Abstract
The geldanamycin derivatives 17-allylamino-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) are promising chemotherapeutic drugs that inhibit heat shock protein 90 (HSP90) function. Previous studies have shown that 17-AAG/DMAG treatment induces the degradation of mutant BRAF (V600E) and inhibits the activation of MAP/ERK1/2 (MEK1/2). We have found, however, that HSP90 inhibition alone is not sufficient for efficient BRAF(V600E) degradation in some cells. HSP90 inhibitors structurally unrelated to geldanamycin, radicicol and novobiocin, while inducing the degradation of the HSP90 client protein RAF-1 fail to induce BRAF(V600E) degradation or inhibit MEK1/2 activation in HT29 human colon cancer cells ‥ Moreover, after treatment with 17-DMAG, the kinase activity of residual, un-degraded BRAF(V600E) was also lost. Incubation of cells with a reactive oxygen species (ROS) scavenger, N-acetyl cysteine (NAC), partially restored kinase activity and also partially prevented BRAF(V600E) degradation due to 17-DMAG treatment. Conversely, treatment with the ROS producing drug menadione clearly inhibited MEK1/2 and reduced BRAF(V600E). These results suggest that in addition to direct inhibition of HSP90, the anti-tumor effect of geldanamycin and its derivatives is also mediated though the production of ROS which may directly inactivate tumorigenic mutant BRAF(V600E).
Keywords: BRAF, MAP kinase, geldanamycin, HSP90, ROS
INTRODUCTION
BRAF, the major extracellular signal-regulated kinase 1/2 (ERK1/2) activator in vertebrates, is required for the maintenance of basal ERK1/2 activity and displays potent transforming activity (1–3). Mutations in BRAF have been identified in 70% of malignant melanomas, 30% of papillary thyroid and serous ovarian carcinomas, 15% of colorectal cancers and at lower frequencies in a wide range of additional human cancers (4, 5). Examination of 126 patients with papillary thyroid cancer indicated that the BRAF mutation correlated significantly with distant metastasis and clinical stage (6). In a systematic evaluation of BRAF and KRAS mutations in 330 colorectal tumors, Rajagopalan et al. (7) identified 32 mutations (10%) in BRAF, 28 with a V600E mutation and 1 each with the R461I, I462S, G463E, or K601E mutations. All but 2 mutations appeared to be heterozygous and, in all 20 cases for which normal tissue was available, the mutations were shown to be somatic. In the same set of tumors there were 169 mutations (51%) in KRAS. Since no tumor was detected with mutations in both BRAF and KRAS, this suggests that BRAF and KRAS mutations are nearly equivalent in their tumorigenic effects and possibly arise at similar phases of tumorigenesis.
The majority of identified BRAF mutations (~90%) lead to substitution of valine 600 by glutamic acid [BRAF(V600E)] which mimics the conformational change induced in the activation segment by T599 and S602 phosphorylation (5, 8). Mutations in KRAS or BRAF can cause constitutive activation of MAP/ERK1/2 kinase (MEK1/2) and ERK1/2 in cancer cells which account for their malignancy. In vitro expression of BRAF(V600E) has been shown to transform fibroblasts and melanocytes as well as to induce haematopoietic dysplasia in transgenic mice and invasive melanomas in p53 −/− zebrafish (4, 9–12). Likewise, the growth of BRAF(V600E)-transformed fibroblasts in xenografts is highly dependent on BRAF(V600E) expression (13) and the suppression of BRAF(V600E) expression in melanoma lines abrogates their transformed phenotype (14, 15). High basal level ERK1/2 activity is observed in more than 60% (combined KRAS and BRAF gene mutation frequency) of colon cancers, thus targeting the BRAF signaling pathway may be an effective approach to control these highly malignant cancers.
HSP90 is a key molecular chaperone that mediates the cellular stress response by regulating the conformation, stability, and function of critical client proteins functioning in the signal transduction, cell cycle, growth control, and apoptosis pathways (16–20). Among such HSP90 client proteins are key oncogenic/growth-stimulating client proteins such as RAF-1, AKT (protein kinase Bα), ERBB2 (Her2), PLK1 (Polo-like kinase 1), MET (hepatocyte growth factor receptor), Aurora B (AIM1), PIM-1, survivin (API4), hTERT, CDK4, and cyclin D1. The benzoquinone ansamycin antibiotic geldanamycin (GA), along with its clinically used analogue 17-allylamino-17-demethoxygeldanamycin (17-AAG) and the water-soluble analogue 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), binds to HSP90 and inhibits its function by masking the ATP-binding site (21, 22). Drug binding to HSP90 by GA or 17-AAG/DMAG inhibits maturation and increases degradation of several cellular survival factors, which contributes to these drugs significant anticancer properties (22).
Treatment with 17-AAG also sensitizes tumor cells to IR exposure (23–27). In Phase I clinical trials, 17-AAG was well tolerated in patients, with only minor cardiac and lung toxicity, even though the drug is also taken up by normal cells. One advantage of using 17-AAG/DMAG as a radiosensitizing agent is the differential effect in normal and tumor cells. In tumor cells, as opposed to normal cells, HSP90 is mainly present in an activated state as part of a multichaperone complex that has a 100-fold higher affinity for 17-AAG (28). Additionally, Eustace et al. found that HSP90α can be secreted extracellularly where it binds to and activates matrix metalloproteinase 2 (MMP2) thereby facilitating the tumor cell invasiveness (28). Inhibition of extracellular HSP90α by 17-AAG/DMAG would decrease both MMP2 activity and tumor invasiveness in addition to radiosensitizing tumor cells, further increasing the potential value of this drug class as chemotherapeutic agents.
Previously we found that indomethacin inhibits ERK1/2 activity in HT29 human colon carcinoma cells, which have high constitutive ERK1/2 activity due to the BRAF(V600E) mutation, and that indomethacin also sensitized cells to radiation (26). Since treatment with 17-AAG further radiosensitized indomethacin-treated HT29 cells (26), we asked whether 17-AAG/DMAG might affect MAP kinase activity. While 17-AAG/DMAG strongly inhibited cellular ERK1/2 activity by destabilizing BRAF(V600E) as reported previously (29, 30), we also found that inhibition of HSP90 alone is not sufficient to induce these two events. This report describes a novel mechanism of BRAF(V600E) kinase inhibition by 17-AAG/DMAG involving reactive oxygen species (ROS) production and proposes that, in addition to HSP90 inhibition, the direct inactivation of tumorigenic mutant BRAF(V600E) by drug induced ROS may contribute to the anti-tumor activity of geldanamycin related drugs as well.
MATERIALS AND METHODS
Cell Culture, Chemicals, Antibodies
HT29, MCF7, SK-MEL-2, SK-MEL-28, and A2058 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in McCoy’s 5A and DMEM medium (Life Technologies), respectively, supplemented with 10% FCS in a humidified incubator atmosphere of 5% CO2-95% air. Cyclohexamide was obtained from Sigma Chemical Co. (St. Louis, MO). Radicicol and novobiocin were obtained from Calbiochem (San Diego, CA). The drugs 17-AAG and 17-DMAG were a gift from the Developmental Therapeutics Program (DTP), NCI, NIH. Primary antibodies against phosphorylated ERK1/2, phosphorylated MEK1/2 and total MEK1/2 were from Cell Signaling Technology (Beverly, MA), BRAF, RAF-1, β-actin and CDC37 were from Santa Cruz (Santa Cruz, CA), HSP90 was from Assay Design (Ann Arbor, MI), p53 was from EMD Chemicals (San Diego, CA).
Western Blotting
Cells were directly lysed in tissue culture wells with NP-40 lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% NP-40) supplemented with proteinase inhibitors (0.5 µg/ml aprotinin, 0.5 µg/ml leupeptin, 0.7 µg/ml pepstatin A) and with phosphatase inhibitors (2 mM sodium pyrophosphate, 50 mM NaF, 5 mM β-glycerophosphate, 1 mM sodium orthovanadate). Lysates were subjected to a brief sonication then clarified by centrifugation (14,000 × g) for 10 min, and the supernatants collected. Protein concentrations were determined by the Bradford method (BioRad, CA). Total cell lysates were separated by SDS-PAGE, and the proteins transferred onto Immobilon-P membranes (Millipore, Bedford, MA). Membranes were incubated with primary antibodies followed by secondary antibodies conjugated with horseradish peroxidase. Signals were detected using SuperSignal West Femto system (Pierce, IL).
Coimmunoprecipitation
MCF7 cells were transfected with BRAF(V600E)-expressing plasmid by electroporation and 48 h later treated with 17-DMAG (1 µM) or radicicol (3 µM) for 4 h then lysed in NP-40 lysis buffer supplemented with proteinase inhibitors and phosphatase inhibitors as described. BRAF(V600E) was immunoprecipitated from cleared lysates with anti-BRAF antibody or normal IgG as a control. Coprecipitated HSP90 and CDC37 were analyzed by western blotting with anti-HSP90 antibody and anti-CDC37 antibody, respectively.
Intracellular Pro-oxidant Production
Cellular pro-oxidant production was determined using the oxidation-sensitive 5- (and-6)-carboxy-2', 7'-dichlorodihydrofluorescein diacetate (C-400, 10 µg/ml) fluorescent probes as described previously (31). Oxidation-insensitive 5- (and-6)-carboxy-2', 7'-dichlorofluorescein diacetate (C-368, 10 µg/ml) fluorescent dyes were used as controls for changes in uptake, ester cleavage, and efflux, so that any changes in fluorescence seen between groups with the oxidation-sensitive dye could be directly attributed to changes in dye oxidation. Both probes were obtained from Invitrogen (Carlsbad, CA) and dissolved in DMSO. Following drug treatment cells were harvested at 37°C using trypsin/EDTA, resuspended in 37°C medium with or without drugs, labeled with the fluorescent dyes for 15 min at 37°C, placed on ice, and analyzed using a FACS440 flow cytometer (BD Biosciences, Mountain View, CA) (excitation 488 nm, emission 535 nm). The mean fluorescence intensity of 20,000 cells was analyzed in each sample and corrected for autofluorescence from unlabeled cells
Immunoprecipitation-kinase assay
Human HT29 cells were treated with 17-DMAG (1 µM) for 16 h then lysed in NP-40 lysis buffer supplemented with proteinase inhibitors and phosphatase inhibitors as described. BRAF(V600E) was immunoprecipitated from cleared lysates with anti-BRAF antibody and used for the in vitro kinase assay. Precipitated beads were resuspended in kinase buffer [20 mM MOPS pH 7.2, 25 mM β-glycerophosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 15 mM MgCl2, 1 mM DTT, 10 µCi of γ-32P ATP, 50 µM ATP] along with bacterially produced recombinant human MEK1 as a substrate and incubated at 30°C for 30 min. The reactions were terminated by addition of 10 µl of 4 × SDS sample buffer, heated at 95°C for 3 min then analyzed by SDS-PAGE. The phosphorylated MEK1 levels were measured by PhosphorImager (BioRad) after resolution by SDS-PAGE. The immunoprecipitated BRAF(V600E) was visualized by western blotting.
RESULTS
Geldanamycin and its derivatives, 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), are HSP90 specific inhibitors under clinical evaluation currently as chemotherapy drugs. Recently we found that 17-AAG enhances indomethacin-induced radiosensitization of HT29 cells (26). In HT29 cells, an oncogenic mutation in the BRAF gene (V600E) (4) leads to constitutive activation of ERK1/2. Preliminary studies from our laboratory (Supplemental Fig. s1), as well as published results (29, 30), indicate that 17-AAG/DMAG treatment decreases cellular BRAF(V600E) levels, without altering BRAF mRNA levels (Supplemental Fig. s2), suggesting loss of HSP90 function increased BRAF degradation. Loss of BRAF(V600E) in 17-AAG/DMAG treated cells also corresponded with decreased MAP activation as determined by measurements of cellular p-MEK and p-ERK levels (Supplemental Fig. s1).
The depletion of cellular BRAF(V600E) and inhibition of MEK1/2 activity by geldanamycin related drugs are not characteristic of all HSP90 inhibitors
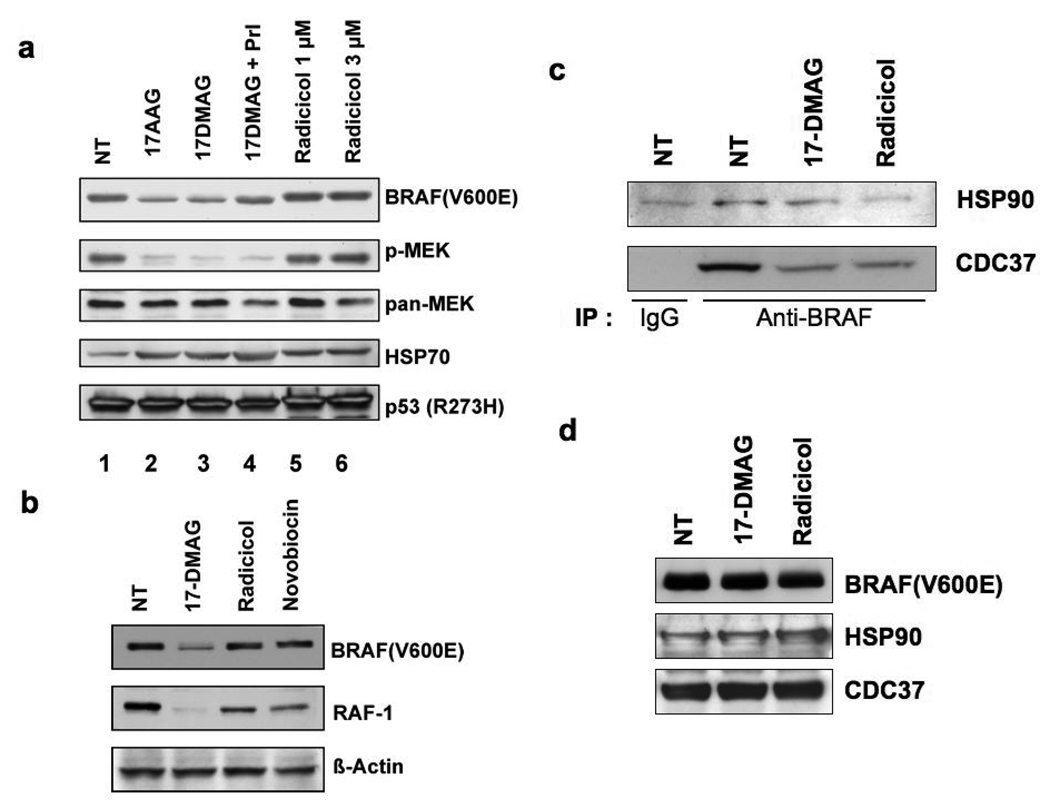
The HSP90 chaperone plays a key role in regulating the cellular stability and activity of its client proteins, therefore, we tested whether inhibition of HSP90 function was the sole mechanism responsible for BRAF(V600E) degradation. Radicicol is a potent inhibitor that, like geldanamycin related drugs, interacts with the HSP90 N-terminal ATPase domain but which has a different chemical structure. HT29 cells were treated with 17-AAG, 17-DMAG, or radicicol for 16 h and cell lysates prepared for western blot analysis of cellular BRAF(V600E), phosphorylated MEK (p-MEK), total MEK1/2 (pan-MEK), HSP70, and mutant p53 (R273H) levels (Fig. 1a). Treatment with 17-AAG or 17-DMAG reduced the amount of BRAF(V600E) (lanes 2 and 3, respectively) as previously described (Supplemental Fig. s1). The reduction in phosphorylated MEK1/2 (p-MEK) was due to the inhibition of MEK1/2 activation since the total amount of MEK1/2 protein was not changed. Radicicol treatment, in contrast, did not cause any reduction in BRAF(V600E) levels or inhibition of MEK1/2 activation (lanes 5 and 6). Accumulation of HSP70 was evident in cells treated with 17-AAG, 17-DMAG, or radicicol (lanes 2, 3, 5, 6) suggesting that all these compounds inhibited HSP90 resulting in activated heat shock factor 1 (HSF1) and HSP70 expression. Addition of proteasome inhibitors (PrI: MG132, ALLN, PSI, lactacystin) to 17-DMAG-treated cells partially inhibited the BRAF(V600E) reduction (lane 4), suggesting that loss of cellular BRAF(V600E) was due, at least in part, to proteosome mediated degradation. There was no degradation of mutant p53 (R273H), in cells treated with either 17-AAG/DMAG or radicicol‥
Fig. 1.
Inhibition of HSP90 alone is not sufficient for MEK1/2 inhibition and reduced BRAF(V600E) levels. (a) Treatment of HT29 cells with the HSP90 inhibitor radicicol does not reduce cellular BRAF(V600E) levels nor inhibit MEK1/2. HT29 cells were treated with 17-DMAG (1 µM) or radicicol (1 or 3 µM) for 16 h. In lane 4, HT29 cells were treated with 17-DMAG (1 µM) together with proteasome inhibitors (PrI; MG132, PSI, ALLN, lactacystin, 10 µM each) for 16 h. Cell lysates were prepared and analyzed by western blotting for the expression of BRAF, phosphorylated MEK1/2 (p-MEK), total MEK1/2 (pan-MEK), HSP70, and p53. Note that radicicol effectively inhibited HSP90 function, as indicated by HSP70 induction, but failed to reduce BRAF(V600E) levels or inhibit MEK1/2 activation. (b) The effect of HSP90 inhibitors on HSP90 client protein levels. HT29 cells were treated with 17-DMAG (1 µM), radicicol (3 µM), or novobiocin (0.8 mM) for 16 h (43, 44). Cell lysates were analyzed by western blotting for the expression of BRAF(V600E), RAF-1, and β-actin. (c) Both 17-DMAG and radicicol dissociate BRAF(V600E)-containing complexes. MCF7 cells were transfected with BRAF(V600E) expression plasmid and 48 h later, transfected cells were treated with 17-DMAG (1 µM) or radicicol (3 µM) for 4 h. Cell lysates were prepared and subjected to immunoprecipitation with anti-BRAF antibody or normal IgG. Precipitated proteins were separated by SDS-PAGE and HSP90 and CDC37 in the complexes were determined by western blotting. (d) Expression of BRAF(V600E), HSP90, and CDC37 in transfected MCF7 cells. The cellular expression levels of BRAF(V600E), HSP90, and CDC37 in transfected MCF7 cells used in (c) were analyzed by western blotting.
The RAF-1 protein is another established HSP90 client protein and when HT29 cells were treated with HSP90 inhibitors (17-DMAG, radicicol, and novobiocin) the cellular levels of RAF-1 were depleted (Fig. 1b), however, only 17-DMAG treatment caused BRAF(V600E) loss. Thus, both radicicol and novobiocin can inhibit HSP90 function in HT29 cells. Heat shock transcription factor 1 (HSF-1) is a client protein activated by inhibition of HSP90 function and 17-DMAG, as well as novobiocin, treated cells contained activated HSF-1 as determined by acquisition of promoter DNA binding capability in electrophoretic mobility shift assays (Supplementary Fig s3). Only weak activation of HSF-1 by radicicol was observed and this is consistent with weak HSP70 induction (Fig. 1a).
Radicicol appeared to only partially inhibit HSP90 function with respect to HSF1 activation, we therefore directly tested whether the BRAF(V600E)-HSP90 complex was dissociated by radicicol or 17-DMAG, since inhibition of HSP90 function is associated with rapid dissociation of bound client proteins (32). Thus, HT29 cells were cultured for 4 h with or without the HSP90 inhibitors then harvested and the cell lysates subjected to immunoprecipitation with BRAF-specific antibody. After only 4 hrs of drug treatment, with either 17-DMAG or radicicol, there was no change in total BRAF(V600E) levels (Fig. 1c). The immunoprecipitated BRAF(V600E) was analyzed for the presence of HSP90 and CDC37, a co-chaperone of HSP90, by western blotting. In control cells BRAF(V600E) was recovered complexed with HSP90 and CDC37. Treatment of cells with either 17-DMAG or radicicol significantly decreased the amount of HSP90/CDC37 complex recovered with immunoprecipitated BRAF, indicating the complexes were equally dissociated by 17-DMAG and radicicol (Fig.1c), even though the latter drug was apparently less effective in disrupting HSP90-HSF1 complexes. More importantly, these results indicate that while each HSP90 inhibitor dissociated BRAF(V600E)-HSP90 complexes only 17-DMAG also induced degradation.
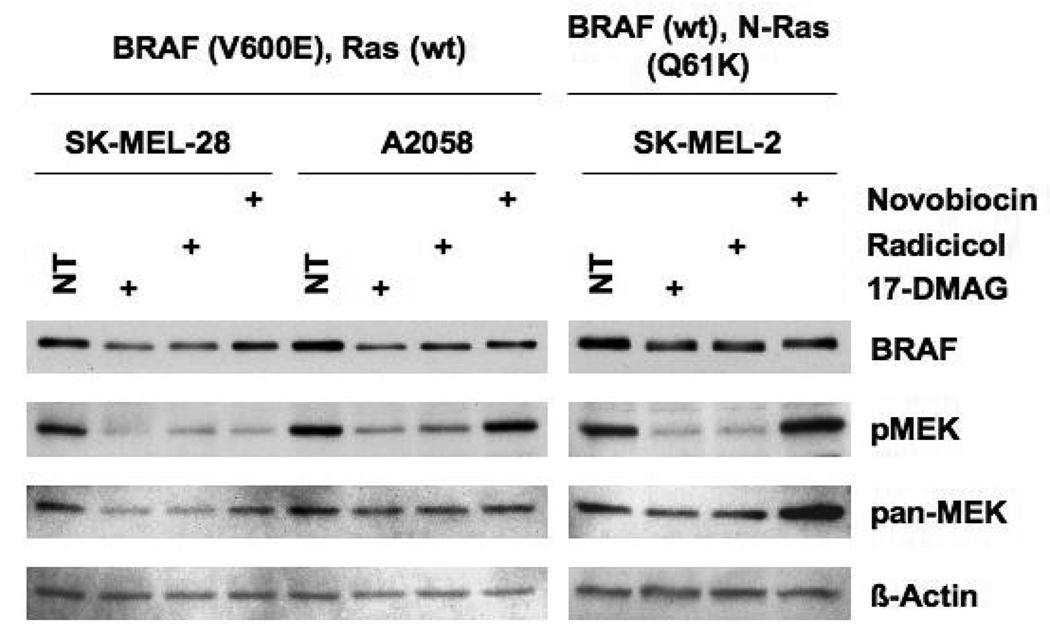
To determine whether the observed effects of HSP90 inhibitors (17-DMAG, radicicol, and novobiocin) on BRAF(V600E) and MEK1/2 activation were specific to HT29 cells, melanoma cell lines expressing either mutant BRAF(V600E) (SK-MEL-28 and A2058) or wild type BRAF (SK-MEL-2) (4) were also examined (Fig 2). Though less effective than in HT29 cells, 17-DMAG treatment reduced BRAF(V600E) levels in both SK-MEL-28 and A2058 but only slightly reduced wild type BRAF levels in SK-MEL-2 cells, as reported previously (29, 30). Interestingly, radicicol was as effective as 17-DMAG in reducing melanoma BRAF(V600E) or wild type BRAF levels and inhibiting MEK1/2 activation. The response to novobiocin treatment, however, was highly variable in melanoma cells. In SK-MEL-28 cells novobiocin inhibited MEK1/2 effectively while only partially reducing BRAF(V600E). In A2058 and SK-MEL-2 cell lines, novobiocin partially reduced BRAF(V600E) or wild type BRAF levels, as did 17-DMAG treatment, but without inhibiting MEK1/2 activities. Inhibition of MEK1/2 activity in melanoma cells by novobiocin, therefore, is not the direct result of reduced BRAF(V600E) or wild type BRAF levels but appears to be dependent upon an unknown, variable cellular factor(s).
Fig. 2.
The effect of HSP90 inhibitors on melanoma cells. SK-MEL-28, A2058 and SK-MEL-2 cells were treated with 17-DMAG (1 µM), radicicol (3 µM), or novobiocin (0.8 mM) for 16 h. Cell lysates were analyzed by western blotting for the expression of BRAF (BRAF(V600E) for SK-MEL-28 and A2058, wild type for SK-MEL-2), active MEK1/2 (p-MEK), total MEK1/2 (pan-MEK), and β-actin.
The anti-oxidant drug N-acetyl-L-cysteine partially protects cellular BRAF(V600E) levels and MEK1/2 activation
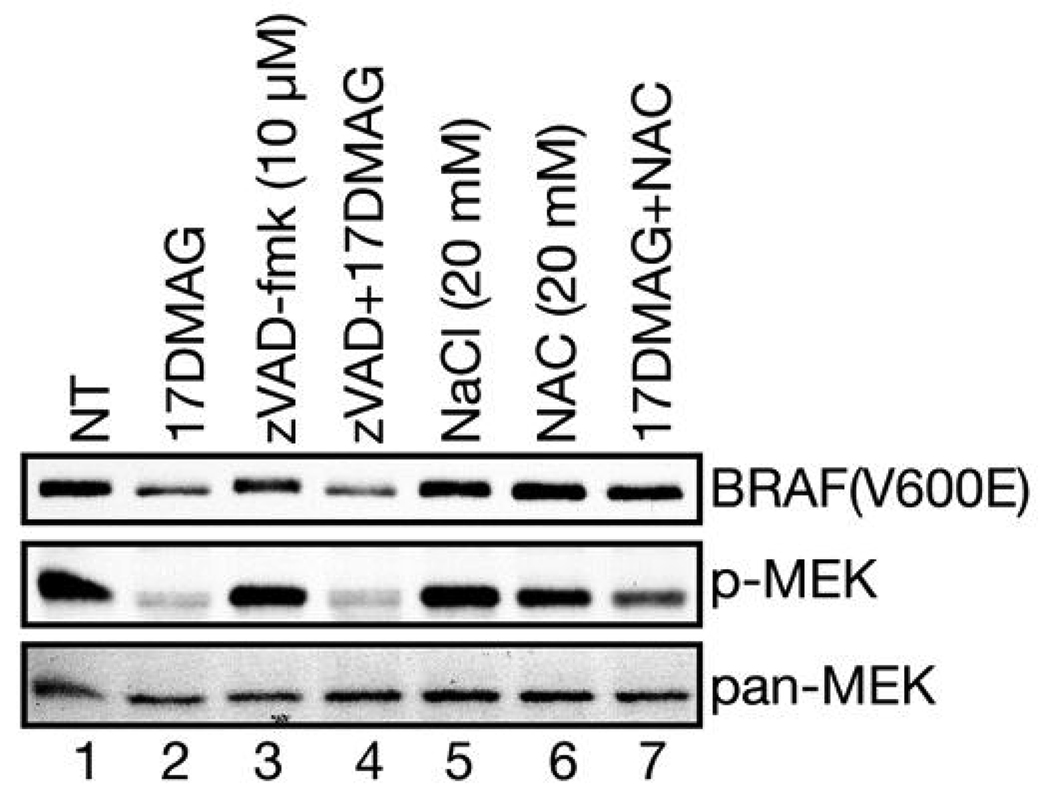
We next investigated whether caspase activation, due to 17-AAG/DMAG induced cell death, could inhibit MEK phosphorylation by degrading BRAF(V600E). Cells (HT29) were incubated with a broadspectrum caspase inhibitor zVAD-fmk together with 17-DMAG for 16 h and cell lysates prepared for western blot analysis (Fig. 3). As before (Fig. 1a), 17-DMAG treatment strongly inhibited MEK1/2 activation and reduced the amount of BRAF(V600E) (lane 2). Inclusion of zVAD-fmk failed to rescue MEK1/2 activation nor protect BRAF(V600E) levels (lane 4). These results clearly demonstrate that both MEK1/2 inhibition and BRAF(V600E) degradation by 17-DMAG treatment are not caspase-dependent.
Fig. 3.
The 17-DMAG induced reduction in BRAF(V600E) levels and inhibition of MEK1/2 activation are ROS-dependent not caspase-dependent. HT29 cells were incubated with the indicated drugs for 16h and cell lysates were prepared. The expression of BRAF(V600E), phosphorylated MEK1/2 (p-MEK) and total MEK1/2 (pan-MEK) were determined by western blotting. Note that zVAD-fmk treatment did not preserve BRAF(V600E) expression levels or MEK1/2 activation (lane 4), while NAC treatment provided significant protection (lane 7).
Geldanamycin and its derivatives, 17-AAG and 17-DMAG, are capable of inducing oxidative stress in cells due to the common benzoquinone structural element (see next section, Fig 4). However, the HSP90 inhibitors radicicol and novobiocin used in the previous experiments, and which lack the benzoquinone moiety, do not induce oxidative stress in cells and fail to inhibit MEK1/2 and to reduce BRAF(V600E) in HT29 cells. Therefore, we also determined the potential role of 17-DMAG induced oxidative stress in MEK1/2 inhibition and BRAF(V600E) reduction. HT29 cells were treated with 17-DMAG with or without added N-acetyl-L-cysteine (NAC), a potent oxidative radical scavenger. In contrast to the lack of any effect observed with caspase inhibition, NAC partially protected MEK1/2 activity and fully protected BRAF(V600E) levels (Fig. 3, lane 7). Neither NAC treatment alone nor NaCl induced osmotic stress had any effect on BRAF(V600E) levels or MEK1/2 activity or cellular MEK levels (lanes 6 and 5). We next determined the relative degree of cellular reactive oxygen species (ROS) produced by 17-DMAG treatment.
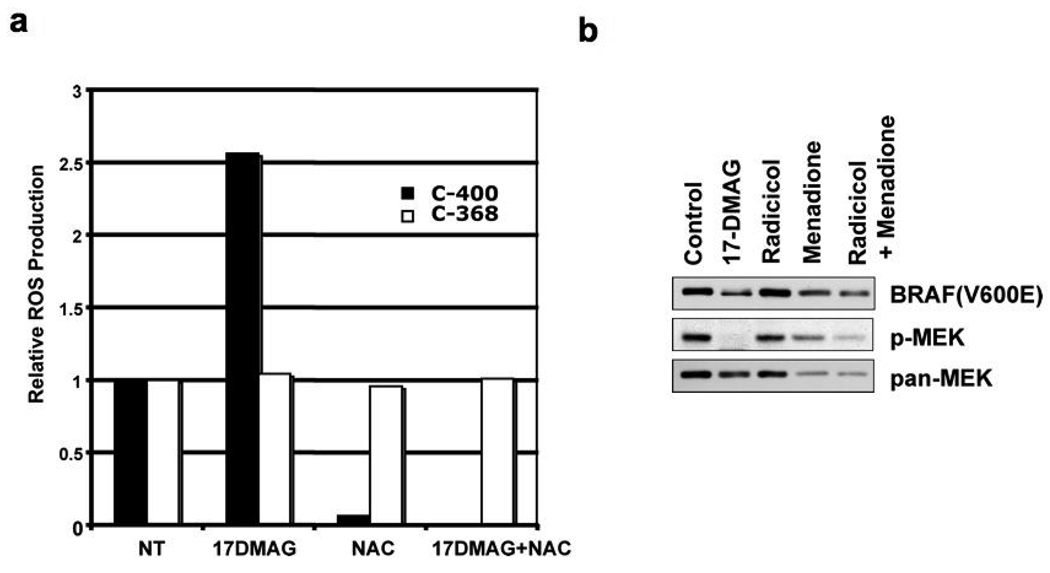
Fig. 4.
Increased production of reactive oxygen species (ROS) in 17-DMAG treated cells. (a) HT29 cells were treated with 17-DMAG (1 µM) and/or NAC (20 mM) for 16 h. Cells were trypsinized and labeled with C-400 or C-368 (10 µg/ml) for 15 min at 37°C. FACS analysis was performed to measure intracellular ROS production as indicated by C-400 dye fluorescenc using the C-368 signal as a reference measurement for drug uptake. Relative ROS production is normalized to non-treated cells. (b) HT29 cells were treated with 17-DMAG (1 µM), radicicol (3 µM), and menadione (50 µM) for 16 h as indicated. Cell lysates were subjected to western blotting analysis for the expression of BRAF(V600E), phosphorylated MEK1/2 (p-MEK) and total MEK1/2 (pan-MEK).
To measure the relative level of ROS in cells, we used carboxy-H2DCFDA (C400, Invitrogen) as a cell-permeant fluorescent indicator for ROS and the ROS-insensitive carboxy-H2DCF (C368, Invitrogen) as a cellular dye-uptake reference. As shown in Fig. 4a, 17-DMAG-treatment increased ROS levels approximately 2.5-fold over levels in non-treated cells. Treatment with NAC nearly completely eliminated basal level ROS as well as the 17-DMAG induced increase in production. Since the uptake of C368 dye did not change significantly during treatment, these results indicated that 17-DMAG indeed increased cellular ROS production as predicted from the molecular structure and NAC was able to remove it. The results from these experiments suggest that 17-AAG/DMAG treatment induces a significant rise in intracellular ROS levels that could partially account for both the decrease in BRAF(V600E) and the inhibition of MEK1/2 activation. This hypothesis was further tested by asking whether forced ROS production in cells also leads to reduced BRAF(V600E) levels and MEK1/2 inhibition. In cells were treated with menadione, a drug widely utilized to induce intracellular ROS production and which also contains a benzoquinone structure (35), drug treatment alone partially reduced cellular BRAF(V600E) levels and significantly inhibited MEK1/2 (Fig. 4b).These effects were enhanced when combined with radicicol treatment while radicicol treatment alone failed to show a clear reduction of BRAF(V600E) or inhibition of MEK1/2 in HT29 cells, strongly supporting the notion that inhibition of HSP90 alone is not sufficient to deplete BRAF(V600E) and inhibit MEK1/2 but without concurrent ROS production.
17-DMAG inactivates BRAF(V600E) kinase activity
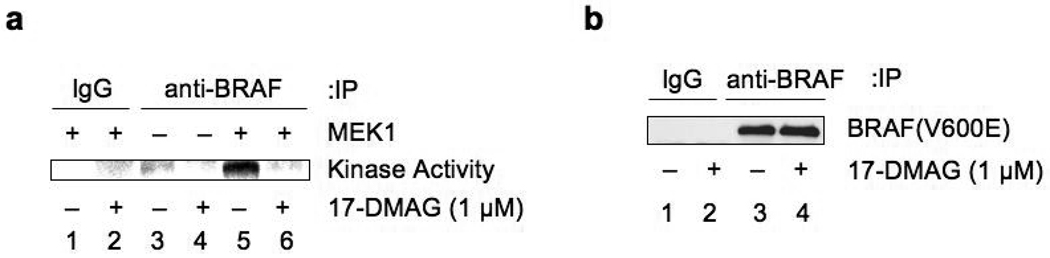
The previous experiments strongly suggest that ROS induced by 17-DMAG contributes to the degradation of BRAF(V600E). We next asked whether 17-DMAG treatment inactivates BRAF(V600E) kinase activity independently of BRAF(V600E) degradation. Cell lysates were prepared from control and 17-DMAG-treated (16 h) HT29 cells and the endogenous BRAF(V600E) protein was recovered by immunoprecipitation with anti-BRAF antibody. Equivalent amounts of BRAF(V600E) were then subjected to an in vitro kinase assay with bacterially produced human MEK1 as substrate. Immunoprecipitated BRAF(V600E) phosphorylated MEK1 protein in vitro (Fig. 5a, lane 5), however, treatment with 17-DMAG clearly inactivated the kinase activity of BRAF(V600E) (Fig. 5a, lane 6), even though equivalent levels of BRAF(V600E) protein were utilized from treated and non-treated cell lysates (Fig. 5b, lanes 3 and 4). Therefore, we conclude that 17-DMAG treatment not only induces BRAF(V600E) degradation but also directly inactivates BRAF(V600E) kinase activity.
Fig. 5.
17-DMAG inactivates BRAF(V600E) kinase activity. (a) HT29 cells were treated with 17-DMAG (1 µM) for 16 h and cell lysates were prepared. Cell lysates (control: 250 µg, drug treated: 500 µg) were immunoprecipitated with anti-BRAF antibody or normal IgG. After extensive washing, an in vitro kinase assay was performed using bacterially produced human MEK1 protein as a substrate, except for lanes 3 and 4 where substrate was omitted in the kinase reaction. The reaction was terminated by adding SDS-sample buffer and the products separated by SDS-PAGE. Incorporated 32P in MEK1 was visualized by PhosphorImager. (b) Input immunoprecipitated BRAF(V600E) used in the kinase assay in (a) was visualized by western blotting. Note that equivalent amounts of immunoprecipitated BRAF(V600E) from control and 17-DMAG-treated cells were utilized for the in vitro kinase assay.
DISCUSSION
Somatic missense mutations in BRAF, such as V600E, have been identified in a wide range of human cancers including malignant melanoma, thyroid cancer, and colon cancer (4). Mutations in BRAF or KRAS cause constitutive activation of MAP kinase pathways in cancer cells, which account for their high malignancy. Indeed, elevated basal ERK1/2 activity is observed in more than 60% of colon cancers (combined mutation frequency in KRAS and BRAF genes), indicating inhibition of the MAP kinase pathway may be a promising means of treating these highly malignant cancers.
We have found that, in addition to indomethacin, the geldanamycin derivatives 17-AAG and 17-DMAG also inhibit the MAP kinase pathway in HT29 human colon carcinoma cells (Supplemental Fig. s1). Mutant BRAF(V600E), unlike the wild-type protein, functions independent of its ability to bind Ras-GTP and is therefore constitutively active (33), as is the MAP kinase pathway in HT29 cells. Analysis of HT29 cells revealed that 17-AAG/DMAG treatment reduced cellular BRAF(V600E) levels and inhibited MEK1/2, in good agreement with recent reports describing BRAF(V600E) as a notable client protein of HSP90 (29, 30). Our results, however, also clearly indicate that inhibition of HSP90 function alone is not sufficient to reduce cellular BRAF(V600E) levels or inhibit MEK1/2 as HSP90 inhibitors structurally unrelated to 17-AAG/DMAG, radicicol and novobiocin, fail to show similar effects (Fig. 1).
Since HSP90 binding regulates client protein activity and/or stability, we compared the effect of HSP90 inhibitors on two additional client proteins: HSF1 and RAF-1. Both 17-DMAG and novobiocin strongly activated HSF1 DNA binding capability, a measure of HSP90-HSF1 complex dissociation, whereas radicicol displayed minimal activation (Supplemental Fig. s3). However, we also found that both 17-DMAG and radicicol equally dissociated BRAF(V600E) complexes containing HSP90 and CDC37 (Fig. 1c). All HSP90 inhibitors tested reduced cellular RAF-1 levels with 17-DMAG again the most effective. In fact, 17-DMAG was in all comparisons made always the most effective drug and the least dependent on cell line examined while both radicicol and novobiocin displayed a greater degree of substrate and cell line variation. Since novobiocin binds to the C-terminal domain of HSP90 (34), it will potentially interact with a different set of substrates than 17-DMAG and radicicol, which bind to the N-terminal ATPase domain. Novobiocin, for example, was recently shown to block the HSP90 carboxy-terminal binding site for PI6K2, leading to constitutive activation, while 17-AAG had no effect (35). Despite the fact that 17-DMAG and radicicol share a common binding domain in HSP90 and the structural and biochemical alterations of HSP90 by these inhibitors are similar (34, 36), they demonstrate different biological properties. Both dissociate BRAF-HSP90/CDC37 complexes in HT29 cells but radicicol is less effective in disrupting HSP90-HSF complexes as well as inhibiting BRAF activity. At least part of this difference in biological effect appears to be due to intra-cellular ROS species generated by the geldanamycin related drugs.
The effect of HSP90 inhibitors on reducing BRAF(V600E) levels and inhibiting MEK1/2 activities was also determined in human melanoma cell lines. As shown in Fig. 2, wild type BRAF expressed in SK-MEL-2 cells was relatively less sensitive to HSP90 inhibitors, which is a good agreement with the previous reports (29, 30). Novobiocin failed to inhibit MEK in this cell line or in A2058 cells, which contain the mutant BRAF(V600E), indicating that BRAF status does not affect novobiocin-dependent MEK inhibition. These results also suggest that even though mutant N-Ras can generate ROS (37) in SK-MEL-2 cells, wild type BRAF is less sensitive to the 17-DMAG-mediated BRAF reduction.
Both 17-AAG and 17-DMAG contain a benzoquinone structure potentially capable of producing ROS species. Cells treated with 17-AAG have previously been observed to produce ROS (38) and we have demonstrated here similar production in cells are treated with 17-DMAG (Fig. 4a). Inhibition of 17-DMAG-dependent ROS production by N-acetyl cysteine (NAC) treatment prevented the reduction of BRAF(V600E) levels and partially prevented MEK1/2 inhibition (Fig. 3).
The benzoquinone moiety in 17-AAG/DMAG is not necessary for inhibition of HSP90 chaperone function and can be reduced to hydroquinone intracellularly by NAD(P)H:quinone oxidoreductase I (NQO1) (39). The level of ROS production in cells by 17-AAG/DMAG, therefore, could be determined by NQO1 expression levels: If so, the activity of NQO1 may determine the effectiveness of 17-AAG/DMAG in cancer cells carrying BRAF(V600E) and possibly other mutations.
We further found that 17-DMAG treatment inactivated the kinase activity of non-degraded BRAF(V600E) (Fig. 5). Since BRAF(V600E) is a constitutively active mutant kinase independent of any posttranslational modifications, such as phosphorylation, these results indicate that 17-DMAG specifically inactivates BRAF(V600E) through a mechanism(s) at least partially dependent upon ROS production. Redox dependent alterations in activity have been reported for many proteins including the c-fos/jun and HSF-1 transcription factors (40, 41). In the former case, REF1 catalyzed reduction of c-fos leads to enhanced DNA binding and transcriptional activity. More interesting, the redox status of key cysteines in HSF-1, an HSP90 client protein, is reported to regulate transcriptional activity (40). The close proximity of both the ROS generating geldanamycin drug and BRAF while simultaneously bound to HSP90 would facilitate ROS induced damage to the client protein. Such damage could enhance both substrate degradation and loss of enzymatic activity. The specific sensitivity of any particular HSP90 substrate under this mechanism could be sequence and structure dependent. In HT29 cells treated with the menadione, BRAF (V600E) levels were only partially reduced while MEK level decreased dramatically (Fig. 4b). What is interesting is that addition of the radicicol HSP90 inhibitor did not further enhance the loss of either protein but did produce a significant additional decline in BRAF activity as reflected in pMEK levels. Thus, inhibition of HSP90 in the presence of ROS production enhanced inhibition of BRAF kinase activity.
Our current understanding of the mechanism(s) by which 17-AAG/DMAG alter the MAP kinase pathways is incomplete even though these drugs have rapidly moved into clinical studies. The anti-tumor effect of 17-AAG/DMAG is certainly mediated by the inhibition of HSP90 which leads to the degradation of critical oncogenic client proteins. However, geldanamycin and its derivatives, 17-AAG/DMAG, also produce ROS. ROS has been shown to induce p38 stress kinase activation (38, 42). In addition, as we demonstrated in this report, ROS produced by 17-AAG/DMAG could also contribute the anti-tumorigenic effect of 17-AAG/DMAG through both the destabilization and the inactivation of BRAF(V600E).
Supplementary Material
ACKNOWLEDGEMENTS
Grant support:
This work was supported by Department of Radiation Oncology, Washington University School of Medicine (N. Horikoshi), National Institute of Health P01CA104457 (M.L. Freeman, R. Higashikubo, N. Horikoshi) and R01CA98666 (N. Horikoshi).
We thank Drs. David Gius and Douglas Spitz for helpful discussions.
REFERENCES
- 1.Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. Embo J. 2002;21:5611–5622. doi: 10.1093/emboj/cdf588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 3.Papin C, Denouel-Galy A, Laugier D, Calothy G, Eychene A. Modulation of kinase activity and oncogenic properties by alternative splicing reveals a novel regulatory mechanism for B-Raf. J Biol Chem. 1998;273:24939–24947. doi: 10.1074/jbc.273.38.24939. [DOI] [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 8.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 9.Ikenoue T, Hikiba Y, Kanai F, et al. Different effects of point mutations within the B-Raf glycine-rich loop in colorectal tumors on mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase and nuclear factor kappaB pathway and celluar transformation. Cancer Res. 2004;64:3428–3435. doi: 10.1158/0008-5472.CAN-03-3591. [DOI] [PubMed] [Google Scholar]
- 10.Mercer K, Giblett S, Green S, et al. Expression of endogenous oncogenic V600EB-raf induces proliferation and developmental defects in mice and transformation of primary fibroblasts. Cancer Res. 2005;65:11493–11500. doi: 10.1158/0008-5472.CAN-05-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patton EE, Widlund HR, Kutok JL, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Wellbrock C, Ogilvie L, Hedley D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–2342. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 13.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani SR, Jacobetz MA, Robertson GP, Herlyn M, Tuveson DA. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res. 2003;63:5198–5202. [PubMed] [Google Scholar]
- 15.Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene. 2004;23:6292–6298. doi: 10.1038/sj.onc.1207785. [DOI] [PubMed] [Google Scholar]
- 16.Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271:22796–22801. doi: 10.1074/jbc.271.37.22796. [DOI] [PubMed] [Google Scholar]
- 17.Stancato LF, Silverstein AM, Owens-Grillo JK, Chow YH, Jove R, Pratt WB. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- 18.An WG, Schnur RC, Neckers L, Blagosklonny MV. Depletion of p185erbB2, Raf-1 and mutant p53 proteins by geldanamycin derivatives correlates with antiproliferative activity. Cancer Chemother Pharmacol. 1997;40:60–64. doi: 10.1007/s002800050626. [DOI] [PubMed] [Google Scholar]
- 19.Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2:3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143:901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munster PN, Srethapakdi M, Moasser MM, Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res. 2001;61:2945–2952. [PubMed] [Google Scholar]
- 22.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 23.Bisht KS, Bradbury CM, Mattson D, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 24.Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res. 2003;9:3749–3755. [PubMed] [Google Scholar]
- 25.Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. Int J Radiat Biol. 2003;79:973–980. doi: 10.1080/09553000310001626135. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Nantz R, Kitamura T, Higashikubo R, Horikoshi N. Combined inhibition of extracellular signal-regulated kinases and HSP90 sensitizes human colon carcinoma cells to ionizing radiation. Oncogene. 2005;24:3011–3019. doi: 10.1038/sj.onc.1208508. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi M, Yu D, Hirayama R, et al. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochem Biophys Res Commun. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 28.Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol. 2004;6:507–514. doi: 10.1038/ncb1131. [DOI] [PubMed] [Google Scholar]
- 29.da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R. Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2005;65:10686–10691. doi: 10.1158/0008-5472.CAN-05-2632. [DOI] [PubMed] [Google Scholar]
- 30.Grbovic OM, Basso AD, Sawai A, et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad IM, Aykin-Burns N, Sim JE, et al. Mitochondrial O2*-and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 32.Li R, Soosairajah J, Harari D, et al. Hsp90 increases LIM kinase activity by promoting its homo-dimerization. Faseb J. 2006;20:1218–1220. doi: 10.1096/fj.05-5258fje. [DOI] [PubMed] [Google Scholar]
- 33.Brummer T, Martin P, Herzog S, Misawa Y, Daly RJ, Reth M. Functional analysis of the regulatory requirements of B-Raf and the B-Raf(V600E) oncoprotein. Oncogene. 2006;25:6262–6276. doi: 10.1038/sj.onc.1209640. [DOI] [PubMed] [Google Scholar]
- 34.Phillips JJ, Yao ZP, Zhang W, et al. Conformational dynamics of the molecular chaperone Hsp90 in complexes with a co-chaperone and anticancer drugs. J Mol Biol. 2007;372:1189–1203. doi: 10.1016/j.jmb.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty A, Koldobskiy MA, Sixt KM, et al. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc Natl Acad Sci U S A. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 37.Kopnin PB, Agapova LS, Kopnin BP, Chumakov PM. Repression of sestrin family genes contributes to oncogenic Ras-induced reactive oxygen species up-regulation and genetic instability. Cancer Res. 2007;67:4671–4678. doi: 10.1158/0008-5472.CAN-06-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell C, Park MA, Zhang G, et al. 17-Allylamino-17-demethoxygeldanamycin enhances the lethality of deoxycholic acid in primary rodent hepatocytes and established cell lines. Mol Cancer Ther. 2007;6:618–632. doi: 10.1158/1535-7163.MCT-06-0532. [DOI] [PubMed] [Google Scholar]
- 39.Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. Formation of 17-allylamino-demethoxygeldanamycin (17-AAG) hydroquinone by NAD(P)H:quinone oxidoreductase 1: role of 17-AAG hydroquinone in heat shock protein 90 inhibition. Cancer Res. 2005;65:10006–10015. doi: 10.1158/0008-5472.CAN-05-2029. [DOI] [PubMed] [Google Scholar]
- 40.Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 42.Dey A, Cederbaum AI. Geldanamycin, an inhibitor of Hsp90 increases cytochrome P450 2E1 mediated toxicity in HepG2 cells through sustained activation of the p38MAPK pathway. Arch Biochem Biophys. 2007;461:275–286. doi: 10.1016/j.abb.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 44.Walerych D, Kudla G, Gutkowska M, et al. Hsp90 chaperones wild-type p53 tumor suppressor protein. J Biol Chem. 2004;279:48836–48845. doi: 10.1074/jbc.M407601200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.