi: 10.1007/s00726-003-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 37.Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, et al. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. doi: 10.1007/s00424-008-0477-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manzanares D, Rodriguez-Capote K, Liu S, Haines T, Ramos Y, Zhao L, et al. Modification of tryptophan and methionine residues is implicated in the oxidative inactivation of surfactant protein B. Biochemistry. 2007;465:604–615. doi: 10.1021/bi062304p. [DOI] [PubMed] [Google Scholar]
- 39.Su WY, Gordon T. Alterations in surfactant protein A after acute exposure to ozone. J Appl Physiol. 1996;80:1560–1567. doi: 10.1152/jappl.1996.80.5.1560. [DOI] [PubMed] [Google Scholar]
- 40.Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G, et al. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008;294:L121–L130. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, et al. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem. 2000;275:38848–38855. doi: 10.1074/jbc.M005322200. [DOI] [PubMed] [Google Scholar]
- 42.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220:72–82. doi: 10.1016/j.taap.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kierstein S, Poulain FR, Cao Y, Grous M, Mathias R, Kierstein G, et al. Susceptibility to ozone-induced airway inflammation is associated with decreased levels of surfactant protein D. Respir Res. 2006;7:85. doi: 10.1186/1465-9921-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer AK, Malkinson AM, Kleeberger SR. Susceptibility to neoplastic and non-neoplastic pulmonary diseases in mice: genetic similarities. Am J Physiol Lung Cell Mol Physiol. 2004;287:L685–L703. doi: 10.1152/ajplung.00223.2003. [DOI] [PubMed] [Google Scholar]
- 45.Prahalad AK, Inmon J, Ghio AJ, Gallagher JE. Enhancement of 2′-deoxyguanosine hydroxylation and DNA damage by coal and oil fly ash in relation to particulate metal content and availability. Chem Res Toxicol. 2000;13:1011–1019. doi: 10.1021/tx000110j. [DOI] [PubMed] [Google Scholar]
- 46.Prahalad AK, Inmon J, Dailey LA, Madden MC, Ghio AJ, Gallagher JE. Air pollution particles mediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem Res Toxicol. 2001;14:879–887. doi: 10.1021/tx010022e. [DOI] [PubMed] [Google Scholar]
- 47.Tokiwa H, Sera N, Nakanishi Y, Sagai M. 8-Hydroxyguanosine formed in human lung tissues and the association with diesel exhaust particles. Free Radic Biol Med. 1999;27:1251–1258. doi: 10.1016/s0891-5849(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 48.Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14:1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- 49.Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ Res. 2003;93:279–284. doi: 10.1016/s0013-9351(03)00041-0. [DOI] [PubMed] [Google Scholar]
- 50.Ito K, Inoue S, Hiraku Y, Kawanishi S. Mechanism of site-specific DNA damage induced by ozone. Mutat Res. 2005;585:60–70. doi: 10.1016/j.mrgentox.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Xie J, Fan R, Meng Z. Protein oxidation and DNA-protein crosslink induced by sulfur dioxide in lungs, livers, and hearts from mice. Inhal Toxicol. 2007;19:759–765. doi: 10.1080/08958370701399885. [DOI] [PubMed] [Google Scholar]
- 52.Williams AS, Issa R, Leung SY, Nath P, Ferguson GD, Bennett BL, et al. Attenuation of ozone-induced airway inflammation and hyper-responsiveness by c-Jun NH2 terminal kinase inhibitor SP600125. J Pharmacol Exp Ther. 2007;322:351–359. doi: 10.1124/jpet.107.121624. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, et al. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol. 2006;34:174–181. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonicelli F, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, et al. Nacystelyn inhibits oxidant-mediated interleukin-8 expression and NF-kappaB nuclear binding in alveolar epithelial cells. Free Radic Biol Med. 2002;32:492–502. doi: 10.1016/s0891-5849(01)00820-6. [DOI] [PubMed] [Google Scholar]
- 55.Dagher Z, Garcon G, Billet S, Verdin A, Ledoux F, Courcot D, et al. Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2007;27:284–290. doi: 10.1002/jat.1211. [DOI] [PubMed] [Google Scholar]
- 56.Valacchi G, Pagnin E, Corbacho AM, Olano E, Davis PA, Packer L, et al. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic Biol Med. 2004;36:673–681. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Churg A, Xie C, Wang X, Vincent R, Wang RD. Air pollution particles activate NF-kappaB on contact with airway epithelial cell surfaces. Toxicol Appl Pharmacol. 2005;208:37–45. doi: 10.1016/j.taap.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 60.Lundborg M, Bouhafs R, Gerde P, Ewing P, Camner P, Dahlen SE, et al. Aggregates of ultrafine particles modulate lipid peroxidation and bacterial killing by alveolar macrophages. Environ Res. 2007;104:250–257. doi: 10.1016/j.envres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Kienast K, Knorst M, Lubjuhn S, Muller-Quernheim J, Ferlinz R. Nitrogen dioxide-induced reactive oxygen intermediates production by human alveolar macrophages and peripheral blood mononuclear cells. Arch Environ Health. 1994;49:246–250. doi: 10.1080/00039896.1994.9937474. [DOI] [PubMed] [Google Scholar]
- 62.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 63.Cuzzocrea S, Zingarelli B, Caputi AP. Peroxynitrate-mediated DNA strand breakage activates poly(ADP-ribose) synthetase and causes cellular energy depletion in a nonseptic shock model induced by zymosan in the rat. Shock. 1998;9:336–340. doi: 10.1097/00024382-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Kirsch M, de Groot H. Ascorbate is a potent antioxidant against peroxynitrite-induced oxidation reactions. Evidence that ascorbate acts by re-reducing substrate radicals produced by peroxynitrite. J Biol Chem. 2000;275:16702–16708. doi: 10.1074/jbc.M909228199. [DOI] [PubMed] [Google Scholar]
- 65.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 66.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 67.Lehnig M. Radical mechanisms of the decomposition of peroxynitrite and the peroxynitrite-CO(2) adduct and of reactions with L-tyrosine and related compounds as studied by (15)N chemically induced dynamic nuclear polarization. Arch Biochem Biophys. 1999;368:303–318. doi: 10.1006/abbi.1999.1268. [DOI] [PubMed] [Google Scholar]
- 68.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD and smokers. Am J Respir Crit Care Med. 1996;159:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 69.Emelyanov A, Fedoseev G, Abulimity A, Rudinski K, Fedoulov A, Karabanov A, et al. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- 70.Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–1307. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- 71.Monteseirin J, Bonilla I, Camacho J, Conde J, Sobrino F. Elevated secretion of myeloperoxidase by neutrophils from asthmatic patients: the effect of immunoptherapy. J Allergy Clin Immunol. 2001;1007:623–626. doi: 10.1067/mai.2001.113566. [DOI] [PubMed] [Google Scholar]
- 72.Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, et al. Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic Biol Med. 2002;33:847–856. doi: 10.1016/s0891-5849(02)00976-0. [DOI] [PubMed] [Google Scholar]
- 73.Nadeem A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 74.Mak JC, Leung HC, Ho SP, Law BK, Lam WK, Tsang KW, et al. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 114:260–264. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione in induced sputum of healthy individual and patients with asthma. Thorax. 2001;56:13–18. doi: 10.1136/thorax.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corradi M, Folesani G, Andreoli R, Manini P, Bodini A, Piacentini G, et al. Aldehydes and glutathione in exhaled breath condensates of children with asthma exacerbations. Am J Respir Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 77.MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 78.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA, Jr, et al. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy. 2008;63:438–446. doi: 10.1111/j.1398-9995.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 79.Inoue K, Takano H, Yanagisawa R, Sakurai M, Abe S, Yoshino S, et al. Effects of components derived from diesel exhaust particles on lung physiology related to antigen. Immunopharmacol Immunotoxicol. 2007;29:403–412. doi: 10.1080/08923970701675002. [DOI] [PubMed] [Google Scholar]
- 80.Wan J, Diaz-Sanchez D. Antioxidant enzyme induction: a new protective approach against the adverse effects of diesel exhaust particles. Inhal Toxicol. 2007;19(Suppl 1):177–182. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- 81.Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003;15:39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- 82.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 83.Chan RC, Wang M, Li N, Yanagawa Y, Onoé K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol. 2006;118:455–465. doi: 10.1016/j.jaci.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 84.Jang AS, Choi IS, Takizawa H, Rhim T, Lee JH, Park SW, et al. Additive effect of diesel exhaust particulates and ozone on airway hyperresponsiveness and inflammation in a mouse model of asthma. J Korean Med Sci. 2005;20:759–763. doi: 10.3346/jkms.2005.20.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 86.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Repir Crit Care Med. 1995;152:S77–S121. American Thoracic Society. [PubMed] [Google Scholar]
- 87.Bts guidelines for the management of chronic obstructive pulmonary disease. The COPD guidelines group of the standards care committee of the BTS. Thorax. 1997;52(Suppl 5):S1–S28. British Thoracic Society. [PMC free article] [PubMed] [Google Scholar]
- 88.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 89.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004:159–170. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 90.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med. 1996;21:669–681. doi: 10.1016/0891-5849(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 91.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159:473–479. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- 92.Gerritsen WB, Asin J, Zanen P, van den Bosch JM, Haas FJ. Markers of inflammation and oxidative stress in exacerbated chronic obstructive pulmonary disease patients. Respir Med. 2005;99:84–90. doi: 10.1016/j.rmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 93.Tomaki M, Sugiura H, Koarai A, Komaki Y, Akita T, Matsumoto T, et al. Decreased expression of antioxidant enzymes and increased expression of chemokines in COPD lung. Pulm Pharmacol Ther. 2007;20:596–605. doi: 10.1016/j.pupt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 94.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 95.Tjalkens RB, Luckey SW, Kroll DJ, Petersen DR. Alpha, beta-unsaturated aldehydes mediate inducible expression of glutathione S-transferase in hepatoma cells through activation of the antioxidant response element (ARE) Adv Exp Med Biol. 1999;463:123–131. doi: 10.1007/978-1-4615-4735-8_15. [DOI] [PubMed] [Google Scholar]
- 96.Sahin U, Unlü M, Ozgüner F, Sütcü R, Akkaya A, Delibas N. Lipid peroxidation and glutathione peroxidase activity in chronic obstructive pulmonary disease exacerbation: prognostic value of malondialdehyde. J Basic Clin Physiol Pharmacol. 2001;12:59–68. doi: 10.1515/jbcpp.2001.12.1.59. [DOI] [PubMed] [Google Scholar]
- 97.Corradi M, Rubinstein I, Andreoli R, Manini P, Caglieri A, Poli D, et al. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;15(167):1380–1386. doi: 10.1164/rccm.200210-1253OC. [DOI] [PubMed] [Google Scholar]
- 98.Nowak D, Kasielski M, Antczak A, Pietras T, Bialasiewicz P. Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: no significant effect of cigarette smoking. Respir Med. 1999;93:389–396. doi: 10.1053/rmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- 99.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 100.Hanta I, Kocabas A, Canacankatan N, Kuleci S, Seydaoglu G. Oxidant-antioxidant balance in patients with COPD. Lung. 2006;184:51–55. doi: 10.1007/s00408-005-2561-4. [DOI] [PubMed] [Google Scholar]
- 101.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 102.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 103.Siler TM, Swierkosz JE, Hyers TM, Fowler AA, Webster RO. Immunoreactive interleukin-1 in bronchoalveolar lavage fluid of high-risk patients and patients with the adult respiratory distress syndrome. Exp Lung Res. 1989;15:881–894. doi: 10.3109/01902148909069633. [DOI] [PubMed] [Google Scholar]
- 104.Kietzmann D, Kahl R, Müller M, Burchardi H, Kettler D. Hydrogen peroxide in expired breath condensate of patients with acute respiratory failure and with ARDS. Intensive Care Med. 1993;19(2):78–81. doi: 10.1007/BF01708366. [DOI] [PubMed] [Google Scholar]
- 105.Roumen RM, Hendriks T, de Man BM, Goris RJ. Serum lipofuscin as a prognostic indicator of adult respiratory distress syndrome and multiple organ failure. Br J Surg. 1994;81:1300–1305. doi: 10.1002/bjs.1800810913. [DOI] [PubMed] [Google Scholar]
- 106.Quinlan GJ, Evans TW, Gutteridge JM. 4-hydroxy-2-nonenal levels increase in the plasma of patients with adult respiratory distress syndrome as linoleic acid appears to fall. Free Radic Res. 1994;21:95–106. doi: 10.3109/10715769409056561. [DOI] [PubMed] [Google Scholar]
- 107.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 108.Upton RL, Chen Y, Mumby S, Gutteridge JM, Anning PB, Nicholson AG, et al. Variable tissue expression of transferrin receptors: relevance to acute respiratory distress syndrome. Eur Respir J. 2003;22:335–341. doi: 10.1183/09031936.03.00075302. [DOI] [PubMed] [Google Scholar]
- 109.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 110.Rosias PP, Den Hartog GJ, Robroeks CM, Bast A, Donckerwolcke RA, Heynens JW, et al. Free radicals in exhaled breath condensate in cystic fibrosis and healthy subjects. Free Radic Res. 2006;40:901–909. doi: 10.1080/10715760500522648. [DOI] [PubMed] [Google Scholar]
- 111.Kettle AJ, Chan T, Osberg I, Senthilmohan R, Chapman AL, Mocatta TJ, et al. Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis. Am J Respir Crit Care Med. 2004;15(170):1317–1323. doi: 10.1164/rccm.200311-1516OC. [DOI] [PubMed] [Google Scholar]
- 112.Jones KL, Hegab AH, Hillman BC, Simpson KL, Jinkins PA, Grisham MB, et al. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr Pulmonol. 2000;30:79–85. doi: 10.1002/1099-0496(200008)30:2<79::aid-ppul1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 113.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006;290:C262–C270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 114.Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol. 2006;35:579–586. doi: 10.1165/rcmb.2005-0473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowley EA, Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol. 2002;15(543):201–209. doi: 10.1113/jphysiol.2002.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynolds WF, Sermet-Gaudelus I, Gausson V, Feuillet MN, Bonnefont JP, Lenoir G, et al. Myeloperoxidase promoter polymorphism -463G is associated with more severe clinical expression of cystic fibrosis pulmonary disease. Mediators Inflamm. 2006:36735. doi: 10.1155/MI/2006/36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aris RM, Christian D, Hearne PQ, Kerr K, Finkbeiner WE, Balmes JR. Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. Am Rev Respir Dis. 1993;148:1363–1372. doi: 10.1164/ajrccm/148.5.1363. [DOI] [PubMed] [Google Scholar]
- 118.Balmes JR, Chen LL, Scannell C, Tager I, Christian D, Hearne PQ, et al. Ozone-induced decrements in FEV1 and FVC do not correlate with measures of inflammation. Am J Respir Crit Care Med. 1996;153:904–909. doi: 10.1164/ajrccm.153.3.8630571. [DOI] [PubMed] [Google Scholar]
- 119.Weinmann GG, Bowes SM, Gerbase MW, Kimball AW, Frank R. Response to acute ozone exposure in healthy men. Results of a screening procedure. Am J Respir Crit Care Med. 1995;151:33–40. doi: 10.1164/ajrccm.151.1.7812569. [DOI] [PubMed] [Google Scholar]
- 120.McDonnell WF, 3rd, Horstman DH, Abdul-Salaam S, House DE. Reproducibility of individual responses to ozone exposure. Am Rev Respir Dis. 1985;131:36–40. doi: 10.1164/arrd.1985.131.S5.S36. [DOI] [PubMed] [Google Scholar]
- 121.Wang L, Pinkerton KE. Air pollutant effects on fetal and early postnatal development. Birth Defects Res (Part C) 2007;81:144–154. doi: 10.1002/bdrc.20097. [DOI] [PubMed] [Google Scholar]
- 122.Foos B, Marty M, Schwartz J, Bennett W, Moya J, Jarabek AM, et al. Focusing on children’s inhalation dosimetry and heath effects for risk assessment: an introduction. J Toxicol Environ Health, Part A. 2008;71:149–165. doi: 10.1080/15287390701597871. [DOI] [PubMed] [Google Scholar]
- 123.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health, Part A. 2008;71:238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 124.Pereira LA, Loomis D, Conceição GM, Braga AL, Arcas RM, Kishi HS, et al. Association between air pollution and intrauterine mortality in São Paulo, Brazil. Environ Health Perspect. 1998;106:325–329. doi: 10.1289/ehp.98106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111:1773–1778. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Srám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ Health Perspect. 2005;113:375–382. doi: 10.1289/ehp.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11:502–511. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 128.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr CA. cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gessner BD, Chimonas MA. Asthma is associated with preterm birth but not with small for gestational age status among a population-based cohort of Medicaid-enrolled children <10 years of age. Thorax. 2007;62:231–236. doi: 10.1136/thx.2005.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108:173–176. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maisonet M, Bush TJ, Correa A, Jaakkola JJK. Relation between ambient air pollution and low birth weight in the northeastern United States. Environ Health Perspect. 2001;109(Suppl 3):351–356. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Slama R, Morgenstern V, Cyrys J, Zutavern A, Herbarth O, Wichmann HE, et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring's term birth weight: a study relying on a land-use regression exposure model. Environ Health Perspect. 2007;115:1283–1292. doi: 10.1289/ehp.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rogers JF, Thompson SJ, Addy CL, McKeown RE, Cowen DJ, DeCoulfe P. The association of very low birth weight with exposures to environmental sulfur dioxide and total suspended particulates. Am J Epidemiol. 2000;151:601–613. doi: 10.1093/oxfordjournals.aje.a010248. [DOI] [PubMed] [Google Scholar]
- 135.Rogers JF, Dunlop AL. Air pollution and very low birth weight infants: a target population? Pediatrics. 2006;118:156–164. doi: 10.1542/peds.2005-2432. [DOI] [PubMed] [Google Scholar]
- 136.Ritz B, Yu F, Fruin S, Chapa G, Shaw GM, Harris JA. Ambient air pollution and risk of birth defects in Southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- 137.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 138.Gilboa SM, Mendola P, Olshan AF, Langlois PH, Savitz DA, Loomis D, et al. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am J Epidemiol. 2005;162:238–252. doi: 10.1093/aje/kwi189. [DOI] [PubMed] [Google Scholar]
- 139.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollutants and mortality in 20 U.S. cities. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 140.Ha EH, Lee JT, Kim H, Hong YC, Lee BE, Park HS, et al. Infant susceptibility of mortality to air pollution in Seoul, South Korea. Pediatrics. 2003;111:284–290. doi: 10.1542/peds.111.2.284. [DOI] [PubMed] [Google Scholar]
- 141.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Does particulate air pollution contribute to infant death? A systematic review. Environ Health Perspect. 2004;112:1365–1371. doi: 10.1289/ehp.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ Health Perspect. 2008;116:110–115. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dales R, Burnett RT, Smith-Doiron M, Stieb DM, Brook JR. Air pollution and sudden infant death syndrome. Pediatrics. 2004;113:628–631. doi: 10.1542/peds.113.6.e628. [DOI] [PubMed] [Google Scholar]
- 144.Tong S, Colditz P. Air pollution and sudden infant death syndrome: a literature review. Paediatr Perinat Epidemiol. 2004;18:327–335. doi: 10.1111/j.1365-3016.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 145.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 146.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 147.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 148.Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358:1700–1711. doi: 10.1056/NEJMra0707601. [DOI] [PubMed] [Google Scholar]
- 149.Cardoso WV. The Lung: Development, Aging and the environment. London: Elsevier Academic Press; 2004. Lung morphogenesis, role of growth factors and transcription factors; pp. 3–11. [Google Scholar]
- 150.Pinkerton KE, Joad JP. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol. 2006;33:269–272. doi: 10.1111/j.1440-1681.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 151.Miller LA. The Lung: Development, Aging and the environment. London: Elsevier Academic Press; 2004. Development of the pulmonary immune system; pp. 169–176. [Google Scholar]
- 152.Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997;8:298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 153.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 154.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 155.Delfino RJ, Staimer N, Tjoa T, Gillen D, Kleinman MT, Sioutas C, et al. Personal and ambient air pollution exposures and lung function decrements in children with asthma. Environ Health Perspect. 2008;116:550–558. doi: 10.1289/ehp.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Etzel RA. How environmental exposures influence the development and exacerbation of asthma. Pediatrics. 2003;112:233–239. [PubMed] [Google Scholar]
- 157.Ko FW, Tam W, Wong TW, Lai CK, Wong GW, Leung TF, et al. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clin Exp Allergy. 2007;37:1312–1319. doi: 10.1111/j.1365-2222.2007.02791.x. [DOI] [PubMed] [Google Scholar]
- 158.Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, et al. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Respir Crit Care Med. 1996;154:300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- 159.Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12:200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 160.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 161.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 162.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 163.Anderson HR, Atkinson RW, Bremner SA, Marston L. Particulate air pollution and hospital admissions for cardiorespiratory diseases: are the elderly at greater risk? Eur Respir J. 2003;21(Suppl 40) doi: 10.1183/09031936.03.00402203. 39s–46s. [DOI] [PubMed] [Google Scholar]
- 164.Kelly FJ. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc. 2005;64:510–526. doi: 10.1079/pns2005457. [DOI] [PubMed] [Google Scholar]
- 165.Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357:2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- 166.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 167.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15:143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- 168.Sunyer J, Schwartz J, Tobías A, Macfarlane D, Garcia J, Antó JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 169.Akbaraly TN, Favier A, Berr C. Total plasma carotenoids and mortality in the elderly: results of the epidemiology of vascular ageing (EVA) study. Br J Nutr. doi: 10.1017/S0007114508998445. in press. [DOI] [PubMed] [Google Scholar]
- 170.Alexeeff SE, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Statin use reduces decline in lung function. VA normative aging study. Am J Respir Crit Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, et al. Downregulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and COPD patients. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2007-0424OC. in press. [DOI] [PubMed] [Google Scholar]
- 172.Alexeeff SE, Litonjua AA, Wright AA, Baccarelli A, Suh H, Sparrow D, et al. Ozone exposure, antioxidant genes, and lung function in an elderly cohort. VA normative aging study. J Occup Environ Med. doi: 10.1136/oem.2007.035253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romieu I, Sienra-Monge JJ, Ramírez-Aguilar M, Moreno-Macías H, Reyes-Ruiz NI, Estela del Río-Navarro B. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 174.McDonnell WF. Intersubject variability in human acute ozone responsiveness. Pharmacogenetic. 1991;1:110–113. doi: 10.1097/00008571-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 175.Kleeberger SR, Bassett DJP, Jakab GJ, Levitt RC. A genetic model for the evaluation of susceptibility to ozone-induced inflammation. Am J Physiol Lung Cell Mol Physiol. 1990;258:L313–L320. doi: 10.1152/ajplung.1990.258.6.L313. [DOI] [PubMed] [Google Scholar]
- 176.Kleeberger SR, Levitt RC, Zhang L-Y, Longphre M, Harkema J, Jedlicka A, et al. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet. 1997;17:475–478. doi: 10.1038/ng1297-475. [DOI] [PubMed] [Google Scholar]
- 177.Kleeberger SR, Reddy SPM, Zhang L-Y, Jedlicka AE. Genetic susceptibility to ozone-induced lung hyperpermeability: role of the toll-like receptor 4 (Tlr4) Am J Respir Cell Mol Biol. 2000;22:620–627. doi: 10.1165/ajrcmb.22.5.3912. [DOI] [PubMed] [Google Scholar]
- 178.Yang IA, Fong KM, Zimmerman PV, Holgate ST, Holloway JW. Genetic susceptibility to the respiratory effects of air pollution. Thorax. 2008;63:555–563. doi: 10.1136/thx.2007.079426. [DOI] [PubMed] [Google Scholar]
- 179.Bergamaschi E, De Palma G, Mozzoni P, Vanni S, Vettori MV, Broeckaert F, et al. Polymorphism of quinone-metabolizing enzymes and susceptibility to ozone-induced acute effects. Am J Respir Crit Care Med. 2001;163:1426–1431. doi: 10.1164/ajrccm.163.6.2006056. [DOI] [PubMed] [Google Scholar]
- 180.Yang IA, Holz O, Jorres RA, Magnussen H, Barton SJ, Rodriguez S, et al. Association of tumor necrosis factor-alpha polymorphisms and ozone-induced change in lung function. Am J Respir Crit Care Med. 2005;171:171–176. doi: 10.1164/rccm.200402-194OC. [DOI] [PubMed] [Google Scholar]
- 181.Winterton DL, Kaufman J, Keener CV, Quigley S, Farin FM, Williams PV, et al. Genetic polymorphisms as biomarkers of sensitivity to inhaled sulfur dioxide in subjects with asthma. Ann Allergy Asthma Immunol. 2001;86:232–238. doi: 10.1016/S1081-1206(10)62697-X. [DOI] [PubMed] [Google Scholar]
- 182.Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med. 2006;173:970–976. doi: 10.1164/rccm.200508-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.David GL, Romieu I, Sienra-Monge JJ, Collins WJ, Ramirez-Aguilar M, del Rio-Navarro BE, et al. Nicotinamide adenine dinucleotide (phosphate) reduced:quinone oxidoreductase and glutathione S-transferase M1 polymorphisms and childhood asthma. Am J Respir Crit Care Med. 2003;168:1199–1204. doi: 10.1164/rccm.200305-684OC. [DOI] [PubMed] [Google Scholar]
- 184.Chen C, Arjomandi M, Tager IB, Holland N, Balmes JR. Effects of antioxidant enzyme polymorphisms on ozone-induced lung function changes. Eur Respir J. 2007;30:677–683. doi: 10.1183/09031936.00160806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cho H, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia: Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- 186.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 187.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 189.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 190.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 191.Kleeberger SR, Schwartz DA. From quantitative trait locus to gene: a work in progress. Am J Respir Crit Care Med. 2005;171:804–805. doi: 10.1164/rccm.2501002. [DOI] [PubMed] [Google Scholar]
- 192.Kleeberger SR, Peden D. Gene-environment interactions in asthma and other respiratory diseases. Annu Rev Med. 2005;56:383–400. doi: 10.1146/annurev.med.56.062904.144908. [DOI] [PubMed] [Google Scholar]