Abstract
We examined the ability to use static line drawings of eye gaze cues to orient visual-spatial attention in children with high functioning autism (HFA) compared to typically developing children (TD). The task was organized such that on valid trials, gaze cues were directed toward the same spatial location as the appearance of an upcoming target, while on invalid trials gaze cues were directed to an opposite location. Unlike TD children, children with HFA showed no advantage in reaction time (RT) on valid trials compared to invalid trials (i.e., no significant validity effect). The two stimulus onset asynchronies (200 ms, 700 ms) did not differentially affect these findings. The results suggest that children with HFA show impairments in utilizing static line drawings of gaze cues to orient visual-spatial attention.
Keywords: Eye gaze, High functioning autism, Orienting, Attention, Cueing
Children with autism have abnormalities in three general domains of functioning: qualitative impairments in social interaction, impairments in communication, and the presence of restrictive, repetitive, and stereotyped patterns of behavior, interests, and activities (APA 1994). Impairments in the quality of social interactions and social communication in children with autism may be related to deficits in the ability to understand the meaning of nonverbal behaviors that are for example, marked by changes in the direction of eye gaze. Children with autism appear to be able to discriminate the direction that eyes are gazing (Baron-Cohen et al. 1995). However, they show deficits in using eye gaze to infer information related to another person's mental states (e.g., desires, goals, Baron-Cohen et al. 1995). Children with autism are also reported to have deficits in using information provided by another person's change in eye gaze to look toward the same target in the periphery (Leekam et al. 1997). The purpose of the present study was to use a simple reaction time task to examine the extent to which children with HFA are able to utilize information provided by static line drawings of eye gaze cues to orient visual-spatial attention compared to TD children.
Posner (1980) and his colleagues developed a cognitive-neuropsychological paradigm that has been used to examine processes that play a key role in visual-spatial attention. The paradigm can vary with regard to the type of cue that is used to direct attention to a location in the periphery (e.g., the brightening of a box in the periphery, a central arrow, eye gaze cue). Studies of healthy adults on “Posner-tasks” examining the effects of eye gaze cues on manual reaction time have shown that adults respond faster to targets appearing at gazed-at locations compared to trials on which eye gaze cues are directed to a location opposite to where the target appears (Friesen and Kingstone 1998; Hietanen and Leppänen 2003; Langton and Bruce 2000). These findings suggest that eye gaze cues can be used to orient visual-spatial attention. The ability to utilize eye gaze cues to orient visual attention has also been examined in adults with autism (Bayliss and Tipper 2005; Ristic et al. 2005; Vlamings et al. 2005).
Developmental studies have also examined the effects of eye gaze cues on the ability to orient visual-spatial attention in typically developing children as young as infants. Studies of normal development suggest that the perception of eye gaze cues emerges in early infancy (Vecera and Johnson 1995), and that the ability to utilize eye gaze cues to orient visual-spatial attention develops shortly thereafter (Farroni et al. 2000; Hood et al. 1998). There have even been studies of gaze cueing in children with autism as young as 2-years-of-age (Chawarska et al. 2003).
To our knowledge, there have been only three studies in the literature that have examined the effects of eye gaze cues on the ability to orient visual-spatial attention in school-aged children with autism (Kylliäinen and Hietanen 2004; Senju et al. 2004; Swettenham et al. 2003). Results from two of those studies showed that children with HFA were able to utilize eye gaze cues to orient visual-spatial attention as well as typically developing children (Kylliainen and Hietanen 2004; Swettenham et al. 2003). Findings from the third study (Senju et al. 2004) showed that differences between children with autism and typically developing controls were related to the stimulus onset asynchrony (SOA, 100, 300, 700, 1,000 ms) between the onset of the cue and the appearance of the target: children with autism and typically developing children showed similar size cueing effects at a 300 ms SOA. However, only the group of children with autism showed a significant cueing effect at the 700 ms SOA. Neither group showed significant cueing effects at 100 ms or 1,000 ms SOAs. Overall, the study by Senju and colleagues suggests that the ability to orient attention in response to eye gaze cues may differ between children with autism and controls, particularly at a 700 ms SOA.
The purpose of the present study was to compare the performance of school-aged children with HFA to TD children on a simple reaction time task involving the static presentation of line drawings of eye gaze cues. The task in the present study is similar to one that was previously used to study the performance of a patient (EVR) following frontal lobe damage (Vecera and Rizzo 2004, 2006).
Methods
Participants
This study involved two groups of children ages 8 through 13 years of age. There were 22 children with high functioning autism (HFA, 16 males, six females; 86.4% Caucasian) and 49 typically developing controls (TD, 25 males, 24 females; 79.6% Caucasian). There were no significant differences in gender ratio between the HFA and TD groups (χ2 = 2.93, p = .10). Participants were recruited from outpatient clinics at the Kennedy Krieger Institute, local Autism Society of America (ASA) chapters, postings at schools, social skills groups, pediatricians’ offices, and by word-of-mouth.
Demographic information for the two groups including mean scores and standard deviations for age, IQ, and SES are presented in Table 1. The two groups did not differ significantly in age. The mean age for the HFA group was 10.47 years, and the mean age for the TD group was 10.41 years. All of the participants received the Wechsler Intelligence Scale for Children. (All were administered the WISC-IV, Wechsler 2003, except two children were tested using the WISC-III, Wechsler 1991.) Of the 71 participants in the study, 70 had Full Scale IQ scores of 80 or above. The remaining child (in the HFA group) had a WISC-IV Full Scale IQ of 76, but his Verbal Comprehension Index and Perceptual Reasoning Index were within the average (VCI of 91) and low average (PRI of 88) range. Table 1 shows that Full-scale IQ scores were significantly higher for children in the TD group (M = 113.5) compared to children in the HFA group (M = 100.6). The two groups did not differ significantly in terms of socio-economic status (SES) based on the Hollingshead (1975). SES data were available for 18 out of 22 children in the HFA group and for 47 out of 49 children in the TD group. There were no significant differences between groups based on SES.
Table 1.
Demographic information
| Age | FSIQ* | SES | |
|---|---|---|---|
| High functioning autism group | |||
| Mean | 10.47 | 100.60 | 56.75 |
| Standard deviation | 1.77 | 15.54 | 8.64 |
| Typically developing group | |||
| Mean | 10.41 | 113.53 | 54.52 |
| Standard deviation | 1.42 | 14.59 | 9.01 |
p < .01
High Functioning Autism Group (HFA)
All children in the HFA group were diagnosed with autism according to the Autism Diagnostic Interview-Revised (ADI-R, Lord et al. 1994; Le Couteur et al. 2003) and the Autism Diagnostic Observation Schedule-Generic (ADOS-G, Lord et al. 2000). The group included only those with idiopathic autism (e.g., no history of Fragile X, encephalitis, or other known medical conditions associated with autism).
The clinician-administered Diagnostic Interview for Children and Adolescents-IV (DICA-IV, Reich et al. 1997) was used to assess the presence of comorbid psychiatric symptoms. Eleven out of the 22 children with autism were determined to have comorbid symptoms including Attention-Deficit/Hyperactivity Disorder, Obsessive Compulsive Disorder, Oppositional Defiant Disorder, Conduct Disorder, Major Depressive Disorder-past, Generalized Anxiety Disorder, and/or Phobias. There were five participants in the HFA group, each known to have a primary diagnosis of autism, who were taking stimulant medications (e.g., Adderall, Concerta, Dexedrine). These stimulant medications were withheld the day prior to testing and on the day of testing. Children in the HFA group who were taking longer lasting medications (e.g., Celexa, Buspar, Prozac, Clonidine, Depakote, Respiridone) did not have those medications withheld.
Typically Developing (TD) Control Group
Children were excluded from the control group if they were suspected of meeting criteria for any of the above listed psychiatric diagnoses on the DICA-IV (Reich et al. 1997), with the exception of a specific phobia. Children in the control group had no history or current use of any psychoactive medication, no history of seizures or other major neurological disorder, and no history of learning disabilities.
The study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained from a parent/guardian and written assent was obtained from all participating children.
Procedure and Stimuli
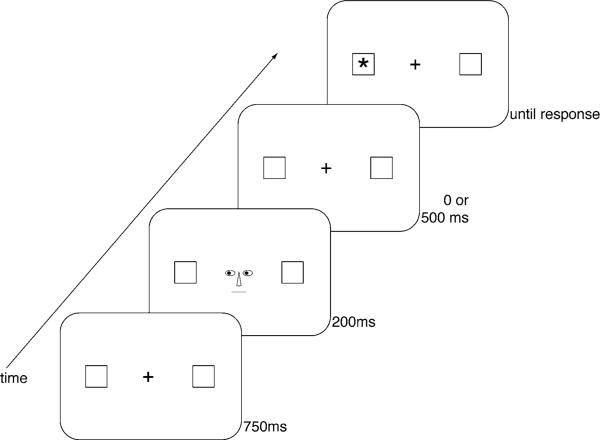
The procedure and stimuli were similar to the descriptions in the papers by Vecera and Rizzo (2004, 2006) with the exception that the schematic line drawings of the eye gaze cues were not surrounded by the outline of a face (see Fig. 1).
Fig. 1.
The stimuli, sequence, and time course of events are depicted. The figure illustrates events on a valid trial. On invalid trials, a target (asterisk) appeared in a peripheral box opposite to the direction of the eye gaze cues
The task was administered using an iMAC OS 9.1 computer with a 14-inch monitor. Participants were seated 80 cm from the computer screen with the keyboard placed within arms’ reach. Participants were instructed to keep looking in the center of the computer screen and press the spacebar on the keyboard as soon as they detected a “star” (an asterisk) in a peripheral box on the computer screen.
The stimuli and time course of events on each trial are depicted in Fig. 1. On each trial, a central fixation point was shown with two empty boxes (0.79° (1.1 cm) high and wide) located 4.82° (6.75 cm) to the right and left of fixation for 750 ms. Next, eye gaze cues, consisting of the schematic drawing of eyes (1.29° or 1.8 cm wide), a nose, and a mouth, were presented for 200 ms. There were two types of eye gaze cues: eyes that were directed to the left visual field and eyes that were directed to the right visual field. The colored portion of the eyes (0.5° or 0.7 cm wide) was moved either 2 mm to the right or left (see Vecera and Rizzo 2004, for details). Following an interstimulus interval of 0 ms (200 ms stimulus onset asynchrony, SOA) or 500 ms (700 ms SOA), a target stimulus, an asterisk (0.64° high by 0.64° wide; 0.9 cm by 0.9 cm), appeared in either the right or left peripheral box until the subject made a response by pressing the spacebar on the keyboard.
Trials in which the eye gaze cues were directed toward the same location as the target were termed valid trials. Trials in which the eye gaze cues were directed in the opposite direction from the target were termed invalid trials. In addition, catch trials were included on which there was no target stimulus following the eye gaze cue. Subjects were told to withhold responding on catch trials. Catch trials were included to make sure that subjects were responding properly to the target stimulus when it appeared. Subjects were presented with auditory feedback (a beep) from the computer when an incorrect response was made on a catch trial. Responses on catch trials were coded as errors.
Each participant completed four blocks of trials, with 60 trials in each block for a total of 240 trials. There was a rest period between each block. In each block, there were 12 catch trials on which no target appeared (20%). In each block, of the 48 trials in which a target appeared, 24 were valid (50%) and 24 were invalid (50%). On half of the trials, the eye gaze cues were directed to the right visual field, and on the other half of the trials, the eye gaze cues were directed to the left visual field. Half of the targets were presented after a 200 ms stimulus onset asynchrony (SOA) and half were presented following a 700 ms SOA. In addition, on half of the target trials, an asterisk appeared in the left box. On the other half, an asterisk appeared in the right box. The order of the blocks was randomized and the order of trials within each block was different for each subject.
Prior to participating in the task, participants were administered a practice session consisting of 10 trials. If more than three errors were made during the practice session, the practice session was repeated until three or fewer mistakes were made. Participants were allowed up to three practice sessions if needed. Practice session data were available for 21 of the 22 children in the HFA group and for 47 of the 49 children in the control group. All except for two participants met criterion on the practice trials in one session. Two of the control subjects took two practice sessions. Upon completion of the task, in order to confirm that participants were able to perceive the direction of the eye gaze cues, participants were shown the eye gaze cues and were asked to report the direction that the eyes were looking on each trial (n = 10 trials). Post-test data were available for 18 out of the 22 children in the HFA group and for 40 out of the 49 children in the TD group. Children in the HFA group and children in the TD group demonstrated an accuracy of seven trials or better (out of 10 trials) on the post-test trials.
Results
Reaction times were measured for all subjects as the time from the onset of the target presentation to the onset of a response on the space bar. Responses shorter than 150 ms (anticipatory errors) and responses longer than 1,500 ms (long latencies) were eliminated. The cut-offs for anticipatory errors and long latencies were determined based upon what has previously been used in the literature (e.g., Bayliss and Tipper 2005; Kylliäinen and Hietanen 2004; Vecera and Rizzo 2004, 2006).
Table 2 displays the percentage of anticipatory errors, long latencies, and responses on catch trials (errors) for participants in each group. T-tests indicated significant differences between groups for anticipatory errors, trials with long latencies, and responses on catch trials (p-values all less than .01). Children in the HFA group made more anticipatory errors, had more trials with long latencies, and made more responses on catch trials compared to children in the TD group.
Table 2.
Means and standard deviations for the percentage of trials with anticipatory errors, trials with long latencies, and responses on catch trials by group
| High functioning autism group |
Typically developing group |
|||
|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | |
| Anticipations* (reaction times shorter than 150 ms) | 2.77 | 3.21 | 1.21 | 1.45 |
| Long latencies* (reaction times longer than 1,500 ms) | 1.80 | 2.39 | .61 | .70 |
| Responses on catch trials* (no target following eye gaze cue) | 21.59 | 16.60 | 10.76 | 9.98 |
p < .01
Mean reaction times were analyzed by using a repeated measures ANCOVA with Group (autism, control) as a between-subject factor and Cue type (valid, invalid) and SOA (200, 700 ms) as within-subjects factors. To statistically account for differences in IQ, Full-scale IQ was included as a covariate factor in the ANCOVA. Table 3 shows the mean reaction times and standard errors for each Cue type (valid, invalid) and SOA (200 ms, 700 ms) for children in the HFA group and for children in the TD group.
Table 3.
Mean reaction time and standard error for Cue type and SOA for each group
| 200 ms SOA |
700 ms SOA |
|||
|---|---|---|---|---|
| Valid | Invalid | Valid | Invalid | |
| High functioning autism group | ||||
| Mean | 500.90 | 504.49 | 504.83 | 504.80 |
| Standard error | 22.61 | 23.89 | 19.04 | 20.24 |
| Typically developing group | ||||
| Mean | 455.85 | 466.11 | 436.20 | 452.43 |
| Standard error | 15.15 | 16.01 | 12.76 | 13.56 |
The Levene's Test of Equality of Error Variances showed a significant difference between groups in the variance on valid trials at the 700 ms SOA (F(1,69) = 8.80, p < .05). Specifically, the TD group showed less variability in their reaction times on valid trials at the 700 ms SOA compared to the HFA group. This suggests that TD children were easily and relatively more consistently able to show an advantage in reaction time from the valid cues at the longer latency. There were no other significant differences between groups according to the Levene's test on invalid trials at the 200 ms and 700 ms SOAs or on valid trials at the 200 ms SOA.
The ANCOVA showed a significant Group (autism, control) by Cue type (valid, invalid) interaction, F(1,68) = 5.74, p < .05. No main effect was found for Group (F(1,68) = 1.44, p > .05), Cue (F(1,68) = 2.54, p > .05), or SOA (F(1,68) = .22, p > .05). The interaction effects of Group by SOA and Cue type by SOA were not significant F(1,68) = 1.69, p = .20 and F(1,68) = .22, p = .64, respectively. In addition, the three-way interaction of Group by Cue type by SOA was not significant F(1,68) = .34, p = .56.
To further analyze the significant Group by Cue type interaction, we conducted a t-test between groups on the validity effect (invalid reaction time minus valid reaction time) collapsed across SOA. The t-test was significant (p < .05). TD children showed significantly larger validity effects (M = 13.24 ms) compared with children with HFA (M = 1.77 ms). To further analyze the effects of the eye gaze cues, we conducted paired t-tests within each diagnostic group on invalid reaction time versus valid reaction time (RT). Results for TD children showed that reaction times on invalid trials were consistently slower than reaction times on valid trials (paired t-test, p < .001; invalid RT M = 459.27, SE = 13.34; valid RT M = 446.03, SE = 12.29). However, for children with HFA, there was no significant difference between reaction times on invalid trials and reaction times on valid trials (paired t test, p = .79; invalid RT M = 504.64, SE = 23.50; valid RT M = 502.86, SE = 23.22). These results show that there was no significant validity effect for children in the HFA group. These findings are illustrated by the differences in the slopes of the lines in Fig. 2.
Fig. 2.
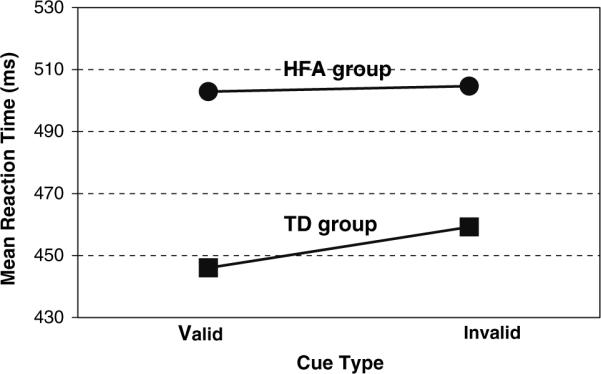
Mean reaction times on valid and invalid trials for children with high functioning autism (HFA) and typically developing children (TD) are illustrated. Note the slopes of the lines differ for children with HFA compared to TD children. Unlike children in the TD group, there was no significant validity effect for children in the HFA group
There was no significant correlation between age and the size of the validity effect for subjects in either group (HFA: r = −.270, p = .23; TD: r = −.269, p = .061). In addition, we inspected whether there was any significant correlation between impairments in social development and the ability to utilize eye gaze cue to shift attention by examining whether there were any significant correlations between the “Qualitative Impairments in Reciprocal Social Interaction” domain scores from the ADI and ADOS instruments and the size of the validity effect. The results showed there were no significant correlations between the size of the validity effect and the “Qualitative Impairments in Reciprocal Social Interaction” domain scores for the ADI (r = .068, p = .77) or for the ADOS (r = .064, p = .79).
Discussion
Under these conditions, in a simple detection task involving the static presentation of line drawings of eye gaze cues, 8 to 13-year-old children with HFA were not able to utilize eye gaze cues to orient visual-spatial attention and respond to the onset of a peripheral target to the same extent as TD children. Specifically, after accounting for IQ in the analyses, unlike TD children, children with HFA showed no advantage from eye gaze cues that were directed to the same spatial location as an upcoming target (valid) compared to eye gaze cues that were directed to an opposite spatial location (invalid). The two stimulus onset asynchronies (200 ms and 700 ms SOAs) did not differentially affect these findings. The failure to find a validity effect at a relatively long SOA (700 ms) argues against the hypothesis that participants with HFA deploy attention in an abnormally slow manner. That is, spatial attention does not appear to be ‘sluggish’ in these individuals.
Our findings are consistent with the results in high functioning adults with autism who were also tested on a simple reaction time task involving schematic line drawings of eye gaze cues (Ristic et al. 2005). Ristic and colleagues found that unlike typical adults, adults with autism did not show significant validity effects in response to eye gaze cues. However, when the eye gaze cues were made to be 80% predictive of the target location, adults with autism showed significant validity effects. Ristic and colleagues interpreted the results to suggest that adults with autism may be relatively more sensitive to changes in stimulus probability compared to normal adults and less sensitive to the “social power of human eyes.” It would be interesting for future studies to examine the performance of children with HFA on a task where the cues were 80% predictive of the target location to determine whether children with HFA perform as well as TD children when the predictive value of the cue is increased.
The results from the present study differ from the results from the studies by Kylliäinen and Hietanen (2004) and Swettenham and colleagues (2003) which found that children with HFA were able to utilize eye gaze cues to orient visual-spatial attention as well as TD children. In the study by Swettenham and colleagues (2003), the colored parts of the eyes (the pupils) appeared to be moving. Perceived motion of the pupils of the eyes may influence reaction time. Having the eyes appear to be moving could possibly facilitate responding to targets at cued locations, and such facilitation might not depend on the gaze direction itself but instead on the sensitivity to directed motion (see Chawarska et al. 2003; Farroni et al. 2000). Farroni and colleagues (2000) reported that directed movement of the pupils of the eyes, compared to static averted eye gaze, facilitates cueing effects in infants on a saccadic reaction time task. Furthermore, Farroni and colleagues (2003) reported for motion to be effective in cueing spatial locations, the motion needs to be preceded by a period of direct mutual gaze (eye contact). Chawarska and colleagues (2003) found that 2-year-old children with autism and typically developing children showed facilitation in saccadic reaction time on valid trials in response to an eye movement cue. Thus, in the study by Swettenham and colleagues (2003), the perception that the eyes were moving could possibly have facilitated the cueing effects. Additionally, different neural mechanisms may be involved in orienting attention guided by moving stimuli compared to static visual stimuli.
In the present study, the finding of no validity effect in the HFA group (when collapsed across the 200 ms and 700 ms SOAs) differs from the significant validity effects reported in the study by Senju and colleagues in children with HFA at the 300 ms and 700 ms SOAs. In the present study, children in the TD group showed a significant validity effect across both 200 and 700 ms SOAs, while the control group in the study by Senju and colleagues showed a significant validity effect only at the 300 ms SOA but not at the 700 ms SOA. The demand of the task (simple detection versus discrimination of target location) is unlikely to be an explanation for the differences in the findings between studies. It should be noted that while previous studies in the literature examining the effects of eye gaze cues on manual reaction time in children with autism involved photorealistic faces (Kylliäinen and Hietanen 2004; Senju et al. 2004; Swettenham et al. 2003), the literature suggests that line drawings of schematic faces and photorealistic faces are processed similarly, at least in gaze cueing studies.
The values for manual reaction times in this manuscript are similar to those reported in prior studies of gaze cueing in children with high functioning autism (Kylliäinen and Hietanen 2004; Senju et al. 2004; Swettenham et al. 2003). There was no significant difference in overall reaction time between children with HFA and TD children. In other words, after accounting for differences in IQ, children with HFA were not slower to respond than TD children. Kylliainen and Hietanen (2004) also reported no significant difference in reaction time between children with HFA and TD children.
Evidence from post-test trials supports the notion that children with HFA looked at the static line drawings of the eye gaze cues. Children with HFA demonstrated on post-test trials that they were able to discriminate and verbally report the direction that the eyes were looking with an accuracy of seven trials or better out of 10 trials (post-test data were available for 18 out of the 22 children with HFA). Thus, the absence of a validity effect in children with HFA is unlikely to be due to impairments in looking at and discriminating the direction of the eye gaze cues. It would be interesting for future studies to include measurements of eye movements.
Our findings in children with HFA are similar to the results shown by the frontal lobe patient EVR (Vecera and Rizzo 2004, 2006). Similar to the findings in children with HFA, EVR failed to show a validity effect in response to eye gaze cues (when tested with 100, 200, and 700 ms SOAs). EVR was also tested under two other cue-type conditions involving peripheral cues and word cues (e.g., RIGHT, LEFT). EVR demonstrated a typical sized validity effect in response to peripheral cues, suggestive of the ability to reflexively shift attention. However, he showed no validity effect in response to cueing mediated by word cues or by the eye gaze cues. These findings suggest that eye gaze cues may influence shifts of attention in a relatively voluntary or controlled manner rather than in an automatic or reflexive manner (Vecera and Rizzo 2004, 2006). Thus, under these conditions, the current findings in high functioning children with autism may suggest impairments in voluntary or controlled orienting of attention. There is evidence in the autism literature to support the suggestion of impairments in the executive control of attention in autism (e.g., Geurts et al. 2004; Goldberg et al. 2005; Hughes et al. 1994; Landa and Goldberg 2005; Ozonoff 1997; Ozonoff et al. 1991, 2004; Pennington and Ozonoff 1996) as well as differences in frontal lobe regions of the brain in individuals with autism (e.g., Bailey et al. 1998; Carper and Courchesne 2000; Girgis et al. 2007; Kemper and Bauman 1998; Ohnishi et al. 2000; Zilbovicius et al.1995).
Posner (1988, 1990) described three mechanisms involved in orienting attention on cueing tasks: attention must be disengaged from the current location, moved to a cued location, and then engaged in a target or stimulus at the new location. The pattern of findings in the present study (i.e., no significant validity effect in children with HFA), in some ways resembles the pattern shown by patients with a form of Parkinsonism called progressive supranuclear palsy (PSP). In patients with PSP, regions of the midbrain are damaged (superior colliculus, pretectum, periaqueductal grey and mesencephalic raphe degenerate) and there are accompanying deficits in producing voluntary saccades, particularly along the vertical meridian compared to the horizontal plane (Posner et al. 1982). Specifically, Posner and colleagues reported on a patient with PSP on a task using a peripheral cue, who showed no validity effect until 1,000 ms after the cue in the vertical direction, but a validity effect early on when tested in the horizontal direction (Posner et al. 1982, Fig. 5). From this pattern of finding, Posner and colleagues proposed that damage to the midbrain affects the latency of covert orienting and reflects a deficit in moving attention (Posner et al. 1982; Posner 1988, 1990).
Wainwright-Sharp and Bryson (1993) reported a similar suggestion that there may be impairments in moving attention in individuals with autism in a paradigm involving central (arrow) cues. That study showed in a sample of high functioning adolescents and adults with autism no significant validity effect at an early, 100 ms SOA, but a significant validity effect at a 800 ms SOA, suggesting a potential deficit in moving visual attention in autism. Future studies are needed comparing the effects of longer SOAs (e.g., 1,000 ms) on the ability to orient attention in our sample of children with HFA in order to be able to conclude whether there is an impairment in moving attention in response to eye gaze cues.
Functional magnetic resonance imaging (fMRI) studies have identified the superior temporal sulcus (STS) as a key neural region involved in eye gaze processing (Hooker et al. 2003; Kingstone et al. 2004; Mosconi et al. 2005; Pelphrey et al. 2003, 2005). Pelphrey and colleagues (2005) found that when typically developing adults and adults with autism viewed a photograph of a character producing shifts in eye gaze, they both activated the STS region. However, when gaze shifts were incongruent with the location of a peripheral target (invalid) compared to when the gaze shifts were congruent (valid), the results showed that only typically developing adults showed increased activity in the STS. In adults with autism, there was no differential activity in the STS between incongruent and congruent gaze shifts. Individuals with autism showed incongruent versus congruent activity in other brain regions including the insular cortex/inferior frontal gyrus and the posterior middle temporal and middle occipital gyri. These findings provide neural evidence for impairments in eye gaze processing in autism and converge with behavioral findings from the current investigation suggesting impairments in utilizing eye gaze cues in autism.
In conclusion, this study demonstrated that compared to TD children, children with HFA showed difficulty in using statically presented line drawings of eye gaze cues to orient visual-spatial attention. This study provides insight into possible mechanisms related to visual-spatial attention that might be disrupted in children with HFA. The similar pattern of performance in children with HFA to the one shown by the frontal lobe patient EVR on the same type of task involving static line drawings of eye gaze cues suggests that impairments to the frontal lobe may potentially contribute to deficits in the voluntary control of attention in individuals with autism. It would be interesting for future fMRI studies to examine whether similar to adults with autism, children with HFA also fail to show differential activity in the STS region of the brain to invalid versus valid eye gaze cues.
In order to be able to address the social implications of the eye gaze cues in the present study, it would be important for future directions to examine whether there are cueing differences between tasks involving line drawings of eye gaze cues and arrow cues in typically developing children. Furthermore, comparisons between static line drawings of eyes, realistic static photographs of real eyes, and videos of eyes moving (in the process of gazing) should be components of future research. Finally, it is of additional importance for future studies to examine the specificity of these findings to individuals with autism by examining whether the pattern of findings also pertains to children with other neurodevelopmental conditions.
Acknowledgments
This research was funded by the following grants: NIH Grants K01 MH 01824 (MCG), R01 NS048527 (SHM), K02 NS 044850 (SHM), HD024061 (Mental Retardation and Developmental Disabilities Research Center), M01 RR00052 (Johns Hopkins General Clinical Research Center); a grant from the National Alliance for Autism Research (SHM); and a National Science Foundation (NSF) award BCS 03-39171 (SPV). We would like to thank the participants and their parents for generously volunteering their time to participate in this study. We would like to thank Megan Roeder for her assistance in recruiting and screening participants for this project.
Contributor Information
Melissa C. Goldberg, Kennedy Krieger Institute, 707 North Broadway, Room 232, Baltimore, MD 21205, USA Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Allison J. Mostow, Kennedy Krieger Institute, 1750 East Fairmount Avenue, Baltimore, MD 21231, USA
Shaun P. Vecera, The University of Iowa, Iowa City, IA, USA
Jennifer C. Gidley Larson, Kennedy Krieger Institute, 707 North Broadway, Room 232, Baltimore, MD 21205, USA.
Stewart H. Mostofsky, Kennedy Krieger Institute, 707 North Broadway, Room 232, Baltimore, MD 21205, USA Johns Hopkins University School of Medicine, Baltimore, MD, USA.
E. Mark Mahone, Kennedy Krieger Institute, 707 North Broadway, Room 232, Baltimore, MD 21205, USA; Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Martha B. Denckla, Kennedy Krieger Institute, 707 North Broadway, Room 232, Baltimore, MD 21205, USA Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington: 1994. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? British Journal of Developmental Psychology. 1995;13:379–398. [Google Scholar]
- Bayliss AP, Tipper SP. Gaze and arrow cueing of attention reveals individual differences among the autism spectrum as a function of target context. British Journal of Psychology. 2005;96:95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain. 2000;123:834–844. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with autism. Child Development. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Brockbank M, Simion F. Infants’ use of gaze direction to cue attention: The importance of perceived motion. Visual Cognition. 2000;7:705–718. [Google Scholar]
- Farroni T, Mansfield EM, Lai C, Johnson MH. Infants perceiving and acting on the eyes: Tests of an evolutionary hypothesis. Journal of Experimental Child Psychology. 2003;85:199–212. doi: 10.1016/s0022-0965(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. Covert and overt orienting to gaze direction cues and the effects of fixation offset. NeuroReport. 1998;14:489–493. doi: 10.1097/00001756-200303030-00039. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Minshew NJ, Melhem NM, Nutche JJ, Keshavan MS, Hardan AY. Volumetric alterations of the orbitofrontal cortex in autism. Progress in Neuropsychopharmacology & Biological Psychiatry. 2007;31:41–45. doi: 10.1016/j.pnpbp.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. Journal of Autism and Developmental Disorders. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Leppänen JM. Does facial expression affect attention orienting by gaze direction cues? Journal of Experimental Psychology Human Perception and Performance. 2003;29:1228–1243. doi: 10.1037/0096-1523.29.6.1228. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University, Department of Sociology; New Haven: 1975. [Google Scholar]
- Hood B, Willen JD, Driver J. Adult's eyes trigger shifts of visual attention in human infant. Psychological Science. 1998;9:131–134. [Google Scholar]
- Hooker CI, Paller KA, Gitelman DR, Parrish TB, Mesulam MM, Reber PJ. Brain networks for analyzing eye gaze. Cognitive Brain Research. 2003;17:406–418. doi: 10.1016/s0926-6410(03)00143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32:477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. Journal of Neuropathology and Experimental Neurology. 1998;57:645–652. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: An fMRI investigation. Brain and Cognition. 2004;55:269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kylliäinen A, Hietanen JR. Attention orienting by another's gaze direction in children with autism. Journal of Child Psychology and Psychiatry. 2004;45:435–444. doi: 10.1111/j.1469-7610.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Goldberg MC. Language and executive functions in high functioning autism: A continuum of impaired to enhanced performance. Journal of Autism and Developmental Disorders. 2005;35:557–573. doi: 10.1007/s10803-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Langton SR, Bruce V. You must see the point: Automatic processing of cues to the direction of social attention. Journal of Experimental Psychology Human Perception and Performance. 2000;26:747–757. doi: 10.1037//0096-1523.26.2.747. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The Autism Diagnostic Interview - Revised (ADI-R) Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Leekam SR, Baron-Cohen S, Perrett D, Milders M, Brown S. Eye-direction detection: A dissociation between geometric and joint attention skills in autism. British Journal of Developmental Psychology. 1997;15:77–95. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Levanthal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: A functional neuroimaging study of social perception in children. NeuroImage. 2005;27:247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Components of executive function deficits in autism and other disorders. In: Russell J, editor. Autism as an executive disorder. Oxford Press; Oxford: 1997. pp. 179–211. [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, Minshew N, Munson JA, Pennington BF, Rogers SJ, Spence MA, Tager-Flusberg H, Volkmar FR, Wrathall D. Performance on Cambridge neuropsychological test automated battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the Collaborative Programs of Excellence in Autism network. Journal of Autism and Developmental Disorders. 2004;34:139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high functioning autistic individuals: Relationship to theory of mind. Journal of Child Psychology and Psychiatry. 1991;32:1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32A:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Structures and functions of selective attention. In: Boll T, Bryant B, editors. Master lectures in clinical neuropsychology. American Psychological Association; Washington: 1988. pp. 173–202. [Google Scholar]
- Posner MI. Hierarchical distributed networks in the neuropsychology of selective attention. In: Carramaza A, editor. Cognitive neuropsychology and neurolinguistics: Advances in models of cognitive function and impairment. Plenum; New York: 1990. pp. 187–210. [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philosophical Transactions of the Royal Society London B: Biological Science. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The diagnostic interview for children and adolescents-IV. Multi-Health Systems; North Tonawanda: 1997. [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack J, Kingstone A. Eyes are special but not for everyone: The case of autism. Cognitive Brain Research. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45:445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Condie S, Campbell R, Milne E, Coleman M. Does the perception of moving eyes trigger reflexive visual orienting in autism? Philosophical Translations of the Royal Society of London. 2003;358:325–334. doi: 10.1098/rstb.2002.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecera S, Johnson MH. Gaze detection and the cortical processing of faces: Evidence from infants and adults. Visual Cognition. 1995;2:59–87. [Google Scholar]
- Vecera S, Rizzo M. What are you looking at? Impaired ‘social attention’ following frontal-lobe damage. Neuropsychologia. 2004;42:1657–1665. doi: 10.1016/j.neuropsychologia.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vecera S, Rizzo M. Eye gaze does not produce reflexive shifts of attention: Evidence from frontal lobe damage. Neuropsychologia. 2006;44:150–159. doi: 10.1016/j.neuropsychologia.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Vlamings PHJM, Stauder JEA, van Son IAM, Mottron L. Atypical visual orienting to gaze- and arrow-cues in adults with high functioning autism. Journal of Autism and Developmental Disorders. 2005;35:267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, Bryson SE. Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23:1–13. doi: 10.1007/BF01066415. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children third edition. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children fourth edition. The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- Zilbovicius M, Garreau B, Samson Y, Remy P, Barthélémy C, Syrota A, Lelord G. Delayed maturation of the frontal cortex in childhood autism. American Journal of Psychiatry. 1995;152:248–252. doi: 10.1176/ajp.152.2.248. [DOI] [PubMed] [Google Scholar]