Abstract
Purpose of review
Measurements of whole-body energy expenditure, body composition, and in vivo metabolic fluxes are required to quantitatively understand involuntary weight loss in cancer cachexia. Such studies are rare because cancer cachexia occurs near the end of life where invasive metabolic tests may be precluded. Thus, models of cancer-associated weight loss are an important tool for helping to understand this debilitating condition.
Recent findings
A computational model of human macronutrient metabolism was recently developed that simulates the normal metabolic adaptations to semi-starvation and re-feeding. Here, this model was used to integrate data on the metabolic changes in patients with cancer cachexia and the resulting computer simulations show how the known metabolic derangements synergize with reduced energy intake to result in a progressive loss of body weight (BW), fat mass (FM), and fat-free mass (FFM). The model was also used to simulate the effects of nutritional support and investigate inhibition of lipolysis versus proteolysis as potential therapeutic approaches for cancer cachexia.
Summary
Computational modeling is a new tool that can integrate clinical data on the metabolic changes in cancer cachexia and provides a conceptual framework to help understand involuntary weight loss and predict the effects of potential therapies.
Keywords: Cancer Cachexia, Weight Loss, Energy Metabolism, Body Composition, Mathematical Model
Introduction
Involuntary weight loss is a hallmark of advanced cancer and culminates in severe depletion of skeletal muscle and adipose tissue [1–4]. This condition, called cachexia, is a significant factor leading to poor quality of life, high mortality and morbidity rates, and poor treatment response in cancer patients [5,6]. Despite substantial progress elucidating the molecular signals underlying the state of cachexia [7,8], measurements of whole-body energy expenditure and metabolic fluxes are required to understand how these signals contribute to weight loss and altered body composition. An integrative analysis of the comparatively simple state of semi-starvation involves complex interactions between food intake, energy expenditure, lipolysis, gluconeogenesis, protein turnover, and substrate utilization [9–13]. Collecting such data in advanced cancer patients is particularly difficult because cachexia occurs in an end-of-life setting where patient vulnerability limits the invasiveness of metabolic tests and disease progression limits the number of patients available for follow-up. Because of these practical difficulties, models of cancer cachexia are required to better understand this debilitating condition. Several animal models have been developed that provide mechanistic insights, but the relevance of these models to the human condition is sometimes questionable [14].
Recently, a computational model was developed that simulates the normal metabolic adaptations to semi-starvation and re-feeding [15]. Here, we adapt this model to integrate clinical data from published metabolic studies in cancer patients. We contrast the state of cancer cachexia with that of semi-starvation by simulating how the known metabolic derangements associated with cancer cachexia synergize with reduced energy intake to result in a progressive loss of body weight (BW), fat mass (FM), and fat-free mass (FFM). We illustrate the utility of the model by simulating the potential therapeutic effect of inhibiting adipose tissue lipolysis in cancer cachexia as was recently suggested [16] and comparing this therapeutic approach with that of inhibiting proteolysis. We also examine the effects of nutritional support introduced at various stages during the progression of cancer cachexia.
Simulation of Semi-starvation
We adapted a previously published computational model of human macronutrient metabolism in healthy young men [15]. The initial conditions of the simulation were selected to realistically represent a cancer patient prior to disease onset: a 69 year old man with an initial body weight of 77.7 kg and 32% body fat corresponding to a body mass index (BMI) of 26.1 kg/m2 and the resting metabolic rate (RMR) was 1606 kcal/d (or 30.6 kcal/kg FFM/d) [17]. We assumed a moderate physical activity level (PAL) of 1.5 and a balanced metabolizable energy intake of 2400 kcal/d consisting of 50% carbohydrate, 35% fat, and 15% protein. These baseline criteria were used as a point of departure to simulate the effects of semi-starvation by linearly decreasing the metabolizable energy intake from the balanced value of 2400 kcal/d at the start of the simulation to 1700 kcal/d after 12 months corresponding to the average energy intake reported for advanced cancer patients [18,19]. Since semi-starvation has been shown to decrease physical activity [12,20] [21], we progressively reduced the corresponding model parameter by 40% which also simulated a realistic reduction of physical activity observed in cancer patients [22–24].
Simulation of Cancer Cachexia
Isotopic tracer methodologies have been used to measure important alterations of whole-body metabolic fluxes associated with cancer cachexia and the average reported values of the primary metabolic changes are listed in Table 1. These metabolic changes were implemented in the computational model to simulate the metabolic derangements of cancer cachexia over-and-above the parallel decreases of energy intake and physical activity with semi-starvation. All other simulated metabolic changes were downstream consequences of these primary defects.
Table 1.
Model parameter changes to simulate the primary metabolic alterations in cancer cachexia
Cancer cachexia results in an increase of whole-body lipolysis by an average of 50% [25–29] and the whole-body proteolysis rate is enhanced by an average of 40% [30–34]. To simulate these changes, the model’s equations for the lipolysis and proteolysis rates, DF and DP respectively, were adapted as follows:
where D̂F and D̂P were the basal daily rates of lipolysis and proteolysis, respectively, and the functions f and g determined how changes of FM, body protein content (P), and carbohydrate intake (CI) altered the daily lipolysis and proteolysis rates (f = g = 1 for a balanced baseline diet and body composition). Normally, the coefficients δF and δP are set equal to zero, but to implement the progressive increase of lipolysis and proteolysis with cancer cachexia we set δF = 0.5 years−1 and δP = 0.6 years−1. Note that the daily lipolysis and proteolysis rates continued to adapt to the changes of body composition and diet as defined by the functions f and g, details of which can be found in a previous publication [15].
Another primary metabolic defect of cancer cachexia is the increased rate of whole-body glycolysis and the concomitantly augmented rate of gluconeogenesis from the produced lactate – an energy requiring process known as the Cori cycle. We modeled the energy cost of Cori cycle activity by accounting for the 6 ATP required for each glucose molecule recycled. The baseline Cori cycle flux was estimated to be 20 g/d, corresponding to 14% of hepatic glucose production, and was linearly increased by 4-fold (along with glycogenolysis) over the 12 month simulation [35].
Rather than allowing the normal decrease of liver mass with weight loss, the liver mass was preserved at its baseline value of 1.8 kg [36] resulting in a constant energy expenditure of 360 kcal/d [37]. The tumor mass was increased to 200 grams [38,39] and we assumed that the tumor specific metabolic rate was 150 kcal/kg/d [40]. The downstream effects of the imposed metabolic changes resulted in dynamic alterations of RMR, substrate utilization, gluconeogenesis (GNG), as well as body weight and composition.
Whole Body Metabolic Fluxes during Semi-Starvation and Cancer
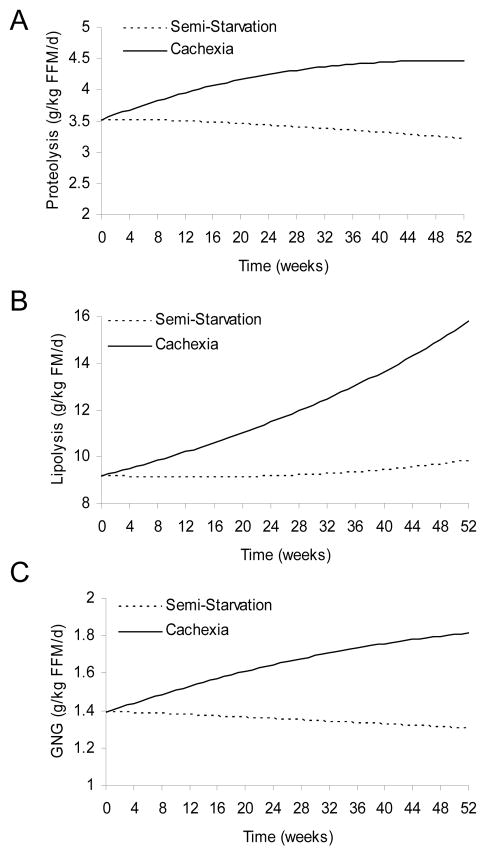
Figure 1 illustrates the simulated changes of whole-body metabolic fluxes that occurred with semi-starvation alone and the additional impact of the cancer-associated metabolic derangements. During weight loss the changes of body composition impact the absolute fluxes of lipolysis and proteolysis as described by the above equations, so we expressed the fluxes per unit body weight. After 12 months the proteolysis rate per kg BW was increased by 40% with cancer cachexia (Fig 1A, solid curves) versus semi-starvation alone (dotted curves). Cancer cachexia increased the whole-body lipolysis rate per kg BW by 50% after 12 months (Fig 1B) versus semi-starvation alone. Whereas these alterations were the direct result of model parameter changes simulating the observed changes in cancer cachexia, their downstream consequence was an increased supply of gluconeogenic precursors and a resulting 40% increase of GNG from amino acids and glycerol (Figure 1C) which is well within the range reported by Tayek et al. [35].
Figure 1.
Simulated whole-body metabolic fluxes during progressive semi-starvation alone (dotted curves) or with the additional metabolic derangements of cancer cachexia (solid curves). Cancer cachexia versus semi-starvation resulted in increased rates of (A) proteolysis, (B) lipolysis and (C) gluconeogenic rates (GNG) from amino acid and glycerol precursors.
Energy Expenditure Changes during Semi-starvation and Cancer
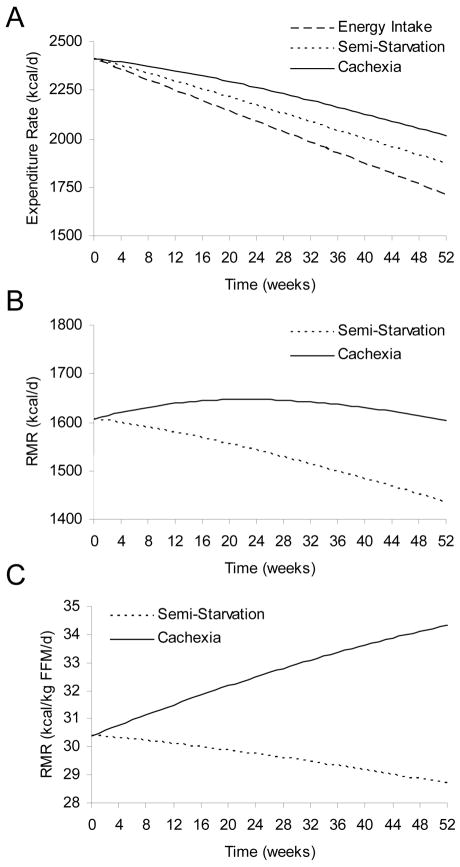
Additional downstream consequences of the primary metabolic changes are depicted in Figure 2, including the relative increase of total energy expenditure and RMR with cancer cachexia (solid curves) versus semi-starvation alone (dotted curves). Figure 2A shows the simulated progressive reduction of energy intake (dashed line) and the acceleration of negative energy balance as the energy intake was linearly decreased from its balanced value of 2400 kcal/d to 1700 kcal/d over the year. The total energy expenditure decreased in both semi-starvation and cancer cachexia simulations, but the widening energy gap between intake and expenditure was more severe with cancer cachexia.
Figure 2.
Simulated changes of energy expenditure during progressive semi-starvation alone (dotted curves) or with the additional metabolic derangements of cancer cachexia (solid curves). (A) Total energy expenditure decreases over time as energy intake was progressively decreased (dashed line), but cancer cachexia resulted in an increased total energy expenditure when compared with semi-starvation alone. (B) Absolute resting metabolic rate (RMR) fell during semi-starvation alone, but was approximately maintained during progression of cancer cachexia. (C) RMR corrected for fat free mass (FFM) markedly increased in cancer cachexia whereas semi-starvation alone caused a modest decline of RMR per kg FFM.
Figure 2B demonstrates that the absolute RMR in semi-starvation decreased over time whereas cancer cachexia resulted in the absolute RMR generally being maintained. Total energy expenditure decreased during semi-starvation both due to reductions of physical activity as well as adaptations of resting metabolism, but total energy expenditure decreased in cancer cachexia primarily because of reduced physical activity. This conforms to published data on total energy expenditure and RMR in patients with cancer cachexia versus healthy control subjects not losing weight [22–24].
Figure 2C demonstrates that the RMR per kg FFM slowly decreased with semi-starvation but progressively increased with cancer cachexia. The relative increase of RMR in the simulation of cancer cachexia was the result of increased substrate turnover and GNG, as well as an increased proportion of the body comprising tissues with a high specific metabolic rate (i.e., tumor and liver) in the face of a concurrent decrease in the mass of tissues with low specific metabolic rate (i.e., muscle and adipose tissue). The simulations compared favorably with the data of Fearon et al. who observed that after 7 months of progressive malignant disease, lung cancer patients had an RMR that was 3 kcal/kg FFM/d greater than weight-losing subjects without cancer [31]. Thus, cancer cachexia results in a persistent state of relative hyper-metabolism and hyper-catabolism [1,2,4], especially in patients with evidence of inflammation [41]. In contrast, semi-starvation causes a decline of RMR that attenuates weight loss [12,20] [21].
Body Composition Changes during Semi-starvation and Cancer
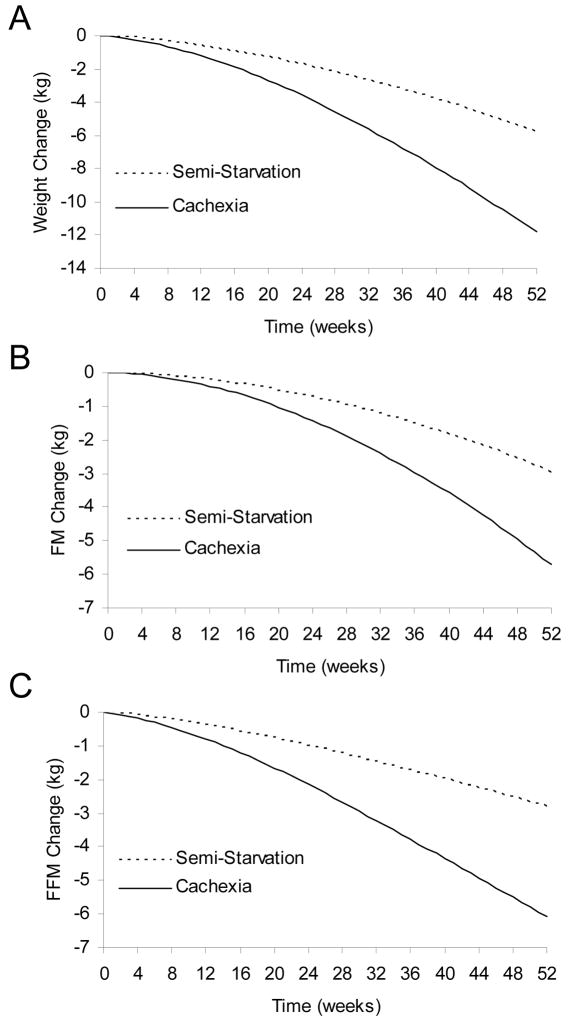
Figure 3A shows that semi-starvation caused a progressive loss of BW reaching 5.8 kg after one year. However, the additional metabolic derangements of cancer cachexia resulted in twice as much weight loss (11.8 kg) over the same interval. About half of the overall weight loss was accounted for by loss of FFM in both semi-starvation and cancer cachexia (Figures 3B, 3C). The relative loss of FFM versus FM can depend on the initial body composition and magnitude of weight loss [42], as well as physical activity or the protein content of the diet [43]. Nevertheless, our simulation results are consistent with the average relative body composition changes observed in patients with cancer cachexia versus normal healthy subjects [36,44–46].
Figure 3.
Simulated changes of body weight and composition during progressive semi-starvation alone (dotted curves), or with the additional metabolic derangements of cancer cachexia (solid curves). (A) Cancer cachexia resulted in twice as much body weight lost versus semi-starvation alone. (B) Cancer cachexia resulted in twice as much body fat mass (FM) lost versus semi-starvation alone. (C) Cancer cachexia resulted in twice as much fat-free mass (FFM) lost versus semi-starvation alone.
The simulated changes of body composition are the consequence of macronutrient imbalances resulting from a mismatch between macronutrient intake and multiple interrelated elements of whole-body metabolism [15]. Whereas the macronutrient intake was the same in semi-starvation and cancer cachexia simulations, the elevated total energy expenditure in cancer cachexia resulted in a greater energy deficit leading to increased tissue catabolism. The amount of fat versus protein catabolism in the model results from the altered metabolic fluxes and substrate competition in metabolically active tissues. These processes are mediated by complex endocrine mechanisms that continue to be actively investigated and the computational model simulates the consequence of these metabolic changes, whatever their underlying mechanism.
Simulated Effect of Lipolysis Inhibition in Cancer Cachexia
The computational model can be used to predict the effects of an intervention that modifies one or more of the whole-body metabolic fluxes. For example, it was recently suggested that a potentially useful therapy for cancer cachexia would be to decrease the abnormally high adipose tissue lipolysis rate via a hormone sensitive lipase inhibitor [16]. We simulated such a therapy by progressively inhibiting lipolysis up to a maximum of 50% after 12 months while maintaining the other metabolic changes of cancer cachexia (i.e., we set the parameter δF = −0.5 years−1).
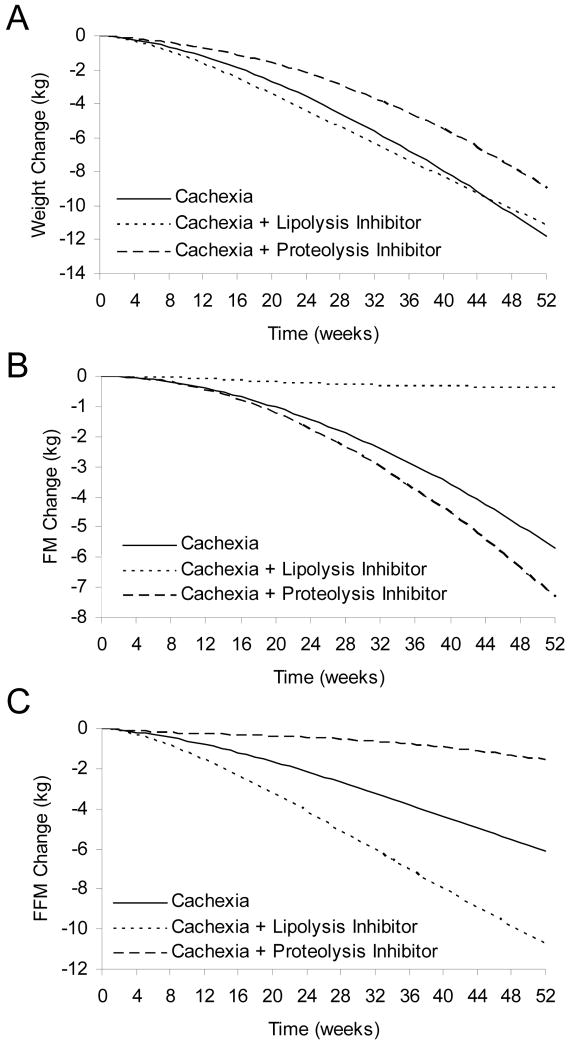
Figure 4A illustrates that inhibiting lipolysis (dotted curve) resulted in no significant attenuation of weight loss versus untreated cachexia (solid curve). However, lipolysis inhibition had the intended effect of preserving body fat mass (Fig 4B), but at the expense of an increased deterioration of lean body mass (Fig 4C). This is a particularly troubling prediction since skeletal muscle wasting would likely contribute the bulk of the additional lean mass loss. Closer examination of the simulation results showed that the underlying physiological mechanism was that lipolysis inhibition reduced the supply of fatty acids for oxidation and therefore increased protein catabolism was required to meet energy needs despite the fact that total energy expenditure was indeed reduced towards that of semi-starvation alone (not shown). This example illustrates that despite having the desired effect on preserving fat mass, lipolysis inhibition may have unintended and adverse metabolic consequences that are predicted using a computational model.
Figure 4.
Simulated changes of body weight and composition during cancer cachexia (solid curves), cancer cachexia with the inhibition of lipolysis (dotted curves), or cancer cachexia with the inhibition of proteolysis (dashed curves). (A) Lipolysis inhibition did not significantly impact the weight loss of cancer cachexia, but proteolysis inhibition attenuated weight loss. (B) Lipolysis inhibition tended to preserve FM during cancer cachexia, but proteolysis inhibition caused a further loss of FM. (C) Lipolysis inhibition exacerbated the loss of FFM during cancer cachexia, but proteolysis inhibition tended to preserve FFM.
Simulated Effect of Proteolysis Inhibition in Cancer Cachexia
We simulated proteolysis inhibition by not allowing the increase of whole body proteolysis that typically occurs along with the other metabolic derangements of cancer cachexia (i.e., we set the parameter δP = 0 years−1). The dashed curve in Figure 4A illustrates that inhibiting proteolysis resulted in an attenuation of weight loss despite a slightly increased deterioration of body fat mass (Fig. 4B). Proteolysis inhibition resulted in a significant preservation of lean body mass (Fig 4C) suggesting that such an intervention may be a good therapy for maintenance of skeletal muscle mass and strength in patients with cancer cachexia. However, such a benefit may come at the price of a slightly increased rate of body fat loss.
Simulated effects of Normalizing Food Intake
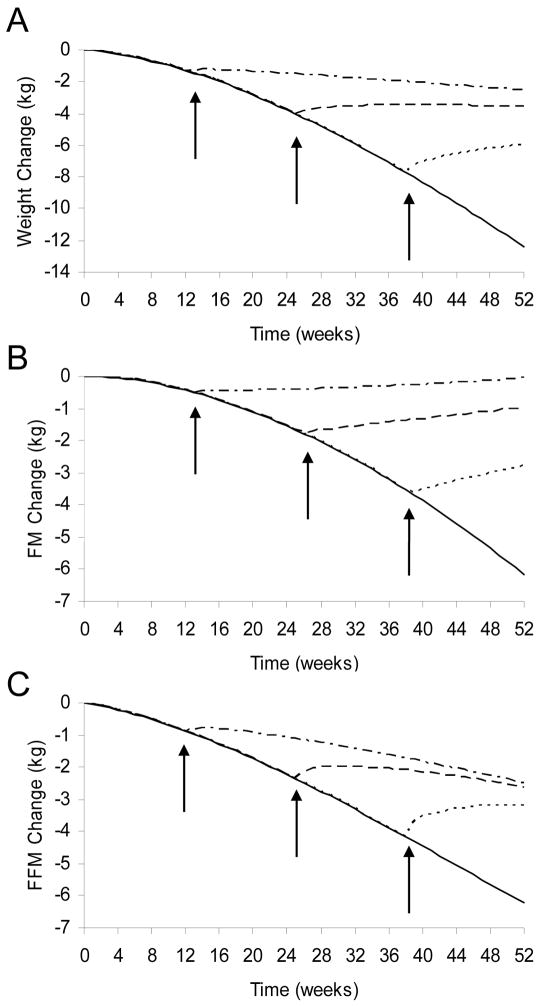
Rather than continuing to allow food intake to fall, we simulated the effects of normalizing intake to the baseline level of 2400 kcal/d after 3, 6, or 9 months of cachexia progression. Normalizing food intake after 3 months (Fig 5A; dashed dotted curve) caused a slowing of weight loss compared with the continued progression of cachexia and anorexia (solid line). Normalization of food intake after 6 months (dashed curves) halted the fall of BW and increased FM, but FFM continued to fall after a transient increase. In contrast, both FM and FFM increased when food intake was normalized after 9 months (dotted curves).
Figure 5.
Simulated effects of normalizing food intake after 3, 6, and 9 months of cancer cachexia. (A) The expected fall of BW with cancer cachexia (solid line) was attenuated when food intake was normalized after 3 months (dashed dotted curve). But weight loss was halted when intake was normalized after 6 months (dashed curve) and BW increased when intake was normalized after 9 months (dotted curve). (B) Normalization of food intake at all three time points caused FM to increase. (C) FFM continued to decrease after food intake was normalized at 3 and 6 months, but FFM increased when intake was normalized after 9 months. The asymptotic BW and composition are mathematically independent of when the nutritional support is delivered.
These simulations suggest that weight loss can be halted or partially reversed depending on the timing of nutritional support. Weight gain was observed when food intake was normalized after 9 months but not after 3 or 6 months. Importantly, the asymptotic final body weight and composition are mathematically independent of when nutritional normalization is initiated. Therefore, weight gain was observed with late nutritional support only because significantly more weight loss was allowed to occur prior to the intervention. Rather than being a sign of therapeutic success, weight gain may reflect a failure to introduce nutritional support soon enough.
Conclusion
There is a paucity of data on the longitudinal changes of whole-body metabolism and body composition in patients with progressive cancer cachexia. Unfortunately, it would be prohibitively difficult and invasive to attempt a comprehensive study in such patients that includes measurements of whole body metabolic fluxes, energy expenditure, physical activity, food intake, and body composition change. In the absence of such a study, the best we can do is piece together information from separate studies to help understand this complex and serious disorder.
We used a computational model to integrate a variety of published data on the primary metabolic changes that occur in cancer cachexia: including increased proteolysis, lipolysis, Cori cycling, tumor growth, and maintenance of liver mass. We introduced these defects linearly over a 12 month period along with reductions of food intake and physical activity. These metabolic changes may be sufficient to explain the observed increases of RMR and GNG in cancer cachexia since the simulated RMR and GNG rates were consistent with existing data [31,35] and were the downstream consequence of the primary metabolic parameter changes. Furthermore, the simulated relative changes of FFM and FM were consistent with body composition changes observed in cancer patients versus normal subjects [36,44–46].
While computational models of cancer cachexia provide a useful conceptual framework for integrating clinical data, we caution against the use of such models to predict the clinical course of an individual patient. In fact, our model suggests that the high inter-individual variability of the primary metabolic defects, along with the variability of food intake, physical activity level and initial body composition, can have a significant impact on the projected severity and pattern of wasting. Since it would be impractical to attempt to measure all of these parameters in an individual patient, the computational model is best applied to help understand and predict average responses and investigate the sensitivity of these responses to changes in model parameters.
Computational models can be used to investigate the potential therapeutic benefits of various interventions. For example, our simulations offered an explanation of the clinical observation that orexigenic drug treatments or aggressive nutritional support tend to attenuate the rate of weight loss, but rarely result in significant weight gain or weight normalization [47,48]. These conclusions underscore the limitations of applying nutritional support as a sole therapy since unabated metabolic changes can undermine their efficacy.
We also simulated the effects of inhibiting proteolysis and lipolysis as potential treatments for cancer cachexia. Importantly, the computational model is not a black box and was used to understand the physiological basis of each prediction. The simulations suggest that proteolysis inhibition may be a reasonable therapeutic target for cancer cachexia in patients with appreciable fat reserves. In contrast, inhibiting lipolysis may not be a good therapeutic target because the decreased supply of fatty acids during negative energy balance necessitated greater protein catabolism to meet the energetic needs and would likely result in significant deterioration of skeletal muscle mass.
Computational model simulations alone should not be used to rule out a potential therapeutic target; however such models provide a useful tool for clinical study design. For example, our simulation of lipolysis inhibition in cancer cachexia suggests that nitrogen balance and urinary creatinine measurements should be performed during any clinical trial of lipolysis inhibition since such measurements may provide an early indication of increased muscle wasting as predicted by the model. Thus, in addition to providing a conceptual framework for integrating clinical data, computational models are also an important tool for helping design clinical investigations as well as to improve our understanding of the complex metabolic state of cancer cachexia.
Acknowledgments
FUNDING INFORMATION: Kevin Hall is supported by the Intramural Research Program of the NIH, NIDDK. Vickie Baracos is supported by grants from the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and the Alberta Cancer Board.
Abbreviations
- ATP
adenosine triphosphate
- BW
body weight
- GNG
gluconeogenesis
- FFM
body fat-free mass
- FM
body fat mass
- PAL
physical activity level
- RMR
resting metabolic rate
References
- 1.Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol. 1999;8:133–141. doi: 10.1016/s0960-7404(99)00045-6. [DOI] [PubMed] [Google Scholar]
- 2.Barber MD, Ross JA, Fearon KC. Disordered metabolic response with cancer and its management. World J Surg. 2000;24:681–689. doi: 10.1007/s002689910110. [DOI] [PubMed] [Google Scholar]
- 3*.Skipworth RJ, Stewart GD, Dejong CH, et al. Pathophysiology of cancer cachexia: Much more than host-tumour interaction? . Clin Nutr. 2007 doi: 10.1016/j.clnu.2007.03.011. This review describes the complex interactions between tumor and host during cancer cachexia and highlights the effect of physical activity and protein kinetics. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. doi: 10.1038/nrc927. [DOI] [PubMed] [Google Scholar]
- 5.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 6.Ovesen L, Hannibal J, Mortensen EL. The interrelationship of weight loss, dietary intake, and quality of life in ambulatory patients with cancer of the lung, breast, and ovary. Nutr Cancer. 1993;19:159–167. doi: 10.1080/01635589309514246. [DOI] [PubMed] [Google Scholar]
- 7.Ramos EJ, Suzuki S, Marks D, et al. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care. 2004;7:427–434. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 8*.Saini A, Al-Shanti N, Stewart CE. Waste management - cytokines, growth factors and cachexia . Cytokine Growth Factor Rev. 2006;17:475–486. doi: 10.1016/j.cytogfr.2006.09.006. This review highlights the many molecular signals believed to play a role in cancer cachexia. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GF., Jr Starvation in man. N Engl J Med. 1970;282:668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 10 **.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. This review highlights the complex metabolic adaptations in humans during starvation and was written by one of the pioneers in the field. [DOI] [PubMed] [Google Scholar]
- 11.Elia M. Hunger disease. Clin Nutr. 2000;19:379–386. doi: 10.1054/clnu.2000.0157. [DOI] [PubMed] [Google Scholar]
- 12.Keys A. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 13.Owen OE, Smalley KJ, D’Alessio DA, et al. Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis] Am J Clin Nutr. 1998;68:12–34. doi: 10.1093/ajcn/68.1.12. [DOI] [PubMed] [Google Scholar]
- 14.Emery PW. Cachexia in experimental models. Nutrition. 1999;15:600–603. doi: 10.1016/s0899-9007(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 15**.Hall KD. Computational model of in vivo human energy metabolism during semistarvation and refeeding. Am J Physiol Endocrinol Metab. 2006;291:E23–37. doi: 10.1152/ajpendo.00523.2005. This paper describes the development of a computational model of human macronutrient metabolism and the relationship to body composition change and integrates the data from many important metabolic studies in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Agustsson T, Ryden M, Hoffstedt J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. This study investigates the cellular mechanisms of enhanced lipolysis in cancer cachexia using primary adipocytes obtained via subcutaneous fat biopsies in cancer patients with and without cachexia. [DOI] [PubMed] [Google Scholar]
- 17.Krems C, Luhrmann PM, Strassburg A, et al. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59:255–262. doi: 10.1038/sj.ejcn.1602066. [DOI] [PubMed] [Google Scholar]
- 18.Bosaeus I, Daneryd P, Lundholm K. Dietary intake, resting energy expenditure, weight loss and survival in cancer patients. J Nutr. 2002;132:3465S–3466S. doi: 10.1093/jn/132.11.3465S. [DOI] [PubMed] [Google Scholar]
- 19.Hutton JL, Martin L, Field CJ, et al. Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am J Clin Nutr. 2006;84:1163–1170. doi: 10.1093/ajcn/84.5.1163. [DOI] [PubMed] [Google Scholar]
- 20.Taylor HL, Keys A. Adaptation to caloric restriction. Science. 1950;112:215–218. doi: 10.1126/science.112.2904.215. [DOI] [PubMed] [Google Scholar]
- 21.Major GC, Doucet E, Trayhurn P, et al. Clinical significance of adaptive thermogenesis. Int J Obes (Lond) 2007;31:204–212. doi: 10.1038/sj.ijo.0803523. [DOI] [PubMed] [Google Scholar]
- 22.Gibney E, Elia M, Jebb SA, et al. Total energy expenditure in patients with small-cell lung cancer: results of a validated study using the bicarbonate-urea method. Metabolism. 1997;46:1412–1417. doi: 10.1016/s0026-0495(97)90140-2. [DOI] [PubMed] [Google Scholar]
- 23.Jebb SA. Sir David Cuthbertson Medal Lecture. Energy metabolism in cancer and human immunodeficiency virus infection. Proc Nutr Soc. 1997;56:763–775. doi: 10.1079/pns19970077. [DOI] [PubMed] [Google Scholar]
- 24.Moses AW, Slater C, Preston T, et al. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002. doi: 10.1038/sj.bjc.6601620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eden E, Edstrom S, Bennegard K, et al. Glycerol dynamics in weight-losing cancer patients. Surgery. 1985;97:176–184. [PubMed] [Google Scholar]
- 26.Jeevanandam M, Horowitz GD, Lowry SF, et al. Cancer cachexia and the rate of whole body lipolysis in man. Metabolism. 1986;35:304–310. doi: 10.1016/0026-0495(86)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Wolfe RR. Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J Clin Invest. 1990;86:1403–1408. doi: 10.1172/JCI114854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legaspi A, Jeevanandam M, Starnes HF, Jr, et al. Whole body lipid and energy metabolism in the cancer patient. Metabolism. 1987;36:958–963. doi: 10.1016/0026-0495(87)90132-6. [DOI] [PubMed] [Google Scholar]
- 29.Zuijdgeest-van Leeuwen SD, van den Berg JW, Wattimena JL, et al. Lipolysis and lipid oxidation in weight-losing cancer patients and healthy subjects. Metabolism. 2000;49:931–936. doi: 10.1053/meta.2000.6740. [DOI] [PubMed] [Google Scholar]
- 30.Eden E, Ekman L, Bennegard K, et al. Whole-body tyrosine flux in relation to energy expenditure in weight-losing cancer patients. Metabolism. 1984;33:1020–1027. doi: 10.1016/0026-0495(84)90231-2. [DOI] [PubMed] [Google Scholar]
- 31.Fearon KC, Hansell DT, Preston T, et al. Influence of whole body protein turnover rate on resting energy expenditure in patients with cancer. Cancer Res. 1988;48:2590–2595. [PubMed] [Google Scholar]
- 32.McMillan DC, Preston T, Fearon KC, et al. Protein synthesis in cancer patients with inflammatory response: investigations with [15N]glycine. Nutrition. 1994;10:232–240. [PubMed] [Google Scholar]
- 33.Melville S, McNurlan MA, Calder AG, et al. Increased protein turnover despite normal energy metabolism and responses to feeding in patients with lung cancer. Cancer Res. 1990;50:1125–1131. [PubMed] [Google Scholar]
- 34.Richards EW, Long CL, Nelson KM, et al. Protein turnover in advanced lung cancer patients. Metabolism. 1993;42:291–296. doi: 10.1016/0026-0495(93)90076-z. [DOI] [PubMed] [Google Scholar]
- 35.Tayek JA, Katz J. Glucose production, recycling, Cori cycle, and gluconeogenesis in humans: relationship to serum cortisol. Am J Physiol. 1997;272:E476–484. doi: 10.1152/ajpendo.1997.272.3.E476. [DOI] [PubMed] [Google Scholar]
- 36.Heymsfield SB, McManus CB. Tissue components of weight loss in cancer patients. A new method of study and preliminary observations. Cancer. 1985;55:238–249. doi: 10.1002/1097-0142(19850101)55:1+<238::aid-cncr2820551306>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Elia M. Organ and tissue contribution to metabolic rate. In: Kinney J, Tucker H, editors. Energy metabolism: Tissue determinants and cellular corollaries. Raven Press; 1992. [Google Scholar]
- 38.Nestle U, Kremp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]
- 39.Zhou SM, Wong TZ, Marks LB. Using FDG-PET activity as a surrogate for tumor cell density and its effect on equivalent uniform dose calculation. Med Phys. 2004;31:2577–2583. doi: 10.1118/1.1779372. [DOI] [PubMed] [Google Scholar]
- 40.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 41.Fearon KC, Barber MD, Falconer JS, et al. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. 1999;23:584–588. doi: 10.1007/pl00012351. [DOI] [PubMed] [Google Scholar]
- 42*.Hall KD. Body fat and fat-free mass inter-relationships: Forbes’s theory revisited. Br J Nutr. 2007;97:1059–1063. doi: 10.1017/S0007114507691946. This paper describes a simple model of the factors contributing to the relative loss of body fat versus fat- free mass with weight loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layman DK, Evans E, Baum JI, et al. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 44.Cohn SH, Gartenhaus W, Sawitsky A, et al. Compartmental body composition of cancer patients by measurement of total body nitrogen, potassium, and water. Metabolism. 1981;30:222–229. doi: 10.1016/0026-0495(81)90145-1. [DOI] [PubMed] [Google Scholar]
- 45.MacFie J, Burkinshaw L. Body composition in malignant disease. Metabolism. 1987;36:290–294. doi: 10.1016/0026-0495(87)90191-0. [DOI] [PubMed] [Google Scholar]
- 46.Watson WS, Sammon AM. Body composition in cachexia resulting from malignant and non-malignant diseases. Cancer. 1980;46:2041–2046. doi: 10.1002/1097-0142(19801101)46:9<2041::aid-cncr2820460923>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jatoi A, Rowland K, Loprinzi CL, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer-associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. J Clin Oncol. 2004;22:2469–2476. doi: 10.1200/JCO.2004.06.024. [DOI] [PubMed] [Google Scholar]